Abstract
Integrin receptors connect the extracellular matrix to the actin cytoskeleton. This interaction can be viewed as a cyclical liaison, which develops again and again at new adhesion sites only to cease at sites of de-adhesion. Recent work has demonstrated that multidomain proteins play crucial roles in the integrin–actin connection by providing a high degree of regulation adjusted to the needs of the cell. In this review we present several examples of this paradigm and with special emphasis on the ILK–PINCH–parvin complex, which amply demonstrates how structural and signalling functions are linked together.
Keywords: actin/focal adhesion/ILK–PINCH–parvin complex/integrin/integrin signalling
Introduction
The integrin family of cell adhesion molecules mediates cellular contacts to the extracellular matrix (ECM) or cell counter receptors, thereby regulating cell motility, cell polarity, cell growth and survival (Brakebusch et al., 2002). The identification and characterization of central players in integrin signalling originated from biochemical, structural and genetic studies. These studies have revealed intimate interplay between integrin and growth factor/cytokine signalling as well as mutual functional dependence between integrins and the actin cytoskeleton (Brakebusch et al., 2002).
Ligand binding to integrins leads to integrin clustering and recruitment of actin filaments and signalling proteins to the cytoplasmic domain of integrins (Hynes, 2002). These specialized, ECM attachment organelles and signalling centres are called focal complexes when they are still nascent and in the process of forming, or focal adhesions (FAs) when they have matured into larger complexes. The formation of cell adhesion complexes assures substrate adhesion as well as targeted location of actin filaments and signalling components, and hence is essential for establishing cell polarity, directed cell migration, and maintaining cell growth and survival.
Recent work has revealed that the integrin–actin cytoskeleton connection is highly dynamic and subject to many regulatory processes. In healing skin wounds for example, integrin-mediated cues promote the reorganization of the cytoskeleton of keratinocytes at the wound edge resulting in directed migration and wound closure. Loss of β1-integrins on keratinocytes leads to impaired as well as non-directed migration resulting in severely delayed re-epithelialization (Grose et al., 2002). Furthermore, it has become clear that the interaction between integrins and the actin cytoskeleton is differentially regulated in different locations of the cell. At the leading edge of migrating cells, integrins bind the ECM, recruit the actin cytoskeleton and initiate local reorganization of the actin network, promoting different types of membrane protrusion. At the rear of the cell, integrins detach from the ECM, dissolve the link to the cytoskeleton and are, at least partially, recycled to the front of the cell (Ballestrem et al., 2001; Laukaitis et al., 2001).
Signalling pathways, which depend on localized integrin activation have also been reported. For example, at the leading edge of cells, integrin signalling dissociates complexes between GTP-bound Rho-GTPases and Rho-GDI to release active Cdc42 and Rac1, resulting in membrane extension (Del Pozo et al., 2002). In astrocytes, integrin binding activates Src-like kinases, which induce the formation of a complex of Cdc42, mPar6 and PKCζ (Etienne-Manneville and Hall, 2001). This complex phosphorylates and inactivates GSK-3β at the leading edge of the cell and thereby allows interaction of adenomatous polyposis coli (Apc) with the plus ends of microtubules. This is essential for the reorientation of the microtubular network and the directed movement of the cells (Etienne-Manneville and Hall, 2003). Finally, complexity is added by the fact that integrin-associated molecules are multifunctional. Integrin-linked actin binding proteins attach to signalling molecules and function as platforms, which brings kinases and substrates together. Integrin-bound signalling molecules, on the other hand, bind to actin binding proteins, enforcing the integrin– cytoskeleton connection. As it turns out for integrin-linked kinase (ILK), such adapter function might be even more important in vivo than the kinase function demonstrated in vitro.
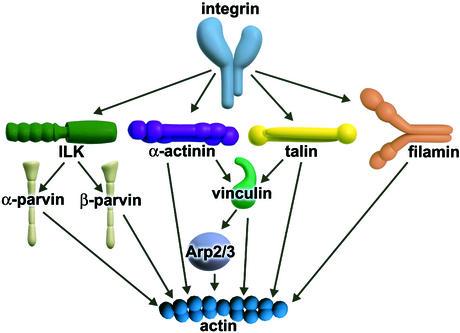
In the present review, we will describe proteins that structurally or functionally link integrins with the actin cytoskeleton, emphasizing their multi-purpose nature (Figure 1).
Fig. 1. Overview of different pathways by which integrin can link to the actin cytoskeleton. The molecules are not drawn to scale.
