Abstract
The emergence of several high profile infectious diseases in recent years has focused attention on our need to understand the ecological factors contributing to the spread of infectious diseases. West Nile virus (WNV) is a mosquito-borne zoonotic disease that was first detected in the United States in 1999. The factors accounting for variation in the prevalence of WNV are poorly understood, but recent ideas suggesting links between high biodiversity and reduced vector-borne disease risk may help account for distribution patterns of this disease. Since wild birds are the primary reservoir hosts for WNV, we tested associations between passerine (Passeriform) bird diversity, non-passerine (all other orders) bird diversity and virus infection rates in mosquitoes and humans to examine the extent to which bird diversity is associated with WNV infection risk. We found that non-passerine species richness (number of non-passerine species) was significantly negatively correlated with both mosquito and human infection rates, whereas there was no significant association between passerine species richness and any measure of infection risk. Our findings suggest that non-passerine diversity may play a role in dampening WNV amplification rates in mosquitoes, minimizing human disease risk.
Keywords: West Nile virus, vector-borne disease, zoonoses, dilution effect, biodiversity, birds
1. Introduction
Placing a value on biological diversity can be difficult, but recent evidence indicating that high species diversity may reduce human exposure to vector-borne diseases suggests a novel function of biodiversity with quantifiable value for human health (Ostfeld & Keesing 2000a,b). A primary mechanism by which biodiversity may moderate disease risk, referred to as the ‘dilution effect’, has been described for Lyme disease (Ostfeld & Keesing 2000b; Schmidt & Ostfeld 2001; LoGiudice et al. 2003) and may also operate for a wide range of other vector-borne diseases (Ostfeld & Keesing 2000a; Holt et al. 2003; Telfer et al. 2005). The dilution effect predicts that infection rates among vectors, and ultimately human infection risk, will be lower in highly diverse host communities where incompetent reservoir hosts dilute rates of disease transmission between vectors and highly competent hosts. Although a number of pathogens spread by generalist vectors might be subject to the dilution effect (Ostfeld & Keesing 2000a), it is unclear how broadly this model applies in natural disease systems. More generally, our understanding of the extent to which patterns of biodiversity affect human disease is still very limited. In this study, we tested associations between host diversity and disease risk focusing on West Nile virus (WNV), an emerging zoonotic disease in the United States.
WNV is a mosquito-borne disease for which wild birds serve as the primary reservoir hosts (Work et al. 1955; Taylor et al. 1956; Hayes 1989). The virus first appeared in the United States in 1999 in New York City (Centers for Disease Control and Prevention (CDC) 1999) and by the end of 2004 had spread to 48 states (CDC 2003, 2004), Canada (Lindsay et al. 2003), Mexico (Estrada-Franco et al. 2003) and the Caribbean (Dupuis et al. 2003; Komar, O. et al. 2003; Quirin et al. 2004). Human WNV infections occur when conditions promote virus amplification within avian and mosquito populations, leading to spillover into incidental host groups. Evidence from both Old World and New World studies suggests that Passeriform (passerine) birds tend to be the most competent avian WNV hosts, whereas non-passerines are much poorer hosts (Work et al. 1955; Komar, N. et al. 2003; Peterson et al. 2004). If non-passerine birds are relatively incompetent WNV hosts, and as suggested by the dilution effect these birds act as alternative blood meal sources for mosquitoes reducing contact rates between vectors and highly competent virus hosts, avian communities composed of high diversities of non-passerine birds may be less able to sustain WNV epizootics. Therefore, based on a dilution effect model, the rate at which vectors acquire and transmit virus should decline with increasing non-passerine diversity, resulting in reduced prevalence of both mosquito infections and human disease.
The predicted negative association between host diversity and disease risk should only hold when individual species act as poor disease hosts. If species tend to be highly competent reservoirs, high species diversity may actually increase disease prevalence. This opposing effect, called a ‘rescue effect’ by Ostfeld & Keesing (2000b), may describe the relationship between passerine diversity and WNV prevalence if multiple passerine species serve as competent virus hosts. To test whether WNV infection rates declined with increasing non-passerine diversity and increased with increasing passerine diversity, we examined associations between WNV infection rates and non-passerine and passerine species richness (number of species) at two spatial scales in Louisiana, USA. WNV was first detected in Louisiana in 2001, and in 2002 the state was a focus of a human WNV disease epidemic that killed 24 people and cost an estimated $20.1 million (Zohrabian et al. 2004). Since Culex mosquitoes are the primary vectors involved in the avian amplification cycle of WNV in North America (Andreadis et al. 2001; Sardelis et al. 2001; Turell et al. 2001), we first looked at associations between Culex infection rates and non-passerine and passerine species richness across a series of study sites located in St Tammany Parish, Louisiana. Next, we explored whether patterns observed in our field study would hold for human infection prevalence at a broader scale by examining associations between human disease incidence and avian species richness at the county level across the entire state.
