Abstract
We explore the role of specialization in supporting species coexistence in high-diversity ecosystems. Using a novel ordination-based method to quantify specialist and generalist feeding structures and diets we examined the relationship between morphology and diet in 120 wrasses and parrotfishes from the Great Barrier Reef. We find that wrasses, despite their morphological diversity, exhibit weak links between morphology and diet and that specialist morphologies do not necessarily equate to specialized diets. The dominant pattern shows extensive overlap in morphology (functional morphospace occupation) among trophic groups; fish with a given morphology may have a number of feeding modes. Such trophic versatility may lay the foundation for both the origins and maintenance of high biodiversity on coral reefs.
Keywords: coral reef fishes, specialist, generalist, functional morphology, trophic ecology
1. Introduction
One of the classical goals in ecology is to explain how certain environments manage to support so many species (Hutchinson 1959; Rosenzweig 1995; Roy et al. 2000). This issue has come to the fore with global threats to biodiversity and recognition of the role of biodiversity in ecosystem function (Naeem et al. 1994; Loreau et al. 2002). Yet, the mechanisms that enable ecosystems to persist with extensive biodiversity are still actively debated. How do coral reefs, for example, manage to support up to 650 coral and 1000 fish species within a single location (Connolly et al. 2003; Bellwood et al. 2005)? Several studies have indicated that local biodiversity is a product of regional biodiversity (Caley & Schluter 1997; Karlson et al. 2004), and that species composition may follow relatively simple assembly rules (Bellwood & Hughes 2001). However, at a community level, where species are capable of interacting directly with one another, the mechanisms of coexistence are still open to question, despite many theoretical and empirical advances (Chesson 2000; Hubbell 2001).
The traditional explanation for the local maintenance of high species diversity is by fine-scale niche partitioning by resource specialists (Dobzhansky 1950). In high-diversity systems one would thus expect to see phenotypic evidence of species being morphologically adapted to their specific niche (Schluter 2000). Reef fishes provide numerous examples of extreme morphologies (Wainwright & Bellwood 2002). Of these, the wrasses (Labridae) are perhaps the best known. With extensive evidence of niche partitioning, particularly with regards to trophic ecology, they are one of the most ecologically and functionally diverse clades of fishes on coral reefs (Wainwright et al. 2004; Westneat et al. 2005), a system characterized by high biodiversity.
Within the labrids, there are numerous examples of specialized structures associated with extreme diets; but how does this relate to the extensive biodiversity in this family of about 600 species? Are these merely the most obvious examples of numerous finely separated trophic niches, or are these exceptions to a different general rule? If functionally based trophic specialization is the key to biodiversity in wrasse communities we would expect to see a close relationship between mechanically significant morphological attributes (of the feeding apparatus) and realized niche utilization patterns (diet). Alternatively, if trophic niche partitioning is relatively unimportant, we would predict a weak relationship between anatomical form and diet. Local biodiversity may have another basis: niche partitioning along another axis (behaviour, habitat), no niche partitioning (lottery hypothesis, recruitment limitation, etc.) or versatility of the feeding mechanism. In the latter two cases, species may be functionally equivalent, with several morphologies being able to exploit the same resources. Interestingly, recent findings have emphasized the potential for broad functional equivalence among species as a central component in Hubbell's neutral model of macro-ecology (Bell 2001; Chave 2004).
In examining a comparable high-diversity system, African Rift Lake cichlids, Liem (1980, 1990) posed the question: why is it that so many apparent specialists are also generalists? Many species with specialized morphologies have generalist diets most of the time. He postulated that the specializations provide a refuge during periods of intense competition when resources are limited. Robinson & Wilson (1998) have since argued that specialization is shaped by optimal foraging theory and the asymmetry between preferred prey items and their availability. Most of the time fish should eat preferred prey, if available, regardless of whether they are morphologically specialized and capable of consuming other taxa in a competitively superior way. Liem's (1980) composite hypothesis of specialization for ‘tough times’ and Robinson & Wilson's (1998) optimal foraging model are both hard to evaluate under ‘normal’ circumstances, but they can both be rejected if the majority of specialists exhibit specialized diets during normal conditions.
In all of the above cases one of the critical questions is how to identify specialized morphologies and diets. This is intuitively obvious in extreme cases but how specialized is specialized? And to what extent does this degree of specialization shape diet during normal times? To investigate the role of trophic specialization as a basis for coexistence in a high diversity system we examined the trophic status of wrasses and parrotfishes (family Labridae) using a novel quantitative measure of specialization.
