Abstract
There is intense debate over the potential impact of seal predation on declining salmon stocks in both the Pacific and Atlantic oceans. However, efforts to model such interactions have been constrained by a lack of data on the functional and numerical responses of these predators. Based upon theory, and data from small-scale terrestrial and freshwater systems, a type 3 functional response is expected to best describe predation by generalist pinnipeds. Similarly, theory also predicts that seal numbers should increase with salmon density in rivers following an aggregative response of predator to prey. We tested these predictions by studying the diet and local density of harbour seals in relation to seasonal variations in the abundance of salmonid in a Scottish river system. As predicted, the abundance of seals in the river was directly related to the abundance of returning salmon, and dietary data supported the type 3 functional response to changes in salmonid abundance. These studies provide empirical support for the use of type 3 response in modelling studies.
Keywords: Phoca vitulina, Salmo spp., predator–prey relationships
1. Introduction
Predation by marine mammals upon commercially important prey species often results in conflict with fisheries, leading to demands for control of predator populations (Harwood & Croxall 1988; Yodzis 2001). As a consequence, considerable effort has been put into gathering data on predator abundance, diet and energy requirements, so that their potential impact upon prey populations can be modelled. However, the resulting model predictions are generally highly uncertain, and have often proved of limited value for informing conservation and resource management (Yodzis 2001). This uncertainty results partly from the inherent difficulty of parameterizing predator–prey models in marine systems. As important, however, is uncertainty over the functional form of these models (Yodzis 1994), where assumptions about how predation changes in response to variations in prey abundance can dramatically affect predicted consumption rates of commercial important prey (Mohn & Bowen 1996).
Theoretical understanding of the functional and numerical responses that link predator and prey populations is well founded (Holling 1959) but, even in more tractable terrestrial systems, field tests of these theories are relatively rare (Abrams & Ginzburg 2000). Consequently, it is not surprising that it has proved difficult to determine the functional response of marine mammals. Where data do exist, these species appear to have diverse diets, and their choice of prey is likely to be a response to changes in the abundance of several different prey species. As such, many marine mammals appear to be generalist predators, and theory would predict that they have a type 3 (sigmoid) functional response that tends to stabilize prey populations, often at suppressed levels (Andersson & Erlinge 1977; Hanski et al. 1991). Ideally, these predictions could be tested by comparing predator diet composition across time or space in relation to data on the relative abundance of their potential prey species. This would be extremely challenging due to the complexity of any analysis of multi-species functional responses. But, more fundamentally, data on changes in the abundance of whole suites of marine prey populations are rarely, if ever, available at spatial scales that are appropriate to marine mammal predators.
An alternative approach, therefore, is to identify a simpler system, where a predator population's response to a single prey type can be studied. The annual return of anadromous salmonids to their freshwater breeding sites provides an ideal opportunity for such studies. Here, intensively managed fisheries can provide relatively fine-scale and high-quality data on changes in prey abundance as the fish move through the coastal and estuarine ranges of marine mammals. The high level of synchrony in the salmonids' return migration means that their abundance can vary dramatically over a period of a few weeks, during which the availability of alternative marine prey can be assumed to remain relatively constant. While this system may not reflect more typical multi-species marine mammal fishery interactions, it has been the subject of intense debate over the potential impacts of seal predation on declining salmonid populations in both the Pacific and the Atlantic oceans (Middlemas et al. 2003; Purcell et al. 2004). Seals and a variety of other predators exhibit aggregative responses to the estuarine and freshwater areas that these fishes pass through (Quinn et al. 2003; Hastie et al. 2004), but there remains much uncertainty over levels of predation and likely impacts on prey stocks (Middlemas et al. 2003). Information on the response of seals to changes in salmonid abundance is, therefore, of particular importance for management of areas that contain protected populations of both seals and salmon.