2. Methods
(a) Field study
(i) Study sites
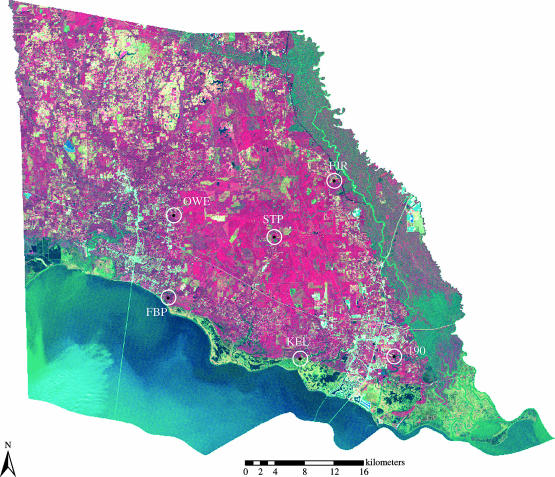
Our six study sites (190, Highway 190; KEL, Keller Road; FBP, Fontainebleau State Park; STP, St Tammany Pipeline; OWE, Owens Road; FJR, Frank Jackley Road) were located in the southern half of St Tammany Parish, Louisiana. Land cover composition varied across sites (figure 1). 190 was situated in an area of commercially developed land interspersed with small fragments of loblolly pine (Pinus taeda) forest and some open marshland. The KEL site comprised almost equal parts brackish marsh and forested cover with a small number of residential buildings. Forest patches were dominated by loblolly pine and Chinese tallow (Sapium sebiferums). FBP was located in a state park with mixed forest dominated by loblolly pine, slash pine (Pinus elliottii) and water oak (Quercus nigra) and large areas of open water (Lake Ponchartrain) and wetland cover. The STP site was located on an active pine plantation consisting of tracts of pine forest at different stages of management and smaller forested wetland areas dominated by water tupelo (Nyssa aquatica) and bald cypress (Taxodium disticum). Lastly, both OWE and FJR were situated in low-density residential areas interspersed with patches of pine forest. FJR also included a relatively small area of wetland habitat.
Figure 1.
Map of St Tammany Parish, Lousiana with the locations of study sites. On the false colour infrared image, vegetated areas appear in shades of red and brown, open water in blue and black, wetland areas in greenish grey to reddish blue, and developed land in light blue and white.
(ii) Mosquito collections and virus testing
We collected data on mosquito WNV infection rates at the six study sites during the summer and early fall of 2003. Adult mosquitoes were collected weekly on a 7 day cycle at each site between 3 June and 28 October 2003. Collections were made using two CDC light traps baited with CO2 (Sudia & Chamberlain 1962) and one gravid trap (Reiter 1983). Traps were placed at a single location in forested areas and the dominant tree species at trapping stations were loblolly pine and/or slash pine. All traps were run from dusk until dawn after which they were retrieved and mosquitoes transported live to the laboratory. Mosquito specimens were stored at −20 °C for approximately 24–36 h, then sorted by collection date and site and identified to species level using a chill table and stereomicroscope. After identification, specimens were pooled by species into groups of between 1 and 60 individuals and shipped on dry ice to the CDC (Fort Collins, CO) where they were stored at −80 °C until virus testing.
To measure mosquito infection rates, mosquito pools were triturated in 1.75 ml of BA-1 diluent using a Mixer Mill apparatus, clarified by centrifugation (Nasci et al. 2002), and then tested for WNV by Vero cell culture plaque assay (Beaty et al. 1989) and by TaqMan Reverse Transcriptase–Polymerase Chain Reaction using WNV-specific primers (Lanciotti et al. 2000). Two parameters describing patterns of infection in the mosquito population were calculated for each study site: (i) mosquito infection prevalence (MLE, maximum-likelihood estimate of the mosquito infection rate) and (ii) density of infected mosquitoes (DIM=mosquitoes/trap night×MLE). We estimated mosquito infection prevalence using the maximum-likelihood (MLE) method (Chiang & Reeves 1962; Walter et al. 1980). Calculations were done using the Microsoft Excel Add-In PooledInfRate 2.0 (Biggerstaff 2004) and infection prevalence is reported as the MLE per 1000 mosquitoes. The DIM was calculated as the total no. of mosquitoes collected/no. of trap nights×MLE. We calculated number of trap nights by summing the number of traps set at a site per day (maximum=3) over the total number of trapping days (22).
(iii) Avian surveys
We used the point transect method to estimate avian species richness at the study sites (Bibby et al. 2000). At each site, the location of the mosquito trap was used as the first transect and nine additional transect points were established within a 1 km radius of the trap for a total of 10 point transects within a 3.14 km2 study area. The study area size for point transects was selected to provide an adequate sample of the avian community within the dispersal range of host-seeking mosquitoes which can range from 1 to 5 km, but is most commonly under 1 km (Service 1997). Transect locations were determined using the random point generator extension (Jenness Enterprises, Flagstaff, AZ) for ArcView 3.3 (ESRI, Redlands, CA), and all transect points were spaced greater than or equal to 200 m apart. A single observer surveyed all 60 transect points once from 24 June to 7 July 2003. Because species composition of breeding birds in the southern US tends to remain relatively stable throughout the summer, we expected that bird surveys done during this time period would provide representative relative species richness estimates of resident birds for comparison with mosquito data. For all sites, surveys began at dawn and were completed by 11.30 h. Each point transect lasted for 10 min during which all bird species seen and heard were recorded. Birds observed visually that could not be identified to species level were classified into taxonomic groupings (e.g. gull, raptor) and the horizontal distances between all birds and the transect point were recorded in intervals of 0–10, 11–20, 21–30, 31–40, 41–50 and 51–100 m. Backup tape recordings of each transect were also made using a Marantz PMD 201 recorder and an AKG D230 omnidirectional microphone. When necessary the tapes were used to verify the species identity of bird songs or calls recorded in the field. For each site, species lists from all 10 point transects were combined and the total number of passerine (order Passeriformes) and non-passerine (all other orders) species detected were used as estimates of relative avian species richness per site. In addition, we used the computer program Distance (Thomas et al. 2005) to estimate the detection probability of non-passerines and passerines across sites and to calculate non-passerine abundance at each site. Across all six study sites, we detected 56 bird species including 35 passerines and 21 non-passerines (electronic supplementary material). The total number of species detected per site ranged from 21 to 31 (electronic supplementary material).