Actin binding proteins as platforms for integrin-mediated signal transduction
Talin
Talin is a large protein of >2500 amino acids, consisting of an N-terminal head region of ∼50 kDa and a large rod region of ∼220 kDa, which contains primarily alanine-rich repeats. Via the Four point one, Ezrin, Radixin, Moesin (FERM) domain in the head, talin binds to integrin, focal adhesion kinase (FAK), phosphatidylinositol phosphate kinase type Iγ (PIPKIγ), Phosphatidylinositol (4,5) bisphosphate (PIP2), non-integrin transmembrane receptors (Horwitz et al., 1986; Chen et al., 1995; Borowsky and Hynes, 1998; Martel et al., 2001; Di Paolo et al., 2002; Ling et al., 2002) and weakly to actin. The rod domain contains a low affinity site for integrin (Yan et al., 2001), three vinculin binding sites and a major actin binding site at the C-terminus (Hemmings et al., 1996). Talin binding to integrin disrupts an intracellular salt bridge between the α and β-integrin subunit, leading to increased integrin affinity, which strengthens the interaction with the ECM (Vinogradova et al., 2002; Garcia-Alvarez et al., 2003).
In vivo, talin is important for the linkage of integrin clusters to the cytoskeleton. In talin-deficient flies, integrins still bind the ECM, but can neither aggregate into clusters nor connect to the cytoskeleton (Brown et al., 2002). These mutants develop a phenotype resembling that of flies lacking integrin βPS, characterized by a failure in germ band retraction and muscle detachment (Leptin et al., 1989; Roote and Zusman, 1995; Schöck and Perrimon, 2003), suggesting that integrin function is highly dependent on talin. However, talin is not essential for integrin signalling resulting in gene expression, since in the absence of zygotic talin integrin-mediated gene repression was still functional (Brown et al., 2002). Mammals have two highly similar isoforms of talin suggesting redundant functions. Mice lacking talin-1 form mesoderm, which fails to migrate (Monkley et al., 2000). Talin-1-deficient embryonic stem (ES) cells show reduced adhesion and spreading on collagen and laminin and are unable to assemble FAs or stress fibres (Priddle et al., 1998). After differentiation, however, vinculin-containing FAs form. Whether this is due to expression of talin-2 (Monkley et al., 2001) or alternative linker molecules can only be revealed by double knockouts of talin-1 and -2.
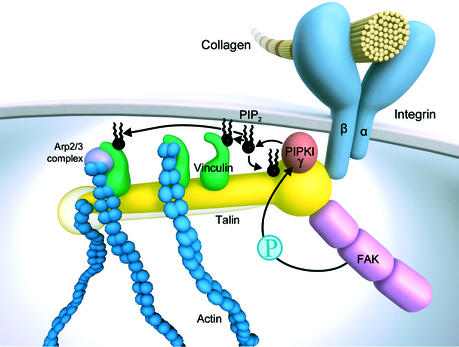
Binding of talin to integrin is increased by PIP2 (Martel et al., 2001). PIP2 also modulates the activity of other cytoskeletal proteins at the plasma membrane, promoting their attachment to the plasma membrane and actin filament assembly. Recent data show that talin is crucial for the localized production of PIP2 at newly engaged integrins. Upon integrin clustering, talin is recruited to focal complexes where it binds and activates the PIP2-producing enzyme PIPKIγ (Di Paolo et al., 2002; Ling et al., 2002). This leads to an increased local concentration of PIP2, which associates with talin and other proteins, facilitating the formation of FAs (Figure 2). This process is subject to regulation. First, interaction of talin with PIPKIγ is dependent on integrin and growth factor signalling (Ling et al., 2002). In suspended cells deprived of growth factors, talin does not associate with PIPKIγ. Secondly, tyrosine phosphorylation of PIPKIγ by FAK increases PIP2 production and the association of PIPKIγ with talin. Interestingly, high expression of PIPKIγ results in a loss of talin from FA and rounding up of the cells.
Fig. 2. Talin associates with PIPKIγ, which produces PIP2. PIP2 binds to talin, strengthening the interaction between talin and integrin. PIP2 also binds to vinculin, which then interacts with talin. PIP2 on vinculin is replaced by actin filaments. PIP2-associated vinculin can transiently bind to activated Arp2/3 complex, which nucleates actin polymerization. Talin is an antiparallel homodimer. For reasons of simplicity, binding partners of the second talin molecules are not shown. The molecules are not drawn to scale.
The association of talin with vinculin, a ubiquitous cytoskeletal protein found at cell–cell and cell–ECM contacts, is also regulated by PIP2. Binding of PIP2 to vinculin unfolds the inactive molecule and exposes binding sites for talin and α-actinin in the head domain and for VASP in the tail domain (Gilmore and Burridge, 1996; Hüttelmaier et al., 1999). Paxillin can bind to the tail in the absence of PIP2 (Steimle et al., 1999). The interaction with talin facilitates vinculin binding to actin (Gilmore and Burridge, 1996), which quite likely requires dissociation of PIP2 due to overlapping binding sites (Steimle et al., 1999). Vinculin can also induce the polymerization of actin monomers by recruiting the Arp2/3 complex, which can initiate actin nucleation (DeMali et al., 2002). This interaction is transient and requires both PIP2 binding to vinculin and activation of the Arp2/3 complex by Rac1. The Arp2/3 complex can nucleate new actin filaments or induce branching of existing filaments resulting in an actin network that pushes the plasma membrane forward. Fibroblasts that lack vinculin or express vinculin mutants unable to recruit the Arp2/3 complex show reduced adhesion, spreading and lamellipodia formation. Thus, vinculin couples newly engaged β1-integrins receptors to actin polymerization and membrane protrusion.