In this study, we explore the functional and aggregative responses of harbour seals (Phoca vitulina) to changes in the abundance of salmonids in an estuarine system in Scotland. In particular, we aim to test whether these generalist marine mammal predators exhibit a sigmoid functional response, by comparing the fit of these data to a type 3 model, and alternative type 1 and 2 models.
2. Material and methods
(a) Study area
The study was carried out in the Cromarty Firth, NE Scotland, where harbour seals come ashore regularly to breed and rest onto inter-tidal sandbanks. These animals represent approximately 10% of a resident breeding population of around 1600 individuals within the inner Moray Firth (Thompson et al. 1997). Several important salmonid rivers flow into the Cromarty Firth, supporting stocks and fisheries for both Atlantic salmon (Salmo salar) and sea trout (Salmo trutta). Historically, this region has seen considerable conflict between seals and salmon fisheries (Rae 1962), and now contains areas that have been designated under the EU Habitats Directive to protect harbour seals, Atlantic salmon and other marine mammal predators of salmon such as bottlenose dolphins (Tursiops truncatus).
(b) Harbour seal diet
Diet composition was determined by identifying and measuring prey hard parts recovered from scats (faeces), and reconstructing prey biomass. Between February and August 2000 we visited the sandbanks used by resting seals at least once a week, except when bad weather prevented access by boat. The site was searched thoroughly and all scat samples collected in individual plastic bags and stored at −20 °C.
Samples were later thawed and each was washed through a nest of sieves (5.0, 1.0, 0.5 and 0.25 mm) to allow collection of hard parts. Prey species were identified from sagittal otoliths and cephalopod beaks using published identification guides (Clarke 1986; Härkönen 1986) and an in-house reference collection. Where it was not possible to identify otoliths to species, they were identified to the lowest possible taxonomic grouping. In particular, this involved pooling data for salmon and sea trout, as digested otoliths of these two species could not be distinguished reliably. The length, width and thickness of each otolith were measured to the nearest 0.01 mm using digital callipers. Where large numbers of otoliths of a single species were found in a single sample, a random sub-sample was measured. This sub-sample consisted of 30 otoliths, or 25% of the total number present, whichever was larger. The upper or lower hood length of each cephalopod beak was also measured to the nearest 0.01 mm with digital callipers.
The percentage, by weight, of prey in the scat samples that contained hard parts was estimated using relationships between otolith size and prey weight. Hard part sizes were first corrected for digestion using correction factors from Tollit et al. (1997a). Original prey sizes were estimated from undigested hard parts sizes using species-specific regression relationships (from Härkönen 1986; Pierce et al. 1991; Brown & Pierce 1998). Finally, weights were summed for each month and adjusted to account for sub-sampling.
(c) Occurrence of seals in freshwater areas
Previous radio-tracking studies indicate that harbour seals from this area typically travel 40–60 km out to sea on foraging trips of several days (Tollit et al. 1998). However, anglers and river managers also report the presence of seals within the freshwater rivers systems, raising the possibility that seals may show an aggregative response as fishes move into the river. We therefore investigated the occurrence of harbour seals in a 450 m stretch of the River Conon, the primary salmon river in this system, for 89 days between June–September 1999 and April–August 2000. In 1997 and 1998, pilot studies on this river had shown that seals were sometimes present around high tide, but never at low tide (χ12=16.7, p<0.001, n=35) and observations were therefore made within 4 h blocks centred on high tide.
Observations were made from a standard point on the river bank throughout each 4 h block. On each occasion that a seal was observed at the surface, the species and time were noted. Any predatory events seen at the surface were also recorded, where possible, also noting the species of prey. The surface condition of the water was assessed using the Beaufort scale and observations were discontinued if the water state reached ‘3’ because seal surfacings were likely to be missed under these conditions.