Our goal in examining trophic functional morphology and diet is not to accept or reject the niche hypothesis, but to quantitatively examine the extent to which morphological and trophic specialization are linked and to evaluate the potential contribution of morphologically based niche partitioning along this resource axis in supporting local biodiversity. To do this we take advantage of a unique combination of datasets based on a detailed examination of 120 labrid species from a single high-diversity biogeographic location, the Great Barrier Reef (GBR). This represents over 90% of the labrid fauna in this region and includes all of the numerically important species (Bellwood & Wainwright 2001; Wainwright et al. 2004). For each species we have fully characterized functional attributes of the trophic apparatus and complementary dietary analysis of over 2000 specimens. These data permit, for the first time, a direct evaluation of the relationship between functional morphology and realized trophic niche for an entire clade within a high diversity ecosystem.
2. Material and Methods
The morphology of the feeding apparatus of each of the 120 species was quantified based on 10 anatomical attributes. These measurements were explicitly chosen because each had known functional implications and were directly associated with variation in feeding performance. These links between morphology and performance are primarily biomechanical and reflect the implications of variation in morphology on feeding performance and diet (e.g. Westneat 1995; Wainwright 1998). An overview of the performance implications is given in Wainwright et al. (2004). The study is specifically restricted to a procurement–diet functional axis. The variables are body mass, mouth gape, premaxillary protrusion, adductor mandibulae muscle mass, sternohyoideus muscle mass, levator posterior muscle mass, anterior jaws 4 bar linkage KT (jaws KT), hyoid apparatus 4 bar linkage KT (hyoid KT), mouth-opening mechanical advantage and mouth-closing mechanical advantage. Measurements were made on 3–4 adult individuals per species and were used to generate species means. The raw data and a detailed description of the variables are provided in Wainwright et al. (2004).
Dietary data were collected from 2053 specimens of 120 species. Most individuals were collected from reefs around Lizard Island in the Northern GBR. These were supplemented by material from other GBR locations, French Polynesia, Papua New Guinea and Indonesia. Specimens were primarily collected using fine barrier nets. All specimens were placed on ice shortly after capture. They were either dissected fresh or the entire alimentary tract was fixed in 10% formalin and preserved in 70% ethanol. Care was taken not to collect more than 20% of the individuals from a single location on a given day; most were collected over months to years and represent a time- and spatially averaged indication of dietary preferences. The contents of the anterior half of the intestine (labrids lack a stomach) were spread on a 10×10 grid on a Petri-dish and viewed under a dissecting microscope. The dominant dietary category was recorded in 40 randomly allocated quadrates. Items were identified to the lowest taxonomic level and allocated to broader groups prior to statistical analyses. Categories were: micro-crustacea (less than 3 mm), macro-crustacea (greater than 3 mm), micro-bivalvia (less than 5 mm), macro-bivalvia (greater than 5 mm), micro-gastropoda (less than 5 mm), macro-gastropoda (greater than 5 mm), Brachyura, Polychaeta, Foraminifera, Echinodermata, Copepoda, gnathiid isopods, Anomura, fish, amorphous organic matter (AOM), black AOM (blood?), algae, coral organic matter and sediment. These categories are, by necessity, broad and trophic specialization within a category remains a possibility. Initial analyses encompassed the entire labrid clade comprising wrasses (Labridae s.s.) and parrotfishes (f. Scaridae). However, the scarids represent a unique and relatively uniform trophic and morphological group (the morphological principal components analysis (PCA) is given in Wainwright et al. 2004)) and were therefore excluded from subsequent analyses. For clarity, the figures presented are restricted to wrasses (95 species, 1800 intestines, with a mean of 19 individuals per species). Diet data were based on the mean of each species (data in electronic supplementary material). Only 15 species had less than five individuals. These species were included if the data were consistent with congenerics or published data using larger sample sizes.
In the analyses, mass variables were cube root transformed to help make variables dimensionally uniform (transformations of morphometric variables follow Wainwright et al. 2004). Morphological variation was explored using a PCA. Because the dataset combines linear and ratio variables we factored the correlation matrix. For comparative purposes, analyses of dietary data mirrored those of the morphological analyses, i.e. using a PCA on the correlation matrix of 19 diet variables. No transformations were required prior to analyses.
Specialization was defined as the relative distance of a species from the centroid in the PCA. We used only those axes that cumulatively explained greater than 85% of variation among species (six for morphology, 13 for diet). The 85% cutoff retained most informative axes and reduced dimensionality. To provide a quantitative estimate of specialization we calculated the distance from the centroid on the first six (or 13) axes using an extension of Pythagoras's Theorem. Two distances were calculated: absolute distance and a standardized distance. In the latter, the loading on each axis was standardized based on the variance explained on that axis (i.e. the score on each PC is multiplied by the proportion of the total variance explained by that PC). In absolute distances, the first six axes are all treated as equally important; for standardized distances the distance along the first axis is disproportionately more important than the second and succeeding axes, etc. Specialization based on distance from the centroid reflects the same characteristics as traditional measures of evenness or trophic diversity (species using many diet groups will lie close to the centre; those that heavily exploit a few resources lie near the perimeter). The main difference is that in the PCA, specialization is more clearly context driven in that it is explicitly defined relative to the group being studied. For comparative purposes dietary specialization was also quantified using a Shannon-Wiener diversity index (H′).