α-actinin
α-actinin connects actin fibrils to the cytoplasmic tail of transmembrane receptors such as integrins, cadherins and ICAMs. In addition, it crosslinks actin filaments to actin bundles and networks. The actin binding domain of α-actinin is located at the N-terminus, whereas the C-terminus consists of one or two EF hands. α-actinin dimerizes in an antiparallel fashion via interaction of the central rod domains. Four isoforms are found in humans and mice. Of the two non-muscle isoforms, α-actinin-1 is ubiquitously expressed and located primarily in FAs (Pavalko and Burridge, 1991), whereas α-actinin-4 is not found at FA or cell–cell junctions, but is present in certain types of membrane ruffles (Honda et al., 1998). α-actinin-4 sems to play a role in endocytosis and tumour cell motility. Muscle-specific α-actinin-2 and -3 crosslink actin filaments in the region of Z discs in striated muscles (Beggs et al., 1992).
Several cytoplasmic proteins interact with actinin including vinculin, zyxin, extracellular signal-regulated kinase 1/2 (Erk1/2), mitogen-activated protein/extracellular signal-regulated kinase kinase 1 (MEKK1), protein kinase N (PKN) and the p85 subunit of phosphatidylinositol-3 kinase (PI3-K), suggesting that α-actinin serves as an important scaffolding protein (Wachsstock et al., 1987; Crawford and Beckerle, 1991; Shibasaki et al., 1994; Mukai et al., 1997; Christerson et al., 1999; Leinweber et al., 1999). These protein–protein interactions can be modulated. PIP2 binding increases the interaction with actin, PKN and PI3-K (Fukami et al., 1994; Shibasaki et al., 1994; Mukai et al., 1997). PIP3, on the other hand, decreases the binding of α-actinin to integrins (Greenwood et al., 2000). Calcium binding to the EF-hands of non-muscle α-actinin reduces the interaction with actin filaments (Witke et al., 1993). Finally, FAK-dependent phosphorylation of α-actinin decreases its association with actin (Izaguirre et al., 2001).
In Drosophila, α-actinin-null mutations are lethal and are characterized by defects in muscle structure and function (Fyrberg et al., 1998). No α-actinin-deficient mice have been reported, but recent experiments using chromophore-assissted laser inactivation of EGFP-coupled α-actinin in Swiss 3T3 cells demonstrated that α-actinin is essential for the integrin–cytoskeleton linkage in FA (Rajfur et al., 2002). The integrity of the FAs themselves was not affected by the laser inactivation.
Filamin
Filamins are dimeric proteins with a head domain containing the actin binding site and a rod domain consisting of 4–24 repetitive units, which resemble the immunoglobulin fold, each consisting of ∼100 amino acids (Stossel et al., 2001; van der Flier and Sonnenberg, 2001). They are non-covalently associated at the C-terminus. In humans, three filamin genes (filamins A, B and C) have been identified, which, by alternative splicing, give rise to several isoforms. While filamins A and B are nearly ubiquitously expressed, filamin-C is found primarily in heart and skeletal muscle.
Filamins link actin filaments in orthogonal networks or parallel bundles. In vitro, the type of actin organization depends on the ratio of filamin to actin. Via different repeat units in the rod, filamins associate with transmembrane proteins such as integrins (Sharma et al., 1995; Loo et al., 1998), signalling molecules such as Rho-GTPases (Ohta et al., 1999), MEKK (Marti et al., 1997) and guanine nucleotide exchange factors (GEFs) (Bellanger et al., 2000), and other proteins.
In a melanoma cell line, filamin expression increased the surface level of β1-integrin as shown by FACS analysis (Meyer et al., 1998). However, this decreased turnover or increased membrane insertion of integrin seems not to be due to direct interaction with filamin, since it could not be coprecipitated with β1-integrin. Beside the regulation of the surface levels of integrins, filamin controls integrin function. In CHO cells, increased filamin binding to integrins by the introduction of point mutations into the cytoplasmic domain of β1 and β7-integrins reduced membrane protrusion, cell polarization and consequently cell migration (Calderwood et al., 2001). Filamin can induce reorganization of the cytoskeleton by different pathways. First, filamin might stimulate changes of the cytoskeleton by providing a platform for simultaneous binding of activators and effectors, thus facilitating signalling. Trio, a GEF specifically activating Rac1, RhoA and RhoG, can bind to filamin and catalyse the transition of filamin-bound Rac1-GDP to Rac1-GTP (Bellanger et al., 2000). Rac1-GTP could then activate the filamin-associated effector molecule PAK1. Alternatively, filamins can activate PAK1 independently of Rho-GTPases. Treatment of MCF7 breast cancer cells with physiological signalling molecules such as heregulin and sphingosin leads to direct association of filamin with PAK1 (Vadlamudi et al., 2002). This results in stimulation of PAK1 kinase activity and phosphorylation of filamin by PAK1 at different serine residues near the C-terminus. The formation of a filamin–PAK1 complex and the PAK1-mediated phosphorylation of filamin is important in vivo, since the membrane ruffling activity of PAK1 in M2 melanoma cells is dependent on filamin expression and the phosphorylation of Ser2152 of filamin.