(d) The abundance of salmonids
Atlantic salmon become available to coastal marine mammals when adults return to their natal rivers, and when smolts leave the rivers for their oceanic feeding grounds in the spring. In this system, almost all adults return as one-sea winter fish (grilse) during the summer. The at-sea distribution of sea trout is less well understood, but this species also moves back into river systems during the summer. Estimates of salmonid abundance are not available for most river systems, but rod and line angling catches can be used to provide an index of relative abundance of returning adult salmon and sea trout (Youngson et al. 2002). Monthly data are collected in commercial confidence, and fluctuations in the catches on the River Conon were therefore expressed using an index in which monthly values were calculated as a percentage of the highest value in the data series. As estimates of seal diet could only be produced for salmonids, we also added monthly catches of salmon and sea trout together and converted to produce an index of adult salmonid abundance.
An index of salmon smolt abundance was produced using information collected by the Conon District Salmon Fisheries Board. Each year salmon smolts are trapped on one tributary of the river (Mills 1964) and released approximately 12 km above the mouth of the river. Using published information on the speed of smolt migrations (Mills 1964; Moore et al. 1995), we estimated that the smolts would reach the study site at the mouth of the river in between 4 and 14 days. The index was therefore created by adding a delay of one week to the number of smolts caught at the trap.
(e) Data analysis
We use the generalized Michaelis–Menton equation to describe the relationship between the percentage by weight in the diet and the abundance of salmonids in the environment:
where F is the maximal feeding rate and G is the affinity constant (Real 1977). Where x=1 (i.e. a model with two parameters and an error term), the equation behaves as a type 2 functional response switching to a sigmoid shaped type 3 response when x>1 (Real 1977). Model fitting was undertaken using the nls (non linear least squares regression) function of S-Plus (S-Plus 2000 release 3, Mathsoft Inc.). A type 1 functional response (straight line: one parameter model) was also fitted to the data. Akaike's information criterion (AIC) and Akaike weights were used to compare fits of the three models as this method adds a penalty proportional to the number of model parameters (Burnham & Anderson 2002).
Generalized linear models (GLM) were used to investigate how changes in the abundance indices affected the probability of sighting seals in the study area, and of salmonid otoliths occurring in seal scats. The probability curve was fitted using the logit link function (Cox & Snell 1989; Milner et al. 1999). The three indices, and their interactions, were included in a GLM specified in S-Plus. Initial model simplification was undertaken using the S-Plus step function that uses the AIC to identify the minimum adequate model (Crawley 2002). Terms were then removed sequentially until only significant terms were left in the model (Crawley 2002). Significance was measured by the change in the deviance (measured using χ2) that occurred when the term was removed from the model (Milner et al. 1999).
3. Results
(a) The functional response
A total of 295 scats were collected from Cromarty Firth sandbanks during the study. 4084 otoliths and 23 cephalopod beaks were identified from the scat samples, from 17 different prey species. The number of samples collected was fairly evenly spread through the season, with over 30 being collected in every month (table 1). The contribution of salmonids to the diet showed a seasonal pattern peaking during July (table 1).
Table 1.
Monthly breakdown of the contribution of salmonids to the diet of seals assessed using scats collected from the Cromarty Firth haulout site. (Information is presented on the number of scats collected and the contribution of salmonids expressed as percentage frequency of occurrence, number of otoliths and percentage by weight.)
| month | n | percentage frequency of occurrence | number of otoliths | percentage by weight |
|---|---|---|---|---|
| February | 34 | 0 | 0 | 0 |
| March | 36 | 0 | 0 | 0 |
| April | 34 | 6 | 2 | 0.3 |
| May | 51 | 12 | 7 | 11.7 |
| June | 41 | 5 | 2 | 0.4 |
| July | 42 | 21 | 18 | 59.0 |
| August | 57 | 7 | 4 | 26.9 |
The fitted Michaelis–Menton equation suggested that the functional response was a sigmoid shape type 3 (x=15; figure 1)
The type 3 model had the lowest AIC value (48.7) followed by type 1 (52.4) and type 2 (54.5). Examination of the Akaike weights provides relatively strong evidence (approximately 80% chance) that the type 3 model describes the data better than the alternatives (Akaike weights: type 1=0.12, type 2=0.04, type 3=0.83).