We explored the relationship between diet and morphology in five ways, comparing: (i) morphological and dietary specialization; (ii) selected anatomical features and major dietary groups (groups are defined as those species with greater than 25% of the intestinal contents within a single dietary category; 64 species can be categorized at a 25% cutoff, falling to 21 at 50% and only 6 at 75%); (iii) dietary groups plotted in functional morphospace; (iv) comparing morphological variance in each dietary group. Variance was calculated following Foote (1997) as the sum of the univariate variances among the study taxa on the selected axes. Group variance (disparity) has the useful property that it does not scale with sample size and finally; (v) we examined the morphological and dietary variables using a canonical correlation analysis. Statistical analyses were undertaken using SPSS (v. 12.0.1) or Statistica (v. 6.1).
3. Results
There was an extremely weak correlation between the degree of morphological specialization and the extent of dietary specialization. Extreme functional morphologies did not reflect extreme diets. Including the Scaridae the relationship between morphological specialization (distance from the centroid on the first six PC axes; explaining 89.0% of variation) and the diet specialization (13 axes; explaining 86.5%) had a coefficient of determination (r2) of 0.005 (0.099 if the Scaridae is excluded). Using standardized data the coefficient was less than 0.001 (0.093 if scarids are removed). In all cases the slope was slightly positive. A comparison of morphological specialization and diet diversity (H′) (excluding the Scaridae) exhibited a negative slope with a coefficient of determination of 0.17 (0.13 for standardized data), indicating a slight tendency for extreme morphologies to have narrow diets.
A plot of the eight main diet groups in functional morphospace based on the first two PC axes (which together explain 54.5% of the variance) reveals a clear pattern (figure 1). Some dietary categories are restricted to peripheral areas of morphospace but for most trophic groups there is extensive overlap, with several feeding types occupying a broad expanse of morphospace near the centroid. Of the eight diet types identified, six occupy an area of morphospace that encloses more species with a different diet type than the type in question. The extreme case is the brachyuran (decapod crab) feeders: there are 25 non-brachyuran feeding species within the morphospace delineated by the six brachyuran feeders. In only two groups, corals and gnathiid isopod feeders, was the specific region of morphospace clearly associated with a particular diet. A comparable plot of the 10 most extreme diet specialists and 10 most generalized diet generalists in morphospace shows extensive overlap with half the generalists lying in specialist space.
Figure 1.
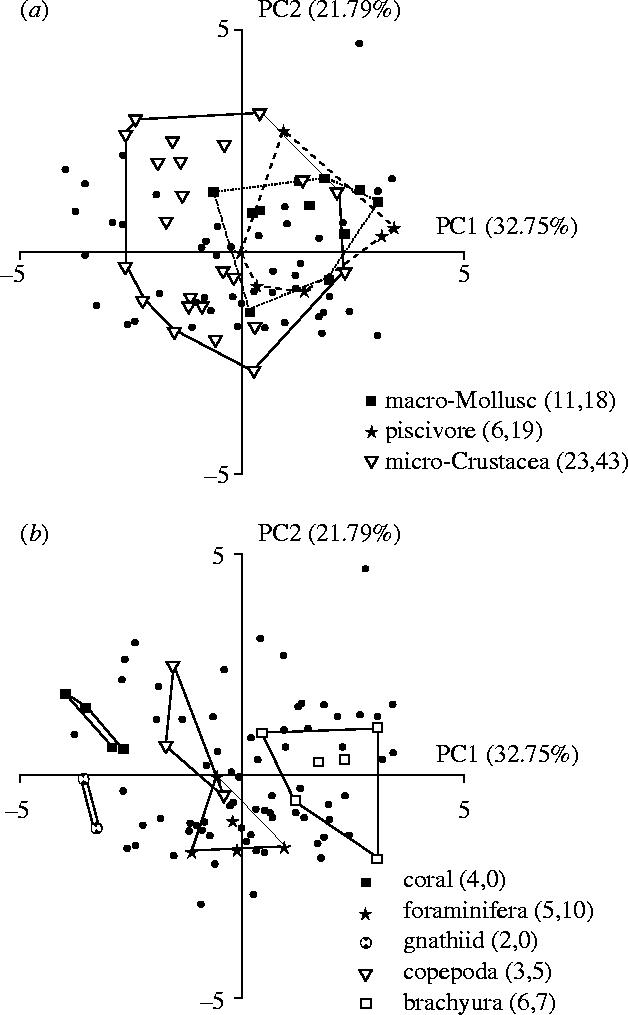
Functional morphospace occupation by eight feeding groups of wrasses. The PCA plot is based on 95 wrasse species, each dot or symbol indicates the location of a species. Points near to the centre are morphologically ‘generalized’, those furthest away are most ‘specialized’. The connected symbols indicate the maximum morphospace occupied by species which feed predominantly (greater than 25% of all items) on a given dietary category. Note the extensive overlap in morphospace. The two sections (a) and (b) are from the same analysis but have been separated for clarity. The numbers in parentheses indicate the number of species in each feeding group followed by the number of other species encapsulated within the two-dimensional morphospace.