Deletion of the filamin orthologue in Dictyostelium, called gelation factor, resulted in impaired photo- and thermosensory responses (Fisher et al., 1997). Whether a general defect of the cytoskeleton occurs is unclear. Filamin and α-actinin seem to have redundant functions. While deletion of α-actinin in Dictyostelium leads only to a minor phenotype, double deletion of α-actinin and gelation factor results in reduced cell size, diminished proliferation, impaired movement and partially defective cytokinesis (Witke et al., 1992; Rivero et al., 1999). Fruit flies deficient for filamin showed female sterility and impaired actin organization of the ring canal structures (Li et al., 1999; Sokol and Cooley, 1999). Inactivation of the X-chromosomal filamin-A gene in humans is embryonic lethal (Fox et al., 1998). Heterozygous filamin-A mutations have been found in patients with periventricular heterotopia, a disease characterized by defective neuronal migration. While males usually die during embryogenesis, female patients have seizures and several non-neuronal defects (Eksioglu et al., 1996). Gain-of-function mutations in the filamin-A gene result in a broad range of congenital malformations, suggesting an important role of filamin- A-dependent signalling pathways in organogenesis (Robertson et al., 2003). Filamins might also be involved in autoimmune diseases, since high serum titres against filamin were found in patients with myasthenia gravis, graft-versus-host disease and Graves disease (Yamamoto et al., 1987; Leedman et al., 1993; Peutz-Kootstra et al., 2000). However, the exact role of filamin in these immune disorders remains unclear.
Signalling to the actin
After ligand binding, integrins activate signalling cascades that affect formation, turnover and linkage of actin filaments. The stimulation of Rho-GTPases is of special importance in this respect. These molecules are essential for the organization of the actin cytoskeleton and promote specialized actin structures such as stress fibres (RhoA), lamellipodia (Rac1) and filopodia (Cdc42) (Etienne-Manneville and Hall, 2002). In addition, Rho-GTPases are involved in cell proliferation, survival, polarity, vesicle transport and various other activities. Integrins can stimulate Rho-GTPases via different pathways, of which those via FAK and Src-like kinases seem to be most important (Schaller, 2001; Arthur et al., 2002). Recently, the integrin-associated molecules integrin cytoplasmic domain-associated protein-1 (ICAP-1) and ILK were suggested to stimulate Rho-GTPases (Degani et al., 2002; Rosenberger et al., 2003). In addition, both molecules affect the linkage of integrins to the cytoskeleton (Wu and Dedhar, 2001; Bouvard et al., 2003).
FAK
FAK is a tyrosine kinase that is present only in FAs. Based on binding of FAK to peptides of the β1 cytoplasmic domain and on co-precipitation experiments, FAK is thought to bind integrins directly (Schaller et al., 1995; Chen et al., 2000). However, since the ‘integrin binding region’ of FAK is not required for localization of FAK to the FA and the ‘FAK binding region’ of β3-integrin is not required for FAK activation, it could be that the in vivo integrin–FAK interaction is indirect (Tahiliani et al., 1997; Shen and Schaller, 1999). This is conceivable since FAK binds strongly to talin and paxillin, which directly and indirectly bind integrin (Chen et al., 1995; Hildebrand et al., 1995). Upon cell attachment to the ECM, FAK becomes autophosphorylated at Tyr397 either directly by integrin clustering or after phosphorylation of tyrosines 576 and 577 by Src, which enhances the catalytic activity of FAK (Schaller, 2001). It is possible that both pathways occur. Src itself might be activated before binding to FAK by a tyrosine phosphatase such as PTP 1B, SHP-2 or PTPα. Activated FAK can bind and phosphorylate a range of different substates, which allows further recruitment of adaptor and signalling molecules. Different regions of the FAK molecule are involved in protein–protein interactions. The phosphorylated Tyr397 can be bound by SH2-domain-containing proteins such as Src-like kinases, Grb7, PI3-K, Shc and PLCγ (Schlaepfer and Hunter, 1997; Han and Guan, 1999; Reiske et al., 1999; Zhang et al., 1999). Grb2 binds the phosphorylated Tyr925. SH3-domain proteins like p130Cas and Rho-GAP protein GRAF interact with proline-rich sequences close to the C-terminus. FAK binds to paxillin and talin via the focal adhesion targeting sequence (FAT) (Chen et al., 1995; Hildebrand et al., 1995).