Figure 1.
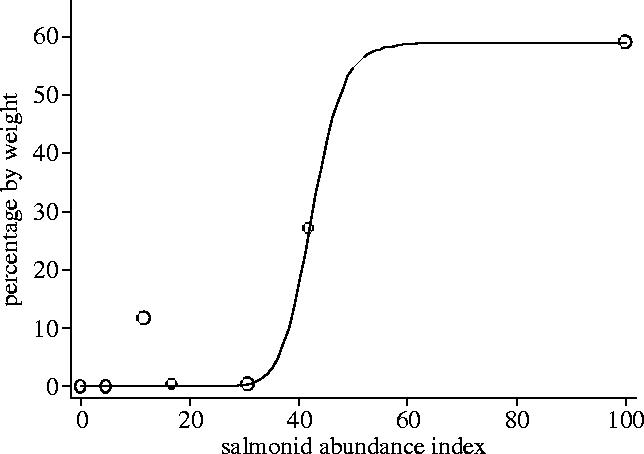
The functional relationship between the abundance of salmonids in the environment and the percentage by weight of salmonids in the diet of harbour seals using the Cromarty Firth during 2000. The fitted Michaelis–Menton function suggests a type 3 functional response.
(b) Aggregative response
Harbour seals were seen in the study area on 26 of the 87 observation periods (table 2) but there was never more than one seal at the surface at any one time. Sighting probability showed a consistent seasonal trend in both years, with sightings peaking during July (table 2). In 6 of 26 occasions on which harbour seals were present they were seen at the surface with a salmonid (identified from the shape and distinctive pink flesh colour; table 2). Seals were not seen at the surface with any other prey item during the study.
Table 2.
Monthly breakdown of the observations undertaken at the mouth of the Conon. (The number of observation periods undertaken (n) are presented along with the number in which harbour seals were observed. The number of observation periods in which harbour seals were seen to consume salmonids is also shown.)
| year | month | n | seals present | sighting probability | observed consuming salmonids |
|---|---|---|---|---|---|
| 1999 | June | 7 | 1 | 0.14 | 0 |
| 1999 | July | 14 | 10 | 0.71 | 2 |
| 1999 | August | 13 | 4 | 0.31 | 2 |
| 1999 | September | 9 | 0 | 0 | 0 |
| 2000 | April | 8 | 1 | 0.12 | 0 |
| 2000 | May | 9 | 0 | 0 | 0 |
| 2000 | June | 9 | 2 | 0.22 | 1 |
| 2000 | July | 9 | 7 | 0.78 | 1 |
| 2000 | August | 9 | 1 | 0.11 | 0 |
| total | 87 | 26 | 0.30 | 6 |
During model simplification the smolt and sea trout indices, along with all the interaction terms, were removed from the model. The results of the GLM suggest that the salmon catch index could be used to predict the probability of sighting a seal in the study area (χ12=28.4, p<0.01; figure 2).
Figure 2.
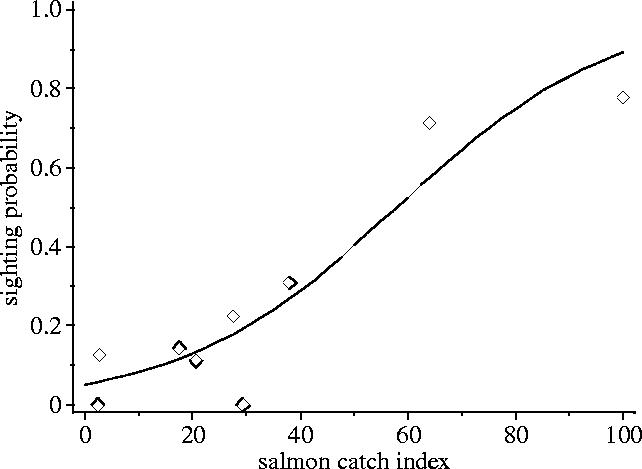
The aggregative response of harbour seals in the mouth of the River Conon to changes in the abundance of adult salmon. Data points represent observed monthly sighting probabilities and the line shows sighting probabilities (p(s)) Predicted by the logistic equation: .