The nature of the relationship between morphology and diet is further exemplified by the patterns of morphological variance or disparity among diet types (figure 2a). Morphological disparity is greatest in the micro-crustacean feeders. Interestingly, the disparity in this group is almost identical to the wrasses as a whole suggesting that this trophic group represents a random sampling of wrasse morphology. In all other groups, the morphological disparity is less than one would expect from a random sample of wrasse cranial morphology, particularly in those groups with more specialized diets. This trend can be seen most clearly by comparing disparity along a gradient based on the mass of the levator posterior, which marks one of the strongest axes in labrid functional morphology from durophageous to gracile feeding structures (figure 2b). The eight diet types exhibit a strong relationship with the levator posterior mass: the highest masses are associated with the most durophageous diets. Overall, disparity is least in the most extreme feeding morphs: foraminifera, gnathiid isopod and coral specialists.
Figure 2.
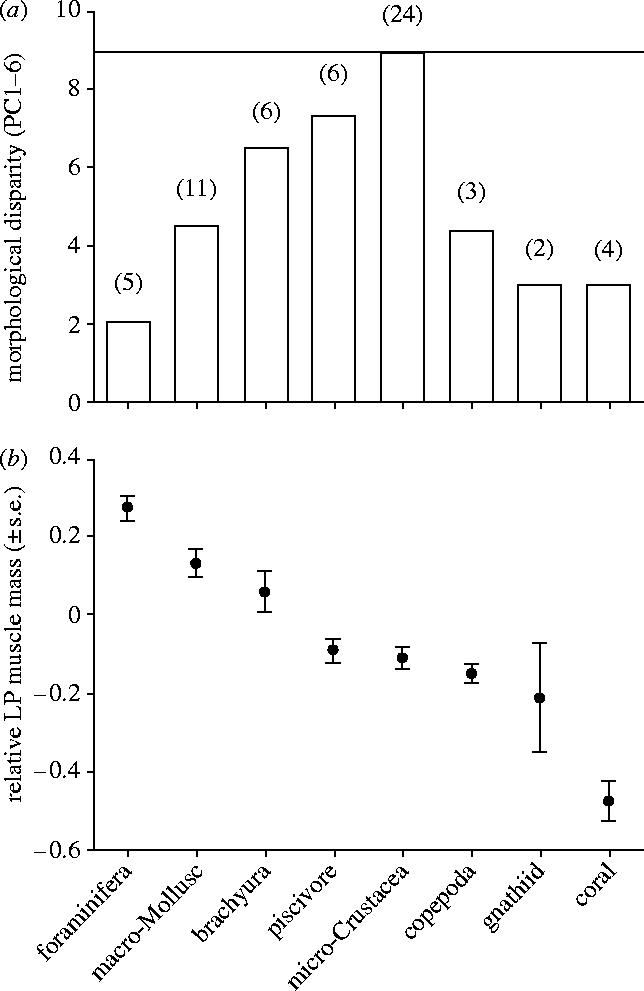
(a) The extent of morphological variation (disparity) within each of the eight feeding groups. The number of species in each group is given in parentheses. The horizontal line indicates the variation in all 95 wrasse species from the Great Barrier Reef. Variation is least in the most specialized feeding groups. (b) The relationship between levator posterior (LP) mass and feeding group in wrasses. The levator posterior is the main crushing muscle of the pharyngeal apparatus. Mass is expressed as the mean residual (±s.e.) of the cube root of the mass (to allow for fish size). Large masses (positive residuals) are associated with hard shelled prey, small masses (negative residuals) with soft prey.
That there is some relationship between morphology and diet is reflected in the canonical correlation analysis. Of the 59% of dietary variance extracted, approximately half, 27%, could be explained by the 10 morphological variables.
4. Discussion
The results present a profound contrast to traditional views of reef fish trophic biology. Rather than a close one-to-one relationship between morphology and diet, labrid fishes, with few exceptions, exhibit no rigid relationship between the functional specializations of the trophic apparatus and their diet. This is particularly striking given that we used the most restrictive range of morphological features, with each having a demonstrable link to jaw and feeding mechanics.