Rho-GTPases can be activated by FAK through several mechanisms. First, a p130Cas–Crk–DOCK180 complex can activate Rac1, which promotes lamellipodia formation (Kiyokawa et al., 1998). Second, PI3-K can stimulate Rho-GTPase-activating GEF molecules via PIP3 production, which in turn stimulates Rho-GTPases (Das et al., 2000). Third, FAK can directly or indirectly interact via paxillin with the adapter GIT1 and with GEFs of the Cool/PIX family, which activate Rac1 and Cdc42 (Turner et al., 1999; Zhao et al., 2000). Finally, Src-like kinases can activate GEFs through phosphorylation (Crespo et al., 1997; Han et al., 1997; Teramoto et al., 1997). In short, integrin activation triggers the formation of various phosphoprotein complexes that can modify the actin cytoskeleton particularly by activating Rho-GTPases.
ICAP-1
ICAP-1 was identified in yeast two-hybrid screens as an integrin binding protein of 200 amino acids containing many putative phosphorylation sites (Chang et al., 1997; Zhang and Hemler, 1999). ICAP-1 binds β1-integrin, but no other integrin β subunits. Overexpression of wild type ICAP-1 increased cell migration on fibronectin and strongly reduced spreading. ICAP-1 is phosphorylated upon attachment to fibronectin. Mutation of Thr38 to aspartic acid, mimicking phosphorylation, reduced cell spreading while mutation to the non-phosphorylatable alanine increased spreading of CHO cells on fibronectin, suggesting that phosphorylation of ICAP-1 modulates integrin function (Bouvard and Block, 1998). Recently, it was shown that ICAP-1 might function as a Rho-GDI by binding Cdc42 and Rac1 and sequestering them in an inactive form in the cytosol (Degani et al., 2002). It was suggested that ICAP-1 binding to β1-integrin releases the inhibition of the Rho-GTPases, thus promoting cell spreading and migration. An alternative model suggests that ICAP-1 binding to β1-integrin prevents talin association and dissolves FAs. This is supported by the observation that ICAP-1 is never found in the FA (Bouvard et al., 2003), rather, prominent ICAP-1 expression occurs at the ruffling edges of migrating cells. These observations suggest that ICAP-1 is a negative regulator of β1-integrin avidity, prevents talin-mediated connection to the cytoskeleton and talin-mediated signalling events. ICAP-1-deficient mice, though viable, develop several defects that could be caused by diminished integrin activity (D.Bouvard and R.Fässler, unpublished data).
The ILK–PINCH–parvin complex as large docking station for actin-regulating proteins
Is the kinase activity of ILK essential in vivo?
ILK was described in 1995 as a Ser/Thr kinase that binds to the cytoplasmic tails of β1, β2 and β3-integrin subunits (Hannigan et al., 1996). It was suggested that ILK stimulation might be crucial for the activation of various integrin signalling pathways. Although the kinase catalytic domain lacks certain amino acids that are usually highly conserved in other Ser/Thr kinases, ILK was shown to readily phosphorylate peptides and model substrates such as myelin basic protein (MBP) in vitro and to induce phosphorylation of the protein kinases PKB/Akt and GSK-3β in cells overexpressing ILK (Delcommenne et al., 1998). Whether ILK also phosphorylates other targets, including the cytoplasmic domains of integrins, was never convincingly shown.
The identification of PKB/Akt and GSK-3β as ILK targets provided an explanation of how integrin-mediated cell proliferation (anchorage-dependent growth) and cell survival could be executed. GSK-3β is a negative regulator of Wnt signalling (Cohen and Frame, 2001), which is inactivated by phosphorylation mediated by Wnt signals or ILK. This leads to the stabilization and increase of β-catenin levels, which permits translocation of β-catenin into the nucleus where it associates with Lef-1/Tcf transcription factors to activate genes including cyclin D1 and c-myc. The formation of Lef-1/Tcf–β-catenin complexes and increased cyclin D1 expression are observed in ILK-overexpressing cells and explain their anchorage-independent growth (Novak et al., 1998) and the formation of tumours, although with long latency, in mice overexpressing ILK in mammary glands (White et al., 2001). PKB/Akt is a Ser/Thr kinase implicated in cell proliferation, survival and growth factor signalling. Full activation of PKB/Akt requires phosphorylation on residues Thr308 and Ser473. The Thr308 kinase is called 3-phosphoinositide-dependent kinase 1 (PDK1) and is well characterized (Lawlor and Alessi, 2001) while the PI3-kinase-dependent kinase that phosphorylates Ser473, termed PDK2, is less well characterized. Several experiments reported by Dedhar and colleagues suggested that ILK is PDK2 (Persad et al., 2001).
However, genetic studies in Drosophila, Caenorhabditis elegans and mouse have cast doubt upon the kinase activity of ILK. Both flies and worms lacking ILK expression show severe muscle abnormalities (Zervas et al., 2001; Mackinnon et al., 2002), but neither patterning defects typical of impaired Wnt signalling nor increased apoptosis typical of impaired PKB/Akt activity were observed in ILK-deficient flies (Zervas et al., 2001). More importantly, the defects in mutant flies and worms could be fully rescued with ILK genes that lacked kinase activity. We made mice lacking ILK expression and found that they die shortly after implantation. Therefore, we established fibroblastoid cell lines in which ILK is deleted in vitro using the Cre/loxP system. These cells have severe adhesion and proliferation defects but normal phosphorylation of PKB/Akt and GSK-3β (Sakai et al., 2003). Similarly, a chondrocyte-specific deletion of ILK in mice did not affect the phosphorylation levels of PKB/Akt and GSK-3β (Grashoff et al., 2003) indicating that in fibroblasts as well as in chondrocytes ILK is not required to phosphorylate PKB/Akt and GSK-3β. Finally, biochemical studies with mammalian cells revealed that ILK is part of a multi-protein complex without intrinsic kinase activity for PKB/Akt (Hill et al., 2002). Whether ILK is functioning as a kinase in vivo is difficult to answer as long as the kinase activity has not been selectively abrogated in mice. Clearly, however, the kinase activity is neither necessary for invertebrate development nor required for PKB/Akt and GSK-3β signalling in several vertebrate cells.