4. Discussion
(a) Functional response
Seals are often considered to be generalist predators (e.g. Härkönen & Heide-Jørgensen 1991). While there is little information on their functional response to changes in prey abundance, switching behaviour in other generalist predators typically produces a type 3 functional response (Fryxell & Lundberg 1994). Our study supports this prediction, since despite being penalized for having the greatest number of parameters, the type 3 functional response is chosen as the best fit for data on harbour seal diet in relation to local changes in the abundance of adult salmonids. The slight outlier to this relationship was the sample from May, where the percentage of salmonids in the diet was underestimated (figure 1). Interestingly, this period coincides with the seaward migration of salmon smolts, which were not accounted for in this abundance index. The functional response is usually associated with feeding rate (Holling 1959) rather than diet composition. However, diet composition is a major component of functional responses, and in the absence of feeding rate data has been used to examine them (e.g. Redpath & Thirgood 1999).
A switching response to changes in salmonid abundance seems likely given the strong seasonal occurrence of these prey in estuaries. Switching between prey types may result from seals adopting different foraging locations, or foraging strategies, as has been suggested for predation on other seasonally available prey in this area such as clupeids and sandeels (Pierce et al. 1991; Tollit et al. 1997b). Bailey & Ainley (1982) also found that a theoretical functional response model that involved switching (i.e. type 3) was a better predictor of the observed occurrence of hake in Californian sea lion diet than a non-switching model (type 2).
Inevitably, in field studies with marine mammals, the numbers of data points available for analysis (figure 1) are much smaller than can be obtained in the smaller-scale terrestrial systems that have been more typically used to derive theory underpinning functional responses. Such small datasets do not allow the existence of alternative functional responses (types 1 and 2) to be rejected with a very high level of confidence. However, examination of the Akaike weights allows the identification of the functional response most likely to produce the data. The main factor that influenced sample size was the sampling intensity. The monthly interval employed in this study was believed to be the most reasonable intensity given the definition of the fish abundance indices. Finer-scale sampling of diet data within a season may be possible at sites where larger numbers of scat samples could be collected and detailed information on fish abundance is available. The alternative approach to increasing quantity of data would be to pool across years. However, this method would have the disadvantage that identifying the response of seals to salmonids could be confounded by greater variability in the abundance of alternative prey (Tollit et al. 1997b) and in the density of predators (Abrams & Ginzburg 2000).
We attempted to focus on a relatively simple system, but were still constrained by uncertainties regarding both the species composition of the salmonid prey in the diet, and the relative abundance of juvenile versus returning adult fish. In future, molecular analysis of scats could be used to focus more directly on predation upon Atlantic salmon (Purcell et al. 2004; Parsons et al. 2005). Not only would this have the advantage that data on the distribution and abundance of this species are of higher quality, but this would also allow us to focus on the interaction of greatest commercial interest.
(b) Aggregative response
The probability of sighting seals within the freshwater river system also showed a strong seasonal pattern that was significantly related to the abundance of returning adult salmon. Interestingly, the sea trout catch index was not a good predictor of seal presence in these areas. Relationships between harbour seal abundance and salmonids have been found in other riverine and estuarine study areas (Brown & Mate 1983; Roffe & Mate 1984; Greenstreet et al. 1993). However, in some of these correlative studies (e.g. Roffe & Mate 1984; Greenstreet et al. 1993) the presence of harbour seals appeared to be related partly to changes in the abundance of alternative prey species. In our study, observations of harbour seals consuming salmonids provide some support for a causal link between seal and salmon abundance, but further work is required to determine whether alternative, more cryptic, prey are also taken in these rivers.