(b) County-level data
We obtained information on human WNV case counts in Louisiana from the Louisiana Office of Public Health (http://www.oph.dhh.state.la.us/) and used these data to calculate disease incidence rates (number of cases per 100 000 population) by county in 2002 and 2003. Additional data on human population size, density and county area were obtained from the United States Census Bureau (http://factfinder.census.gov). Datasets on state-wide bird distributions were obtained from the Louisiana breeding bird atlas for which ground surveys were conducted in 1996–1998 (Wiedenfeld & Swan 2000). Using the Atlas dataset, we compiled a list of all bird species recorded as possible, probable or confirmed breeders within each county. The total numbers of passerine and non-passerine bird species occurring in each county were used as estimates of avian species richness.
(c) Statistical analyses
We tested associations between mosquito infection rates and measures of avian species richness or abundance (non-passerine species richness, passerine species richness, non-passerine abundance) using simple linear regression analyses. We also repeated each test using multivariate regression analyses to control for the effects of mosquito abundance. For all analyses, measures of avian and mosquito abundance were log-transformed to normalize data distributions. The significance level was set at 0.05, but to reduce the probability of committing Type 1 errors we adjusted α for multiple comparisons using the sequential Bonferroni method (Rice 1989), where k is the number of analyses testing the same null hypothesis.
To test associations between avian diversity and human disease incidence we focused on counties that had reported at least one human WNV case, since a number of non-avian related factors (e.g. geography, climate, demography) can influence the presence or absence of human disease in a county (e.g. Ruiz et al. 2004). To test our predictions, we used multiple regression analysis with model simplification (Crawley 2002). Initial (maximal) models included four potential predictor variables: two measures of avian diversity (passerine species richness, non-passerine species richness); human population density, which can influence both mosquito densities and host–vector contact rates; and county area, since area was positively correlated with overall bird species richness (F1,62=53.4, r2=0.46, p<0.0001). To find minimal adequate models, the least significant predictor variables were deleted from the full models in a stepwise process until all variables remaining had p≤0.05. At each step, the Aikaike information criterion was used to evaluate competing models. Separate analyses were performed for 2002 and 2003, and we report minimal adequate models for both years. For all tests, disease incidence, human population density and county area were log-transformed to normalize data distributions. In addition, tests for spatial autocorrelation were performed using the S-Plus SpatialStats module (Insightful Corp., Seattle, WA).
3. Results
(a) Avian species richness and mosquito infection rates
WNV was detected in 20 Culex species mosquito pools at five out of six study sites (table 1). Seventeen out of the 20 positive pools came from Culex nigripalpus, so we calculated virus infection rates for (i) all Cx. species combined and (ii) Cx. nigripalpus only (table 1). In simple linear regression tests, non-passerine species richness was significantly negatively correlated with infection prevalence for all Cx. species (F1,4=19.3, r2=0.83, p=0.01; figure 2a) and Cx. nigripalpus (F1,4=15.9, r2=0.80, p=0.02; figure 2b). Similarly, there was a significant negative correlation between non-passerine species richness and the density of infected Cx. mosquitoes (F1,4=14.9, r2=0.79, p=0.02; figure 2c) and Cx. nigripalpus (F1,4=43.4, r2=0.92, p=0.003; figure 2d). By contrast, passerine species richness was not significantly correlated with either MLE (Cx. spp.: F1,4=1.5, r2=0.27, p=0.29; Cx. nigripalpus: F1,4=1.8, r2=0.32, p=0.25; figure 2e,f) or DIM (Cx. spp.: F1,4=0.75, r2=0.16, p=0.43; Cx. nigripalpus: F1,4=0.04, r2=0.01, p=0.86; figure 2g,h). Given the effects of non-passerine species richness, we also tested associations between non-passerine abundance and mosquito infection rates. Like non-passerine species richness, non-passerine abundance was significantly negatively correlated with both MLE (Cx. spp.: F1,4=19.7, r2=0.83, p=0.01; Cx. nigripalpus: F1,4=16.5, r2=0.81, p=0.02; figure 2i,j) and DIM (Cx. spp.: F1,4=19.4, r2=0.83, p=0.01; Cx. nigripalpus: F1,4=13.5, r2=0.77, p=0.02; figure 2k,l).
Table 1.