ILK drives actin reorganization/dynamics in vivo
If ILK has no essential kinase activity in vivo, how can ILK exert its function? Answers came from a combination of genetic studies in flies, worms and mice and screens for ILK interactors using mammalian cells, which point to a prominent role of ILK in modulating the actin cytoskeleton. The absence of ILK expression in Drosophila leads to a severe defect in integrin-mediated adhesion. Both ILK- and βPS integrin-deficient flies exhibit muscle detachment, but for different reasons (Zervas et al., 2001). In muscles lacking βPS integrin expression, the plasma membrane detaches from the ECM, but still anchors actin filaments. In ILK-deficient muscle the plasma membrane is attached to the ECM but fails to connect to actin filaments, suggesting that ILK is a structural component that links the cytoskeleton and the plasma membrane at sites of integrin-mediated adhesion. In C. elegans the homologue of mammalian ILK is found in body-wall muscles where it concentrates together with β-integrin/PAT3 at FA-like muscle attachment sites (dense bodies). Loss of ILK expression leads to an embryonic-lethal phenotype characterized by muscle detachment. The phenotype is called PAT4 (Paralysed and Arrested elongation at the Two-fold stage) and resembles the loss-of-function phenotype of β-integrin/PAT-3 (Mackinnon et al., 2002). Since the actin cytoskeleton has not been analysed in β-integrin/PAT-3 mutant worms, it is not known whether abnormal F-actin attachment to adhesion sites is causing the muscle phenotype.
In mouse, loss of ILK expression leads to peri-implantation lethality. Around implantation the primitive endoderm develops on the surface of the inner cell mass (ICM) of the blastocyst and lays down a basement membrane (BM) that is required for adjacent ICM cells to polarize and establish the columnar epiblast (primitive ectoderm), and for the remaining ICM cells to undergo apoptosis resulting in the establishment of the proamniotic cavity. Cell differentiation in embryoid bodies (EBs) closely mimics the events occurring at peri-implantation (Li et al., 2003) and therefore, we used ILK-null EBs to delineate the reason for the peri-implantation lethality caused by loss of ILK. In ILK-deficient EBs the primitive endoderm differentiates and produces a BM but the polarization of ICM cells to form columnar epiblast cells and subsequent cavitation is severely impaired. This defect is different from β1-integrin-deficient mice, where the primitive endoderm does not express laminin α1 and hence cannot form a BM, resulting in peri-implantation lethality (Aumailley et al., 2000; Li et al, 2002). If this defect is rescued by exogenous addition of laminin, the β1-null EBs lay down a BM, develop a normal epiblast and form cavities (Li et al., 2002). These data indicate that β1-integrins can act independently of ILK, and ILK independently of β1-integrins.
The underlying cause of the polarization defect of ILK-deficient epiblast is abnormal F-actin reorganization (Sakai et al., 2003). Normal ICM cells adjacent to the endodermal BM distribute F-actin evenly beneath their plasma membrane. During elongation and polarization of the epiblast cells the F-actin locates to the apical side facing the cavity. In ILK-null EBs, F-actin accumulates in ICM cells at sites of integrin attachment to the BM already prior to polarization. In few EBs, a few ICM cells gain columnar shape with some F-actin in an apical belt while a significant amount, however, remains at the basal side where integrins attach the polarized epiblast cells to the BM. These findings indicate that a major function of ILK in epiblast cells is to prevent F-actin accumulation at integrin adhesion sites to the BM. A similar requirement of ILK to reorganize F-actin is also evident in ILK-deficient fibroblasts and chondrocytes, which accumulate abnormal aggregates of F-actin beneath the plasma membrane and display several other abnormal actin-based processes such as diminished cell spreading and delayed formation of stress fibres and FAs (Grashoff et al., 2003; Sakai et al., 2003).
Loss of ILK expression leads to a severe actin-based defect in fly and mouse epiblast as well as fibroblasts and chondrocytes. While mammalian cells accumulate abnormal amounts of F-actin at sites of integrin adhesion, ILK-deficient muscle cells in flies fail to link actin filaments to integrin adhesion sites in muscle cells resulting in a detachment from, rather than an accumulation of F-actin at, the cell membrane (Zervas et al., 2001). Although this is a striking difference, the F-actin accumulation at the membrane might also occur during muscle development. When contraction starts, however, the F-actin will be detached from the membrane due to weak interaction with for example, intergins. Therefore, F-actin distribution should be determined prior to muscle cell contraction. In addition, F-actin localization should be analysed in ILK-deficient wing epithelial cells that detach from the ECM, which results in blister formation. However, it cannot be excluded that ILK exerts different or opposing functions in different cell types.