These results provide some evidence for an aggregative response linking the use of rivers by harbour seals to salmon abundance. However, even though nearby haul-out sites were used by 100–200 harbour seals (Thompson et al. 1997; Middlemas 2003), we never saw more than one individual at a time during our observations on the river. While other seals were undoubtedly present elsewhere in the river system, reports from fisheries managers indicate that observations are typically of a single animal and overall numbers are low. This suggests that any aggregative response is relatively weak. This could be because overall abundance of salmonids is low relative to alternative marine prey, and only small numbers of seals would be predicted to use these areas under the ideal free distribution (Sutherland 1996). Alternatively, the number of seals using these freshwater sites could be constrained by social factors. For example, studies of brown bear (Ursus arctos) predation on chum (Oncorhynchus keta) and pink salmon (Oncorhynchus gorbuscha) have shown that streams generally support one large socially dominant bear (Gende & Quinn 2004).
(c) Implications for management of seals–fisheries interactions
In the absence of information on functional responses, models of interactions between marine mammals and fisheries should ideally be developed to incorporate a range of forms for these relationships (Harwood & Stokes 2003). However, where this is not possible, our data provide empirical support for theory that proposes that type 3 functional responses are appropriate for these generalist predators. With respect to the particular interaction between coastal seals and salmonids, further work is now required to determine whether the seals' within-season type 3 response also reflects responses to inter-annual variation in salmonid abundance. If so, such predation would be unlikely to drive prey populations to extinction but could constrain populations in a ‘predator pit’ (Walters 1986). Alternatively, predator density or inter-annual variability in the availability of alternative prey could have a stronger impact on prey choice, leading to more stochastic variation in predation upon salmonids. To develop appropriate measures to reduce impacts of seals on protected salmonid populations, further work is now required to assess the relative impact of salmon density, alternative prey density and predator social interactions on their foraging behaviour.
Acknowledgments
We thank John Hislop and Simon McKelvey for help throughout this project together with all those who helped with sample collection. We also thank Gordon Hastie, Robin Cook and John Fryxell for comments on earlier versions of this manuscript. S.J.M. was supported by a Fisheries Research Services studentship, and additional funding was provided by the HDH Wills Trust and Talisman Energy (UK) Ltd.
References
- Abrams P.A, Ginzburg L.R. The nature of predation: prey dependent, ratio dependent or neither? Trends Ecol. Evol. 2000;15:337–341. doi: 10.1016/s0169-5347(00)01908-x. 10.1016/S0169-5347(00)01908-X [DOI] [PubMed] [Google Scholar]
- Andersson M, Erlinge S. Influence of predation on rodent populations. Oikos. 1977;29:591–597. [Google Scholar]
- Bailey K.M, Ainley D.G. The dynamics of California Sea Lion predation on Pacific hake. Fish. Res. 1982;1:163–176. 10.1016/0165-7836(81)90018-7 [Google Scholar]
- Brown E.G, Pierce G.J. Monthly variation in the diet of harbour seals in inshore waters along the southeast Shetland (UK) coastline. Mar. Ecol. Prog. Ser. 1998;167:275–289. [Google Scholar]