Summary of mosquito collections and infection rate estimates at study sites. (MLE, maximum-likelihood estimate of mosquito infection prevalence; DIM, density of infected mosquitoes.)
| Culex species | Culex nigripalpus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| site | total no. collected | pools tested (pos. pools) | MLE (per 1000) | 95% CI | DIM (per trap night) | total no. collected | pools tested (pos. pools) | MLE (per 1000) | 95% CI | DIM (per trap night) |
| 190 | 10 050 | 299 (2) | 0.20 | 0.04–0.65 | 33.5 | 4554 | 118 (2) | 0.44 | 0.08–1.45 | 33.4 |
| KEL | 22 259 | 703 (1) | 0.04 | 0.00–0.22 | 13.5 | 2502 | 106 (1) | 0.40 | 0.02–1.94 | 15.2 |
| FBP | 2775 | 215 (0) | 0 | 0.00–1.36 | 0 | 1132 | 71 (0) | 0 | 0.00–3.23 | 0 |
| STP | 3901 | 169 (7) | 1.86 | 0.82–3.69 | 110 | 1388 | 89 (7) | 2.45 | 1.08–4.84 | 51.5 |
| OWE | 2494 | 158 (8) | 3.34 | 1.57–6.32 | 126 | 3003 | 57 (5) | 3.85 | 1.44–8.54 | 175 |
| FJR | 1187 | 112 (2) | 1.69 | 0.30–5.50 | 30.4 | 826 | 50 (2) | 2.42 | 0.44–7.87 | 30.3 |
Figure 2.
Associations between measures of avian diversity or abundance and mosquito infection rates; r2=coefficient of determination for linear regression tests with *p≤0.05 and **p≤0.01. (a) Non-passerine species richness versus Cx. species infection prevalence (MLE). (b) Non-passerine species richness versus Cx. nigripalpus MLE. (c) Non-passerine species richness versus density of infected Cx. species (DIM). (d) Non-passerine species richness versus Cx. nigripalpus DIM. (e) Passerine species richness versus Cx. species MLE. (f) Passerine species richness versus Cx. nigripalpus MLE. (g) Passerine species richness versus Cx. species DIM. (h) Passerine species richness versus Cx. nigripalpus DIM. (i) Non-passerine abundance versus Cx. species MLE. (j) Non-passerine abundance versus Cx. nigripalpus MLE. (k) Non-passerine abundance versus Cx. species DIM. (l) Non-passerine abundance versus Cx. nigripalpus DIM.
Since vector abundance may play a role in determining mosquito infection rates, we repeated the analyses above controlling for the effects of mosquito abundance using multivariate regression tests. Each analysis included two predictor variables, a measure of avian species richness and either Cx. species or Cx. nigripalpus abundance. Non-passerine richness remained significantly negatively correlated with all measures of mosquito infection rate, and non-passerine abundance was significantly correlated with Cx. species and Cx. nigripalpus MLE but only marginally correlated with both measures of DIM (table 2). Even after controlling for mosquito abundance, passerine species richness was not significantly associated with either MLE or DIM (table 2). There was also no significant correlation between mosquito abundance and MLE or DIM except in one case where Cx. nigripalpus abundance was negatively correlated with Cx. nigripalpus MLE (table 2).
Table 2.
Associations between avian species richness and abundance, mosquito abundance and measures of mosquito infection rate. (Table shows regression results for measures of avian diversity and abundance included in multiple regression models with mosquito (Cx. species or Cx. nigripalpus) abundance as a covariate. r is the partial regression coefficient, significant p-values are in bold and * denotes significance levels that became non-significant after correcting for multiple comparisons with the sequential Bonferroni adjustment (k=2).)
| non-passerine richness+log mosquito abundance | passerine richness+log mosquito abundance | log non-passerine abundance+log mosquito abundance | ||||
|---|---|---|---|---|---|---|
| mosquito infection rate | non-passerine species richness | mosquito abundance | passerine species richness | mosquito abundance | non-passerine abundance | mosquito abundance |
| Cx. species MLE | r=−0.81 | r=−0.35 | r=0.16 | r=−0.45 | r=−0.82 | r=−0.22 |
| p=0.01 | p=0.09 | p=0.86 | p=0.62 | p=0.04 | p=0.40 | |
| Cx. nigripalpus MLE | r=−1.07 | r=−0.47 | r=1.01 | r=0.64 | r=−0.97 | r=−0.32 |
| p=0.0002 | p=0.002 | p=0.17 | p=0.35 | p=0.01 | p=0.19 | |
| Cx. species DIM | r=−0.88 | r=−0.02 | r=0.54 | r=0.18 | r=−0.98 | r=0.16 |
| p=0.05 | p=0.93 | p=0.60 | p=0.86 | p=0.03* | p=0.58 | |
| Cx. nigripalpus DIM | r=−0.95 | r=−0.02 | r=0.73 | r=0.88 | r=−0.84 | r=0.17 |
| p=0.01 | p=0.91 | p=0.34 | p=0.26 | p=0.05* | p=0.57 | |
(b) Avian species richness and human disease incidence
Out of 64 Louisiana counties, 41 reported human WNV cases in 2002 and 33 reported human cases in 2003. We constructed multivariate regression models for both years, beginning with four predictor variables: non-passerine species richness, passerine species richness, human population density and county area, and used model simplification to find minimal adequate models explaining significant variation in disease incidence. Only non-passerine richness entered the minimal adequate model for 2002 (F1,39=14.3, r2=0.27, p=0.0005; figure 3a), while both non-passerine richness and human population density were included in the model for 2003 (model: F2,30=16.6, r2=0.52, p<0.0001; population density: r=−0.53, p=0.0005; non-passerine richness: r=−0.34, p=0.019; figure 3b).