There is a clear distinction between the possession of functional specializations, which may provide the potential to use extreme or specialized dietary resources, and the requirement to do so or the regular expression of these abilities. Likewise, generalized morphologies do not preclude specialization. The results do not refute the hypotheses of Liem (1980) or Robinson & Wilson (1998), but go further to suggest that a lack of specialization may be a critical factor in supporting coral reef biodiversity. In many ways this supports the neutral theory of macro-ecology (Hubbell 2001), where local communities may be structured by local metapopulational dynamics and regional species extinction probabilities rather than local niche-partitioning and assembly rules.
Despite the appearance of broad functional equivalence, in several cases morphological specialization has resulted in trade-offs and constraints which limit feeding options. Corallivory and molluscivory, for example, have led to specialized morphologies that correlate with specialized diets. It is these groups that define the outer limits of functional morphospace. However, for the vast majority of species their trophic morphology permits extensive versatility in diet. We see multiple mapping of functional morphology to diets. Fish with different morphologies feed on similar prey, and many different diets correspond to a similar morphology. Relatively few feeding modes have a restricted range of morphologies (e.g. corallivores, gnathiid parasite feeders, foraminifera specialists). In these cases, successful exploitation of the resource is associated with a limited range of morphologies. In marked contrast, other groups (feeding on small crustacea, brachyura, fish, etc.) are exploited by taxa with a wide range of morphologies. What is particularly striking is that even in some of the more specialized groups the functional morphospace invariably encloses numerous other species with different diets. The functional morphospace of mollusc feeders, piscivores and brachyuran feeders all enclose 2–6 times more species than represented by the groups themselves.
A critical issue in this regard is the extent to which the functional morphospace occupied by each group is phylogenetically constrained. Although all trophic groups contain multiple genera, the three dietary groups exhibiting the least morphological variation are represented by just two lineages. In these groups, at least, phylogenetic constraints may be significant. Phyletic history notwithstanding, broad representation of genera within a trophic group may be a key element in supporting biodiversity. Such trophic versatility supports biodiversity by permitting numerous different morphologies to exploit the resource. The roles of versatility in supporting diversity are manyfold:
Versatility in skull design reduces constraints along other resource axes. For example, Thalassoma, Gomphosus, Choerodon and Novaculichthys all feed on crabs but Thalassoma and Gomphosus are gracile and extensively modified for high mobility and dominate high wave energy locations, while Choerodon and Novaculichthys are more robust and occupy lower energy locations (Bellwood & Wainwright 2001). Crab feeding morphology does not restrict head shape or locomotor mode and, thus diversification along other axes is not constrained; this provides opportunities for behavioural specialization or mechanical specialization in other functional systems (cf. Ghalambor et al. 2003).
Versatility also permits several species to use a given resource or niche. In some respects this represents a trophic version of the lottery hypothesis (Sale 1977; Munday 2004), where any one of a number of species with comparable competitive abilities can use a common resource. In these instances, coexistence depends on environmental variability or stochastic events underpinning differential survival of the species in other spatial or temporal arenas (Chesson & Warner 1981; Chesson 1985). The ability of multiple designs to use a single resource may relax or diffuse interspecific competition (by permitting extensive prey switching and opportunistic exploitation), thereby facilitating local cooccurrence and boosting local species richness. This attribute, in particular, is most consistent with the key assumptions of limited niche differentiation in the neutral model of community composition.
Versatility in resource use also permits repeated invasion of niche space by different lineages. Versatility means that few lineages are strongly constrained by morphological history. Preliminary phylogenetic analyses (Barber & Bellwood 2005; Westneat et al. 2005) and existing taxonomic hierarchies suggest that the central region of labrid diet space (micro-crustacea) has representatives from at least six different labrid lineages on the GBR alone. In evolutionary terms, versatility permits ready access to trophic niches.
Colonization potential will be enhanced by versatility. Reefs are frequently widely separated and survival often depends on colonization ability. Versatility would reduce barriers to colonization in such fragmented habitats because of the wide range of suitable trophic resources that a particular jaw design can exploit. Tropically versatile species may be resistant to extinction because of their capacity to capitalize on local variation in prey availability and community structure. We anticipate the possibility that future analyses will reveal marked variation in patterns of prey use among populations of wrasse species (Turingan et al. 1995).
To what extent do the results conflict with previous ecomorphological analyses of reef fishes? A great deal of effort and a major part of the research on functional morphology has demonstrated the clear links between morphology and diet (reviewed by Wainwright & Bellwood 2002). The current study, rather than conflicting with these previous works, puts them into perspective and offers a more pluralistic view of the role that functional morphology plays in shaping patterns of resource use. Previous work has focused on the identification of constraints and trade-offs that shape the potential diet of species that possess a particular morphology.