- Brown R.F, Mate B.R. Abundance, movements, and feeding-habits of harbor seals, Phoca vitulina, at Netarts and Tillamook Bays, Oregon. Fish. Bull. 1983;81:291–301. [Google Scholar]
- Burnham K.P, Anderson D.R. Springer; New York: 2002. Model selection and inference: a practical information-theoretic approach. [Google Scholar]
- Clarke M.R. 1st edn. Clarendon Press; Oxford, UK: 1986. A handbook for the identification of cephalopod beaks. [Google Scholar]
- Cox D.R, Snell E.J. 2nd edn. Chapman & Hall; London: 1989. Analysis of binary data. [Google Scholar]
- Crawley M.J. 1st edn. Wiley; Chichester, UK: 2002. Statistical computing: an introduction to data analysis using S-Plus. [Google Scholar]
- Fryxell J.M, Lundberg P. Diet choice and predator–prey dynamics. Evol. Ecol. 1994;8:407–421. 10.1007/BF01238191 [Google Scholar]
- Gende S.M, Quinn T.P. The relative importance of prey density and social dominance in determining energy intake by bears feeding on Pacific salmon. Can. J. Zool. 2004;82:75–85. 10.1139/Z03-226 [Google Scholar]
- Greenstreet S.P.R, Morgan R.I.G, Barnett S, Redhead P. Variation in the numbers of shags Phalacrocorax aristotelis and common seals Phoca vitulina near the mouth of an Atlantic salmon Salmo salar river at the time of the smolt run. J. Anim. Ecol. 1993;62:565–576. [Google Scholar]
- Hanski I, Hansson L, Henttonen H. Specialist predators, generalist predators, and the microtene rodent cycle. J. Anim. Ecol. 1991;60:353–367. [Google Scholar]
- Härkönen T.J. Danbiu ApS; Hellerup, Denmark: 1986. Guide to the otoliths of the bony fishes of the Northeast Atlantic. [Google Scholar]
- Härkönen T.J, Heide-Jørgensen M.P. The harbor seal Phoca vitulina as a predator in the Skagerrak. Ophelia. 1991;34:191–207. [Google Scholar]
- Harwood J, Croxall J.P. The assessment of competition between seals and commercial fisheries in the North-Sea and the Antarctic. Mar. Mammal. Sci. 1988;4:13–33. [Google Scholar]
- Harwood J, Stokes K. Coping with uncertainty in ecological advice: lessons from fisheries. Trends Ecol. Evol. 2003;18:617–622. 10.1016/j.tree.2003.08.001 [Google Scholar]
- Hastie G.D, Wilson B, Wilson L.J, Parsons K.M, Thompson P.M. Functional mechanisms underlying cetacean distribution patterns: hotspots for bottlenose dolphins are linked to foraging. Mar. Biol. 2004;144:397–403. 10.1007/s00227-003-1195-4 [Google Scholar]
- Holling C.S. The components of predation as revealed by a study of small mammal predation of the European pine sawfly. Can. Entomol. 1959;91:293–320. [Google Scholar]
- Middlemas, S. J. 2003 Interactions between harbour seals (Phoca vitulina) and salmonids (Salmo spp.) in estuarine environments. Ph.D. thesis, University of Aberdeen, Scotland.
- Middlemas S.J, Armstrong J.D, Thompson P.M. The significance of marine mammal predation on salmon and sea trout. In: Mills D.H, editor. Salmon at the edge. Blackwell Science; Oxford, UK: 2003. pp. 42–60. [Google Scholar]
- Mills D.H. The ecology of young stages of of the Atlantic salmon in the River Bran, Ross-shire. Fresh. Salm. Fish. Res. Scot. 1964;32:58. [Google Scholar]
- Milner J.M, Elston D.A, Albon S.D. Estimating the contributions of population density and climatic fluctuations to interannual variation in survival of soay sheep. J. Anim. Ecol. 1999;68:1235–1247. 10.1046/j.1365-2656.1999.00366.x [Google Scholar]
- Mohn R, Bowen W.D. Grey seal predation on the Eastern Scotian shelf: modelling the impact on Atlantic cod. Can. J. Fish. Aquat. Sci. 1996;53:2722–2738. 10.1139/cjfas-53-12-2722 [Google Scholar]