Figure 3.
Relationship between human WNV disease incidence by county and non-passerine species richness in (a) 2002 and (b) 2003. In minimum adequate multiple regression models, non-passerine species richness was the sole predictor of disease incidence in 2002 (r=−0.52, t=−3.79, p<0.001), and one of two predictors of disease incidence in 2003 (r=−0.34, t=−2.49, p<0.05).
Since spatial autocorrelation in aggregated epidemiological data can bias the results of ordinary least-squares regression (Cressie 1991), we examined whether spatial non-independence may have influenced our regression analyses using the Moran's I statistic. A Moran's I value close to zero indicates spatial independence, whereas values approaching −1 or +1 indicate negative or positive spatial autocorrelation. We found no evidence of strong spatial autocorrelation in raw WNV incidence rates across infected counties in 2002 (I=0.07) or 2003 (I=0.10) and in both cases the null hypothesis of spatial randomness could not be rejected (p=0.36). When we checked for spatial autocorrelation in the residuals of minimal adequate models for both years, we obtained similar patterns (2002: I=0.04, p=0.53; 2003: I=0.15, p=0.18) suggesting that spatial non-independence did not bias our results.
4. Discussion
Our results show a strong association between non-passerine species richness and WNV infection rates. WNV activity in Culex mosquitoes, including the proportion and density of infected mosquitoes, declined with increasing non-passerine species richness suggesting that virus amplification rates were lower at sites with more non-passerine species. Since Culex mosquitoes are thought to be the primary vectors involved in the avian cycle of WNV (Andreadis et al. 2001; Sardelis et al. 2001; Turell et al. 2001) and members of this genus likely serve as important bridge vectors moving virus from birds to humans (Fonseca et al. 2004), any reduction in Culex infection rates may dampen the size of WNV epizootics, potentially reducing human disease risk. The pattern we observed for all Culex species was in large part driven by a single species, Cx. nigripalpus, which was the most frequently infected mosquito in our study. When we looked solely at Cx. nigripalpus, non-passerine species richness was once again strongly associated with reduced mosquito infection prevalence and density. Culex nigripalpus is an opportunistic biter that has been implicated in human transmission of St Louis encephalitis (Zyzak et al. 2002; Shaman et al. 2003) and may also be an important epizootic and epidemic vector of WNV in certain regions of the United States (Sardelis et al. 2001; Rutledge et al. 2003; Turell et al. 2005).
Reductions in WNV infection rates in mosquitoes should result in lower human infection rates, since fewer contacts between humans and infected vectors will occur. If non-passerines have a dampening effect on WNV transmission in mosquito populations, this effect should also be apparent in humans. Therefore, given our field study results, we expected that human WNV disease incidence would also be negatively correlated with non-passerine species richness. In support of this prediction, we found a negative association between human disease incidence and non-passerine species richness in both 2002 and 2003. While these results suggest that non-passerine species richness plays a role in minimizing human infection risk, species richness alone explained much less of the variance in human infection rates across counties (27%) than in mosquito infection rates across sites (79–92%). Because the dataset we used to calculate avian species richness scores for the county analyses was compiled between 4 and 7 years before the WNV outbreaks of 2002–2003 (see §2), it is possible that the effects of non-passerine species richness on human disease are under- or overestimated in our models. Several other factors besides avian diversity are also likely to influence the incidence of human disease.
Human population density was the most important predictor of human WNV disease incidence in our model for 2003. Infection rates decreased with increasing population density, suggesting that people living in more populated urban areas have a lower risk of WNV infection. Human population density and non-passerine species richness, together, explained 52% of the variance in WNV incidence, indicating that factors affecting contact rates between humans and vectors such as urbanization and human behaviour are likely to combine with ecological variables to determine risk of human infection. Other studies have shown that demographic factors, including age, income and race, can be important predictors of WNV infection risk in humans (e.g. Ruiz et al. 2004). Environmental variables, such as climate, habitat and land cover, often associated with the prevalence of other vector-borne diseases (e.g. Beck et al. 1994; King et al. 2004; Zhou et al. 2004), may also play a role in WNV dynamics and could account for some of the unexplained variance in our models. Finally, many of the mosquito species implicated in the transmission of WNV have very catholic feeding habits, feeding on birds as well as a wide variety of mammals (Apperson et al. 2002; Gomes et al. 2003). Thus, local diversity of mammalian hosts, which we did not include in our analyses, may have also influenced human WNV infection risk.
In contrast with the patterns we observed for non-passerines, there was no significant correlation between passerine species richness and either mosquito infection rates or human disease incidence. Since the dilution effect should only hold when individual species are poor virus hosts, the lack of an association between passerine species richness and virus infection rates supports the idea that passerines, as a group, are more competent virus hosts than are non-passerines. Experimental work on reservoir competence in 25 bird species showing 7 out of 10 passerine species to be highly competent WNV hosts (reservoir competence index>1) compared to only 1 out of 15 non-passerine species (Komar, N. et al. 2003) supports this view. However, despite this evidence of high reservoir competence in multiple passerine species our prediction that infection rates would increase with increasing passerine species richness was not supported, possibly because the key species driving WNV transmission were common birds that occurred at all field sites and in all counties.