It is in the boundary areas that the clearest limits to functional design can be seen. For example, the size of the pharyngeal apparatus limits crushing strength and the hardness of prey that can be eaten (Clifton & Motta 1998; Wainwright & Bellwood 2002). Some of these marginal regions have distinct trade-offs while others are more accommodating. Coral mucous feeding requires a small mouth and strength modified jaws (jaw speed is lost as a trade-off). In these species the diet is almost exclusively mucous. In contrast, the macro-mollusc specialists can, and do, consume a large range of taxa. Choerodon anchorago, for example, is one of the most powerful gastropod crushing species yet 15% of the diet is fishes. In this case pharyngeal strength and strong oral jaws do not preclude preying on elusive prey.
In contrast to these specialists, many other trophic modes have a many-to-one mapping (cf. Alfaro et al. 2004), where several anatomical designs are capable of feeding on a given item. For some, the number of designs are limited, e.g. piscivory which has just two discrete modes (jaw ram feeding Epibulus versus body ram in Oxycheilinus, Hologymnosus, Cheilio, etc.). Other areas such as micro-crustacean feeding appear to be accessible by a vast array of morphologies with the variance among taxa feeding in this group being equal to the variance found among taxa in the whole family. The corollary to this ‘many-morphologies-to-one-diet’ pattern is the occurrence of many diets exploited by a single region of morphospace. This is particularly evident in the upper right hand quadrant (figure 1) where a small region of morphospace contains species which feed on four of the eight major dietary categories. Interestingly the two left-hand quadrants do not reveal the same level of overlap. A comparable pattern of many-morphologies-to-one-functional mechanical property has been described in wrasse jaws (Alfaro et al. 2004) and may emerge as a widespread phenomenon in physiological and ecomorphological analyses (Ghalambor et al. 2004; Wainwright et al. 2005).
Character displacement, one of the clearest indicators of competition, requires a strong link between the morphology and the trophic niche. Presumably, if these fishes do compete there are multiple potential niche axes, and in most species trophic morphology may not reflect the change. The traditional view of a one-to-one relationship between morphology and diet, as exemplified by the relationship between levator posterior mass and prey shell hardness (Wainwright 1988) (reviewed by Wainwright & Bellwood 2002), may be overly simplistic. The reality may encompass more flexible relationships. For example, multiple morphological configurations may have similar functional properties (Alfaro et al. 2004), alternatively systems may exhibit phenotypic plasticity (Turingan et al. 1995) or modifications related to minor dietary components (Benkman 2003). In all cases this lays the foundation for a broader, more pleuralistic, view of ecomorphology. In this framework, morphology and functional constraints may set limits to patterns of resource use and define the primary axes of variation within lineages, but the vast majority of species operate well within their maximal prey capture and handling capacity, taking advantage of a highly versatile feeding mechanism that allows fishes to respond to local biotic and abiotic influences in their choice of prey resources.
A clear alternative to the niche-based hypotheses is the stochastic models where niche-partitioning is negligible and where coexistence depends on stochastic processes encompassing alternatives such as the Lottery Hypothesis (Sale 1977; Chesson & Warner 1981) and the Neutral Model (Bell 2001; Hubbell 2001). If versatility operates as a functional equivalent of the lottery hypothesis it merely highlights the lack of determinism in the arena studied. But, as noted by Chesson (1985), it still permits deterministic processes to operate in other arenas. In reef fish populations this may be away from the reef in the plankton where high mortality and complex behaviour may be the ultimate environmental or biological determinants of local species composition (Munday 2004).
Finally, one must consider the extent to which the observations on wrasses may reflect a more general phenomenon. The results seem to stand in marked contrast to low diversity systems such as temperate freshwater lakes, where character displacement and competition are apparent (Schluter 2000). However, it may have broader application in other high diversity groups and ecosystems. The pattern seen in labrids appears to be reflected, but to an even greater extent, in the coral reef damselfishes (Pomacentridae) and cardinalfishes (Apogonidae), where extremely limited morphological variation (Emery 1973; Barnett 2004) presents no barrier to taxonomic diversification; both families rival or exceed the wrasses in both abundance and diversity on coral reefs (Ackerman & Bellwood 2000). A comparable situation appears to exist in both African and Central American cichlids, where the high species richness and morphological diversity can rival that seen on coral reefs (Keenleyside 1991). In both locations the diversity of cichlids appears to rest to a considerable degree on trophic versatility. Perhaps, as for wrasses on coral reefs and fishes in other high-diversity systems, the key to local richness, at least from a trophic perspective, lies in versatility—a community composed of numerous jacks-of-all-trades with the boundaries being pushed by just a handful of masters.