- Moore A, Potter E.C.E, Milner N.J, Bamber S. The migratory behavior of wild Atlantic salmon (Salmo salar) smolts in the estuary of the River Conwy, North Wales. Can. J. Fish. Aquat. Sci. 1995;52:1923–1935. [Google Scholar]
- Parsons K.M, Piertney S.B, Middlemas S.J, Hammond P.S, Armstrong J.D. DNA-based identification of salmonid prey species in seal faeces. J. Zool. 2005;266:275–281. 10.1017/S0952836905006904 [Google Scholar]
- Pierce G.J, Thompson P.M, Miller A, Diack J.S.W, Miller D, Boyle P.R. Seasonal-variation in the diet of common seals (Phoca vitulina) in the Moray-Firth area of Scotland. J. Zool. 1991;223:641–652. [Google Scholar]
- Purcell M, Mackey G, LaHood E, Huber H, Park L. Molecular methods for the genetic identification of salmonid prey from Pacific harbor seal (Phoca vitulina richardsi) scat. Fish. Bull. 2004;102:213–220. [Google Scholar]
- Quinn T.P, Gende S.M, Ruggerone G.T, Rogers D.E. Density-dependent predation by brown bears (Ursus arctos) on sockeye salmon (Oncorhynchus nerka) Can. J. Fish. Aquat. Sci. 2003;60:553–562. 10.1139/f03-045 [Google Scholar]
- Rae B.B. The effect of seal stocks on Scottish marine fisheries. In: LeCren, Holdgate, editors. The exploitation of natural animals populations. Blackwell Scientific; Oxford, UK: 1962. pp. 305–311. [Google Scholar]
- Real L.A. The kinetics of functional response. Am. Nat. 1977;111:289–299. 10.1086/283161 [Google Scholar]
- Redpath S.M, Thirgood S.J. Numerical and functional responses in generalist predators: hen harriers and peregrines on Scottish grouse moors. J. Anim. Ecol. 1999;68:879–892. 10.1046/j.1365-2656.1999.00340.x [Google Scholar]
- Roffe T.J, Mate B.R. Abundances and feeding-habits of pinnipeds in the Rogue River, Oregon. J. Wildl. Manage. 1984;48:1262–1274. [Google Scholar]
- Sutherland W.J. 1st edn. Oxford University Press; Oxford, UK: 1996. From individual behaviour to population ecology. [Google Scholar]
- Thompson P.M, Tollit D.J, Wood D, Corpe H.M, Hammond P.S, Mackay A. Estimating harbour seal abundance and status in an estuarine habitat in north-east Scotland. J. Appl. Ecol. 1997;34:43–52. [Google Scholar]
- Tollit D.J, Steward M.J, Thompson P.M, Pierce G.J, Santos M.B, Hughes S. Species and size differences in the digestion of otoliths and beaks: implications for estimates of pinniped diet composition. Can. J. Fish. Aquat. Sci. 1997a;54:105–119. 10.1139/cjfas-54-1-105 [Google Scholar]
- Tollit D.J, Greenstreet S.P.R, Thompson P.M. Prey selection by harbour seals, Phoca vitulina, in relation to variations in prey abundance. Can. J. Zool. 1997b;75:1508–1518. [Google Scholar]
- Tollit D.J, Black A.D, Thompson P.M, Mackay A, Corpe H.M, Wilson B, Van Parijs S.M, Grellier K, Parlane S. Variations in harbour seal, Phoca vitulina, diet and dive-depths in relation to foraging habitat. J. Zool. 1998;244:209–222. 10.1017/S0952836998002064 [Google Scholar]
- Walters C.J. 1st edn. Macmillan Publishing Company; New York: 1986. Adaptive management of renewable resources. [Google Scholar]
- Yodzis P. Predator–prey theory and management of multispecies fisheries. Ecol. Appl. 1994;4:51–58. [Google Scholar]
- Yodzis P. Culling predators to protect fisheries: a case of accumulating uncertainties—response. Trends Ecol. Evol. 2001;16:282–283. 10.1016/S0169-5347(01)02159-0 [Google Scholar]
- Youngson A.F, MacLean J.C, Fryer R.J. Rod catch trends for early-running MSW salmon in Scottish rivers (1952–1997): divergence among stock components. ICES J. Mar. Sci. 2002;59:836–849. 10.1006/jmsc.2002.1195 [Google Scholar]