The pattern we observed for non-passerine species richness and WNV infection rates could be driven by two distinct mechanisms: a density-dependent dilution effect where dilution occurs because increased species richness leads to a decline in the density of competent hosts, or a frequency-dependent dilution effect where dilution is the direct result of increased biodiversity (Rudolf & Antonovics 2005). If our results were due to a density effect, then we would expect increased non-passerine species richness to be associated with reduced passerine (competent host) abundance. We found no association between non-passerine species richness and passerine abundance (F1,4=0.21, r2=0.05, p=0.67; V. O. Ezenwa 2005, unpublished data), which suggests that the effect we observed is more likely to be frequency dependent.
With a frequency-dependent effect, we expected that associations between non-passerine abundance and mosquito infection rates would be even stronger than the patterns we reported for non-passerine richness, because non-passerine abundance should be a better estimate of the proportion of individuals in the community that divert mosquito blood meals away from competent hosts. Non-passerine abundance was consistently negatively correlated with mosquito infection rates in the univariate tests, and in most cases patterns were as strong as those for non-passerine species richness, but not stronger (see figure 2). In the multivariate tests controlling for mosquito abundance, non-passerine abundance was significantly correlated with mosquito infection prevalence (MLE), but only marginally associated with the DIM after we applied a correction for multiple comparisons. In contrast, non-passerine richness was significantly correlated with both MLE and DIM in the multi-regression tests. At present, it is unclear whether these results indicate a real difference in the magnitude of the effects of non-passerine species richness versus abundance, since several other factors may account for why the abundance effects were not stronger. First, true relative bird abundance across sites may have been difficult to quantify and a high degree of uncertainty in our abundance estimates could have influenced our results. Second, our bird surveys did not detect nestlings which are often important blood meal sources for mosquitoes (Kale et al. 1972). Third, since abundance can change over short time periods, particularly during the breeding season, our measures of avian abundance which were based on single surveys done in June–July may not have reflected actual non-passerine abundances over the entire period of mosquito collections (June–October). All of these factors could have introduced significant biases in our abundance estimates, weakening associations between non-passerine abundance and WNV infection rates. However, the fact that our measures of non-passerine abundance did show relatively strong negative associations with mosquito infection rates supports the idea that non-passerines act as WNV dilution hosts via a frequency-dependent dilution effect.
The results of this study indicate a link between non-passerine diversity and WNV infection risk, supporting the hypothesis that increased biodiversity can moderate disease risk. We suggest that the dilution effect can at least partially explain the patterns we report here, but since the complex interactions among WNV host and vector ecology and virus dynamics are only beginning to be understood (Marra et al. 2004), this effect may be only one of several mechanisms accounting for observed patterns of this disease. For example, in our field study, the two sites with the lowest mosquito infection rates (FBP, KEL; see table 1) and highest non-passerine species richness were both located in areas with large and undisturbed natural wetlands (see figure 1). Although we did not find a significant correlation between wetland area and virus prevalence across study sites (V.O. Ezenwa, M.S. Godsey, R.J. King, S.C. Guptill 2005, unpublished data), land cover variation may be another potential factor driving the patterns we describe. A critical test for confirming the role of the dilution effect in WNV epidemiology will include demonstrating that shifts in avian diversity and community composition directly affect vector infection rates (e.g. LoGiudice et al. 2003). Nevertheless, the concordance between the patterns we observed in our field and county-level studies suggests that avian diversity and community composition have important implications for WNV transmission in humans.
Acknowledgments
We thank C. Palmisano, V. Taylor and the entire St Tammany Parish Mosquito Abatement District for invaluable assistance. We also thank D. Wesson, N. Lyons and S. Schwamberger for help with mosquito collections; B. Massery for assistance with avian fieldwork; K. Burkhalter, D. Charnetzky, L. Colton, C. Hodge, J. Lamb, J. Magowitz and S. Price for technical support; and S. Price, L. DeCola, L. Milheim and M. Coffey for helpful discussion. The Louisiana GAP Analysis Project provided access to the LA Breeding Bird Atlas GIS database. This work was funded by the United States Geological Survey and was performed while V.O.E. held a National Research Council Research Associateship Award.
Supplementary Material
References
- Andreadis T.G, Anderson J.F, Vossbrinck C.R. Mosquito surveillance for West Nile virus in Connecticut, 2000: isolation from Culex pipiens, Cx. restuans, Cx. salinarius, and Culiseta melanura. Emerg. Infect. Dis. 2001;7:670–674. doi: 10.3201/eid0704.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apperson C.S, et al. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J. Med. Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- Beaty B.J, Calisher C.H, Shope R.S. Arboviruses. In: Schmidt N.J, Emmons R.W, editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. American Public Health Association; Washington, DC: 1989. pp. 797–856. [Google Scholar]
- Beck L.R, et al. Remote sensing as a landscape epidemiologic tool to identify villages at high risk for malaria transmission. Am. J. Trop. Med. Hyg. 1994;5:271–280. doi: 10.4269/ajtmh.1994.51.271. [DOI] [PubMed] [Google Scholar]
- Bibby C.J, Burgess N.D, Hill D.A, Mustoe S.H. Bird census techniques. 2nd edn. Academic Press; London: 2000. [Google Scholar]
- Biggerstaff, B. J. 2004 PooledInfRate, version 2.0. An Excel Add-In to compute infection rates from pooled data: Division of Vector-Borne and Infectious Diseases, Centers for Disease Control and Prevention, Fort Collins, Colorado.