Binding partners of ILK
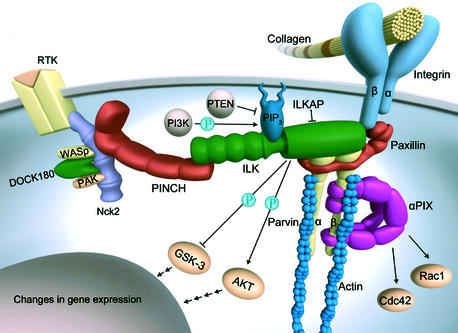
Much progress has been made in identifying ILK-interacting proteins and surprisingly, each of them can modulate actin either directly or in an indirect manner (Figure 3). In addition to the C-terminal putative kinase domain, ILK is composed of an N-terminal array of four ankyrin repeats and a pleckstrin homology (PH) domain. While it has not been determined whether the PH domain interacts directly with phospholipids, the C-terminal region has been shown to bind paxillin (Nikolopoulos and Turner, 2001), and the N-terminal region has been shown to bind the double zinc finger domain (LIM)-only adapter proteins PINCH-1 and PINCH-2 (Zhang et al., 2002a; Braun et al., 2003), which interact with the SH2/SH3-containing adaptor protein Nck2 (or Grb4; Tu et al., 2001a). Nck2 can bind through its SH2 domain phosphorylated tyrosine residues of receptor tyrosine kinases such as epidermal growth factor receptor (EGFR) or platelet-derived growth factor (PDGFR) and thereby possibly linking integrin and growth factor signalling. In addition, the SH3 domains of Nck2 can recruit actin-regulatory proteins such as WASp (Wiskott–Aldrich Syndrome protein), DOCK180 (180 kDa protein downstream of Crk) and PAK (p21-activated kinase) suggesting a role for actin reorganization/actin dynamics.
Fig. 3. ILK recruits several adaptor proteins that modulate actin dynamics or actin attachment to the integrin adhesion site either directly or indirectly. In addition, ILK can be located to the plasma membrane through interactions with phospholipids. Membrane binding activates the kinase function, subsequent to the phosphorylation of Akt and GSK3. RTK, receptor tyrosine kinase; ILKAP, ILK-associated phosphatase. The molecules are not drawn to scale.
The putative kinase domain of ILK can recruit a new family of F-actin binding proteins. This family has three members and was identified independently in several laboratories and has multiple names. Wu and colleagues identified the calponin homology-ILK binding protein (CH-ILKBP; Tu et al., 2001b), which is identical to actopaxin described by Nikolopoulos and Turner (2000) or α-parvin identified by Olski et al. (2001). In addition to α-parvin, Olski et al. (2001) described the primary sequence of β-parvin, which is identical to the ILK-binding protein affixin (Yamaji et al., 2001), and γ-parvin, which was not tested for ILK binding. We will call the family members α-, β- and γ-parvins throughout the review to underline their structural similarity.
Overexpression of mutant forms of ILK, PINCH or parvin, which are unable to interact with each other, revealed that the formation of a ternary complex of ILK, PINCH and parvins is essential for their recruitment to FAs (Zhang et al., 2002b). The significance of the ILK–PINCH–parvin complex for cell adhesion, cell spreading, FA assembly and stress fibre formation has been shown in several cell culture studies. The ILK–parvin interaction, for example, was investigated in a myoblast cell line, in which disruption of the ILK–α-parvin complex retarded formation of stress fibres as well as FAs and delayed cell spreading (Tu et al., 2001b). It seems that the formation of the ILK–PINCH–parvin complex, however, is not sufficient for FA localization and that interactions with additional proteins are necessary for FA recruitment. A possible candidate for such an additional interactor is paxillin, which binds integrins, ILK and α-parvin (Nikolopoulos and Turner, 2002). Besides the recruitment of the ILK–PINCH–parvin complex into FAs, paxillin may also further reinforce F-actin interactions through recruitment of vinculin. Overexpression of a mutant parvin defective for paxillin binding impairs cell adhesion and cell spreading, supporting the hypothesis that the recruitment of parvin and hence the entire complex to integrins is important for actin filament assembly/recruitment at cell adhesion sites (Nikolopoulos and Turner, 2000). Recent yeast two-hybrid studies identified the guanine exchange factor αPIX [Pak-interacting exchange factor, also called Cool2 (cloned out of library) or ARHGEF6 (Rosenberger et al., 2003)] as a new β-parvin-interacting protein. αPIX contains a Dbl homology domain, which can activate the small GTPases Rac and Cdc42.