Acknowledgments
We thank numerous colleagues, including H. Choat, M. Marnane, I. Stobutzki and F. Walsh for kind donations of material and S. Connolly and three referees for helpful comments. JCU Animal Ethics Approval A650. Centre for Coral Reef Biodiversity contribution no. 141; supported by the Australian Research Council.
Supplementary Material
References
- Ackerman J.L, Bellwood D.R. Reef fish assemblages: a re-evaluation using enclosed rotenone stations. Mar. Ecol. Prog. Ser. 2000;206:227–237. [Google Scholar]
- Alfaro M.E, Bolnick D.I, Wainwright P.C. Evolutionary dynamics of complex biomechanical systems: an example using the four-bar mechanism. Evolution. 2004;58:495–503. [PubMed] [Google Scholar]
- Barber P.H, Bellwood D.R. Biodiversity hotspots: evolutionary origins of biodiversity in wrasses (Halichoeres: Labridae) in the Indo-Pacific and New World tropics. Mol. Phylogenet. Evol. 2005;35:235–253. doi: 10.1016/j.ympev.2004.10.004. 10.1016/j.ympev.2004.10.004 [DOI] [PubMed] [Google Scholar]
- Barnett, A. 2004 The trophic and reproductive implications of mouthbrooding in apogonid fishes. Hons thesis, Department of Marine Biology, James Cook University, Townsville.
- Bell G. Neutral macroecology. Science. 2001;293:2413–2418. doi: 10.1126/science.293.5539.2413. 10.1126/science.293.5539.2413 [DOI] [PubMed] [Google Scholar]
- Bellwood D.R, Hughes T.P. Regional-scale assembly rules and biodiversity of coral reefs. Science. 2001;292:1532–1534. doi: 10.1126/science.1058635. 10.1126/science.1058635 [DOI] [PubMed] [Google Scholar]
- Bellwood D.R, Wainwright P.C. Locomotion in labrid fishes: implications for habitat use and cross-shelf biogeography on the Great Barrier Reef. Coral Reefs. 2001;20:139–150. 10.1007/s003380100156 [Google Scholar]
- Bellwood D.R, Hughes T.P, Connolly S.R, Tanner J. Environmental and geometric constraints on Indo-Pacific coral reef biodiversity. Ecol. Lett. 2005;8:643–651. 10.1111/j.1461-0248.2005.00763.x [Google Scholar]
- Benkman C.W. Divergent selection drives the adaptive radiation of crossbills. Evolution. 2003;57:1176–1181. doi: 10.1111/j.0014-3820.2003.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Caley J.M, Schluter D. The relationship between local and regional diversity. Ecology. 1997;78:70–80. [Google Scholar]
- Chave J. Neutral theory and community ecology. Ecol. Lett. 2004;7:241–253. 10.1111/j.1461-0248.2003.00566.x [Google Scholar]
- Chesson P.L. Coexistence of competitors in spatially and temporally varying environments: a look at the combined effects of different sorts of variability. Theor. Popul. Biol. 1985;28:263–287. 10.1016/0040-5809(85)90030-9 [Google Scholar]
- Chesson P.L. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000;31:343–366. 10.1146/annurev.ecolsys.31.1.343 [Google Scholar]
- Chesson P.L, Warner R.R. Environmental variability promotes coexistence in lottery competitive systems. Am. Nat. 1981;117:923–943. 10.1086/283778 [Google Scholar]
- Clifton K.B, Motta P.J. Feeding morphology, diet, and ecomorphological relationships among five Caribbean labrids (Teleostei, Labridae) Copeia. 1998;1998:953–966. [Google Scholar]
- Connolly S.R, Bellwood D.R, Hughes T.P. Geographic ranges and species richness gradients: a re-evaluation of coral reef biogeography. Ecology. 2003;84:2178–2190. [Google Scholar]
- Dobzhansky T. Evolution in the tropics. Am. Sci. 1950;38:209–221. [Google Scholar]
- Emery A.R. Comparative ecology and functional osteology of fourteen species of damselfish (Pisces: Pomacentridae) at Alligator Reef, Florida Keys. Bull. Mar. Sci. 1973;23:649–770. [Google Scholar]
- Foote M. Sampling, taxonomic description, and our evolving knowledge of morphological diversity. Paleobiology. 1997;23:181–206. [Google Scholar]
- Ghalambor C.K, Walker J, Reznick D.N. Multi-trait selection, adaptation, and constraints on the evolution of burst swimming performance. Int. Comp. Biol. 2003;43:431–438. doi: 10.1093/icb/43.3.431. [DOI] [PubMed] [Google Scholar]