- Centers for Disease Control and Prevention. Outbreak of West Nile-like viral encephalitis—New York. Morb. Mortal. Wkly Rep. 1999;48:845–849. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. West Nile virus activity—United States, November 20–25, 2003. Morb. Mortal. Wkly Rep. 2003;52:1160. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. West Nile virus activity—United States, September 15–21, 2004. Morb. Mortal. Wkly Rep. 2004;53:875–876. [PubMed] [Google Scholar]
- Chiang C.L, Reeves W.C. Statistical estimation of virus infection rates in mosquito vector populations. Am. J. Hyg. 1962;75:377–391. doi: 10.1093/oxfordjournals.aje.a120259. [DOI] [PubMed] [Google Scholar]
- Crawley M.J. Wiley; Chichester, UK: 2002. Statistical computing: an introduction to data analysis using S-Plus. [Google Scholar]
- Cressie N.A.C. Statistics for spatial data. Wiley; New York: 1991. [Google Scholar]
- Dupuis A.P, II, Marra P.P, Kramer L.D. Serologic evidence of West Nile virus transmission, Jamaica, West Indies. Emerg. Infect. Dis. 2003;9:860–863. doi: 10.3201/eid0907.030249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Franco J.G, et al. West Nile virus in Mexico: evidence of widespread circulation since July 2002. Emerg. Infect. Dis. 2003;9:1604–1607. doi: 10.3201/eid0912.030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca D.M, Keyghobadi N, Malcolm C.A, Mehmet C, Schaffner F, Mogi M, Fleischer R.C, Wilkerson R.C. Emerging vectors in the Culex pipiens complex. Science. 2004;303:1535–1538. doi: 10.1126/science.1094247. 10.1126/science.1094247 [DOI] [PubMed] [Google Scholar]
- Gomes A.C, Silva N.N, Marques G.R.A.M, Brito M. Host feeding patterns of potential human disease vectors in the Paraba Valley region, state of São Paulo, Brazil. J. Vect. Ecol. 2003;28:74–78. [PubMed] [Google Scholar]
- Hayes C.G. West Nile fever. In: Monath T.P, editor. Arboviruses: epidemiology and ecology. CRC Press; Boca Raton: 1989. pp. 59–88. [Google Scholar]
- Holt R.D, Dobson A.P, Begon M, Bowers R.G, Schauber E.M. Parasite establishment in host communities. Ecol. Lett. 2003;6:837–842. 10.1046/j.1461-0248.2003.00501.x [Google Scholar]
- Kale H.W, Edman J.D, Webber L.A. Effect of behavior and age of individual Ciconiiform birds on mosquito feeding success. Mosq. News. 1972;32:343–350. [Google Scholar]
- King R.J, Campbell-Lendrum D.H, Davies C.R. Predicting geographic variation in cutaneous leishmaniasis, Colombia. Emerg. Infect. Dis. 2004;10:598–607. doi: 10.3201/eid1004.030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York strain of West Nile virus. Emerg. Infect. Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar O, et al. West Nile virus transmission in resident birds, Dominican Republic. Emerg. Infect. Dis. 2003;9:1299–1302. doi: 10.3201/eid0910.030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R.S, et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay R, et al. Rapid antigen-capture assay to detect West Nile virus in dead corvids. Emerg. Infect. Dis. 2003;9:1406–1410. doi: 10.3201/eid0911.030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGiudice K, Ostfeld R.S, Schmidt K.A, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl Acad. Sci. USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. 10.1073/pnas.0233733100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra P.P, et al. West Nile virus and wildlife. Bioscience. 2004;54:393–402. [Google Scholar]
- Nasci R.S, Gottfried K.L, Burkhalter K.L, Kulasekera V.L, Lambert A.J, Lanciotti R.L, Hunt A.R, Ryan J.R. Comparison of vero cell plaque assay, TaqMan reverse transcription RNA assay, and Vectest antigen assay for detection of West Nile virus in field-collected mosquitoes. J. Am. Mosq. Control Assoc. 2002;18:294–300. [PubMed] [Google Scholar]
- Ostfeld R.S, Keesing F. Biodiversity series: the function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. Zool. 2000a;78:2061–2078. 10.1139/cjz-78-12-2061 [Google Scholar]
- Ostfeld R.S, Keesing F. Biodiversity and disease risk: the case of Lyme disease. Conserv. Biol. 2000b;14:722–728. 10.1046/j.1523-1739.2000.99014.x [Google Scholar]
- Peterson A.T, Komar N, Komar O, Navarro-Sigüenza A, Robbins M.B, Martínez-Meyer E. West Nile virus in the New World: potential impact on bird species. Bird Conserv. Int. 2004;14:215–232. 10.1017/S0959270904000309 [Google Scholar]
- Quirin R, Salas M, Zientara S, Zeller H, Labie J, Murri S, Lefrancois T, Petitclerc M, Martinez D. West Nile virus, Guadeloupe. Emerg. Infect. Dis. 2004;10:706–708. doi: 10.3201/eid1004.030465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter P. A portable battery-powered trap for collecting gravid Culex mosquitoes. Mosq. News. 1983;43:496–498. [Google Scholar]
- Rice W.R. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Rudolf V.H.W, Antonovics J. Species coexistence and pathogens with frequency-dependent transmission. Am. Nat. 2005;166:112–118. doi: 10.1086/430674. 10.1086/430674 [DOI] [PubMed] [Google Scholar]
- Ruiz M.O, Tedesco C, McTighe T.J, Austin C, Kitron U. Environmental and social determinants of human risk during a West Nile virus outbreak in the greater Chicago area. Int. J. Health Geogr. 2004;3:8. doi: 10.1186/1476-072X-3-8. 10.1186/1476-072X-3-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge C.R, Day J.F, Lord C.C, Stark L.M, Tabachnick W.J. West Nile virus infection rates in Culex nigripalpus (Diptera: Culicidae) do not reflect transmission rates in Florida. J. Med. Entomol. 2003;40:253–258. doi: 10.1603/0022-2585-40.3.253. [DOI] [PubMed] [Google Scholar]
- Sardelis M.R, Turell M.J, Dohm D.J, O'Guinn M.L. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg. Infect. Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K.A, Ostfeld R.S. Biodiversity and the dilution effect in disease ecology. Ecology. 2001;82:609–619. [Google Scholar]
- Service M.W. Mosquito (Diptera: Culicidae) dispersal—the long and short of it. J. Med. Entomol. 1997;34:579–588. doi: 10.1093/jmedent/34.6.579. [DOI] [PubMed] [Google Scholar]
- Shaman J, Day J.F, Stieglitz M. St. Louis encephalitis virus in wild birds during the 1990 south Florida epidemic: the importance of drought, wetting conditions, and the emergence of Culex nigripalpus (Diptera: Culicidae) to arboviral amplification and transmission. J. Med. Entomol. 2003;40:547–554. doi: 10.1603/0022-2585-40.4.547. [DOI] [PubMed] [Google Scholar]
- Sudia W.D, Chamberlain R.W. Battery-operated light trap, an improved model. Mosq. News. 1962;22:126–129. [PubMed] [Google Scholar]
- Taylor R.M, Work T.H, Hurlbut H.S, Rizk F. A study of the ecology of West Nile virus in Egypt. Am. J. Trop. Med. Hyg. 1956;5:579–620. doi: 10.4269/ajtmh.1956.5.579. [DOI] [PubMed] [Google Scholar]
- Telfer S, Brown K.J, Sekules R, Begon M, Hayden T, Birtles R. Disruption of a host–parasite system following the introduction of an exotic species. Parasitology. 2005;130:661–668. doi: 10.1017/s0031182005007250. 10.1017/S0031182005007250 [DOI] [PubMed] [Google Scholar]
- Thomas, L. et al 2005 Distance 5.0. Release Beta 3. Research Unit for Wildlife Population Assessment, University of St Andrews, UK. http://www.ruwpa.st-and.ac.uk/distance/
- Turell M.J, O'Guinn M.L, Dohm D.J, Jones J.W. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J. Med. Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Turell M.J, Dohm D.J, Sardelis M.R, O'Gunn M.L, Andreadis T.G, Blow J.A. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Walter S.D, Hildreth S.W, Beaty B.J. Estimation of infection rates in populations of organisms using pools of variable size. Am. J. Epidemiol. 1980;112:124–128. doi: 10.1093/oxfordjournals.aje.a112961. [DOI] [PubMed] [Google Scholar]
- Wiedenfeld D.A, Swan M.M. Louisiana Sea Grant College Program, Louisiana State University; Baton Rouge: 2000. Louisiana breeding bird atlas. [Google Scholar]
- Work T, Hurlbut H, Taylor R.M. Indigenous wild birds of the Nile delta as potential West Nile virus circulating reservoirs. Am. J. Trop. Med. Hyg. 1955;4:872–888. doi: 10.4269/ajtmh.1955.4.872. [DOI] [PubMed] [Google Scholar]
- Zhou G, Minakawa N, Githeko A, Yan G. Association between climate variability and malaria epidemics in the East African highlands. Proc. Natl Acad. Sci. USA. 2004;101:2375–2380. doi: 10.1073/pnas.0308714100. 10.1073/pnas.0308714100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohrabian A, Meltzer M.I, Ratard R, Billah K, Molinari N.A, Roy K, Scott R.D, Peterson L.R. West Nile virus economic impact, Louisiana 2002. Emerg. Infect. Dis. 2004;10:1736–1744. doi: 10.3201/eid1010.030925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyzak M, Loyless T, Cope S, Wooster M, Day J.F. Seasonal abundance of Culex nigripalpus Theobald and Culex salinarius Coquillett in north Florida, USA. J. Vector Ecol. 2002;27:155–162. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.