- Ghalambor C.K, Reznick D.N, Walker J.A. Constraints on adaptive evolution: the functional trade-off between reproduction and fast start escape performance in the guppy (Poecilia reticulata) Am. Nat. 2004;164:38–50. doi: 10.1086/421412. 10.1086/421412 [DOI] [PubMed] [Google Scholar]
- Hubbell S.P. Princeton University Press; Princeton, NJ: 2001. A unified neutral theory of biodiversity and biogeography. [Google Scholar]
- Hutchinson G.E. Homage to Santa Rosalina, or why are there so many kinds of animals? Am. Nat. 1959;93:145–159. 10.1086/282070 [Google Scholar]
- Karlson R.H, Cornell H.V, Hughes T.P. Coral communities are regionally enriched along an oceanic biodiversity gradient. Nature. 2004;429:867–870. doi: 10.1038/nature02685. 10.1038/nature02685 [DOI] [PubMed] [Google Scholar]
- Keenleyside M.H.A. Chapman & Hall; London: 1991. Cichlid fishes behaviour, ecology and evolution. [Google Scholar]
- Liem K.F. Adaptive significance of intra- and interspecific differences in the feeding repertoires of cichlid fishes. Am. Zool. 1980;20:295–314. [Google Scholar]
- Liem K.F. Aquatic versus terrestrial feeding modes: possible impacts on the trophic ecology of vertebrates. Am. Zool. 1990;30:209–221. [Google Scholar]
- Loreau M, Naeem S, Inchausti P. Oxford University Press; Oxford, UK: 2002. Biodiversity and ecosystem functioning: synthesis and perspectives. [Google Scholar]
- Munday P.L. Competitive coexistence of coral-dwelling fishes: the lottery hypothesis revisited. Ecology. 2004;85:623–628. [Google Scholar]
- Naeem S, Thompson L.J, Lawler S.P, Lawton J.H, Woodfin R.M. Declining biodiversity can alter the performance of ecosystems. Nature. 1994;368:734–737. 10.1038/368734a0 [Google Scholar]
- Robinson B.W, Wilson D.S. Optimal foraging, specialization, and a solution to Liem's paradox. Am. Nat. 1998;151:223–235. doi: 10.1086/286113. 10.1086/286113 [DOI] [PubMed] [Google Scholar]
- Rosenzweig M.L. Cambridge University Press; Cambridge, UK: 1995. Species diversity in space and time. [Google Scholar]
- Roy K, Jablonski D, Valentine J.W. Dissecting latitudinal diversity gradients: functional groups and clades of marine bivalves. Proc. R. Soc. B. 2000;267:293–299. doi: 10.1098/rspb.2000.0999. 10.1098/rspb.2000.0999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale P.F. Maintenance of high diversity in coral reef fish communities. Am. Nat. 1977;111:37–359. 10.1086/283164 [Google Scholar]
- Schluter D. Oxford University Press; Oxford, UK: 2000. The ecology of adaptive radiation. [Google Scholar]
- Turingan R.G, Wainwright P.C, Hensley D.A. Interpopulation variation in prey use and feeding biomechanics in Caribbean triggerfishes. Oecologia. 1995;102:296–304. doi: 10.1007/BF00329796. 10.1007/BF00329796 [DOI] [PubMed] [Google Scholar]
- Wainwright P.C. Morphology and ecology: the functional basis of feeding constraints in Caribbean labrid fishes. Ecology. 1988;69:635–645. [Google Scholar]
- Wainwright P.C, Bellwood D.R. Ecomorphology of feeding in coral reef fishes. In: Sale P.F, editor. Coral reef fishes. Dynamics and diversity in a complex ecosystem. Academic Press; San Diego: 2002. pp. 33–55. [Google Scholar]
- Wainwright P.C, Bellwood D.R, Westneat M.W, Grubrich J.R, Hoey A.S. A functional morphospace for the skull of labrid fishes: patterns of diversity in a complex biomechanical system. Biol. J. Linn. Soc. 2004;82:1–25. 10.1111/j.1095-8312.2004.00313.x [Google Scholar]
- Wainwright P.C, Alfaro M.E, Bolnick D.I, Hulsey C.D. Many-to-one mapping of form to function: a general principle in organismal design? Int. Comp. Biol. 2005;45:256–262. doi: 10.1093/icb/45.2.256. [DOI] [PubMed] [Google Scholar]
- Westneat M.W. Feeding, function and phylogeny: analysis of historical biomechanics in labrid fishes using comparative methods. Syst. Biol. 1995;44:361–383. [Google Scholar]
- Westneat M.W, Alfaro M.E, Wainwright P.C, Bellwood D.R, Grubich J.R, Fessler J.L, Clements K.D, Smith L.L. Local phylogenetic divergence and global evolutionary convergence of skull function in reef fishes of the family Labridae. Proc. R. Soc. B. 2005;272:993–1000. doi: 10.1098/rspb.2004.3013. 10.1098/rspb.2004.3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