Altogether these reports show that the ILK– PINCH–parvin complex can modulate the actin cytoskeleton at several levels. First, proteins such as the parvins can recruit F-actin either directly or indirectly through paxillin and vinculin. Secondly, the recruitment of Nck2 to PINCH can modulate actin polymerization through WASp and the Arp2/3 complex. Thirdly, Nck2 or parvin can bind GEFs such as DOCK180 or αPIX and effector proteins of small GTPases such as PAK and thereby regulate actin turnover. In this respect it is interesting to note that the expression of a dominant-negative Rac in MDCK epithelial cells leads to inverted deposition of F-actin (O’Brien et al., 2001). Instead of apical distribution, F-actin is often found at the basal site of the cells. This phenoype resembles the defects that we have observed in the ILK-null EBs where F-actin is present at the integrin–BM adhesion site (Sakai et al., 2003).
The availability of mutant mice and cells, GFP-tagged proteins that allow imaging of live cells ex vivo and in vivo, and specific antibodies for biochemistry should make it possible now to unravel the many remaining mysteries of the ILK–PINCH–parvin complex. In particular, they should enable us to determine whether the ILK– PINCH–parvin complex is ‘just’ an actin modulator that affects integrins indirectly, like each actin modulator would do, or whether it is indeed used by the integrins to transduce messages into and receive messages from within cells.
Concluding remarks
More than 20 molecules are currently described that interact directly with the intracellular domain of integrins. Many more are indirectly linked and participate in the formation of big multiprotein complexes that form around the integrins, linking them to the cytoskeleton and inducing cytoskeletal rearrangement. Such protein complexes allow a highly graduated response of the cell to external stimuli, which is crucial for cell polarity and migration. Furthermore, multiprotein complexes enable many levels of regulation with respect to complex composition, protein–protein interaction and activation status of individual components. Via such regulatory processes information from other signalling pathways can also be integrated. This high degree of regulation allows many cell-specific responses.
Acknowledgments
Acknowledgements
We thank Drs Rupert Timpl, Oliver Brandau, Takao Sakai, Randi Bordoy and Kathryn Rodgers for critically reading the manuscript. Our work was supported over the past years by the Swedish Research Foundation, Swedish Cancer Foundation, the Göran Gustafsson Foundation for Research in Natural Science and Medicine, the DFG, the Max Planck Society and the Fonds der Chemischen Industrie.
References
- Arthur W.T., Noren,N.K. and Burridge,K. (2002) Regulation of Rho family GTPases by cell–cell and cell–matrix adhesion. Biol. Res., 35, 239–246. [DOI] [PubMed] [Google Scholar]
- Aumailley M., Pesch,M., Tunggal,L., Gaill,F. and Fässler,R. (2000) Altered synthesis of laminin 1 and absence of basement membrane component deposition in β1 integrin-deficient embryoid bodies. J. Cell Sci., 113, 259–268. [DOI] [PubMed] [Google Scholar]
- Ballestrem C., Hinz,B., Imhof,B.A. and Wehrle-Haller,B. (2001) Marching at the front and dragging behind: differential αvβ3-integrin turnover regulates focal adhesion behavior. J. Cell Biol., 155, 1319–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs A.H., Byers,T.J., Knoll,J.H., Boyce,F.M., Bruns,G.A. and Kunkel,L.M. (1992) Cloning and characterization of two human skeletal muscle α-actinin genes located on chromosomes 1 and 11. J. Biol. Chem., 267, 9281–9288. [PubMed] [Google Scholar]
- Bellanger J.M., Astier,C., Sardet,C., Ohta,Y., Stossel,T.P. and Debant,A. (2000) The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat. Cell Biol., 2, 888–892. [DOI] [PubMed] [Google Scholar]
- Borowsky M.L. and Hynes,R.O. (1998) Layilin, a novel talin-binding transmembrane protein homologous with C-type lectins, is localized in membrane ruffles. J. Cell Biol., 143, 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard D. and Block,M.R. (1998) Calcium/calmodulin-dependent protein kinase II controls integrin α5β1-mediated cell adhesion through the integrin cytoplasmic domain associated protein-1α. Biochem. Biophys. Res. Commun., 252, 46–50. [DOI] [PubMed] [Google Scholar]
- Bouvard D., Vignoud,L., Dupe-Manet,S., Abed,N., Fournier,H.N., Vincent-Monegat,C., Retta,S.F., Fässler,R. and Block,M.R. (2003) Disruption of focal adhesions by integrin cytoplasmic domain associated protein-1α. J. Biol. Chem., 278, 6567–6574. [DOI] [PubMed] [Google Scholar]
- Brakebusch C., Bouvard,D., Stanchi,F., Sakai,T. and Fässler,R. (2002) Integrins in invasive growth. J. Clin. Invest., 109, 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A., Bordoy,R., Stanchi,F., Moser,M., Kostka,G., Ehler,E., Brandau,O. and Fässler,R. (2003) PINCH2 is a new 5 LIM domain protein, homologous to PINCH and localized to focal adhesions. Exp. Cell Res., 284, 237–248. [DOI] [PubMed] [Google Scholar]
- Brown N.H., Gregory,S.L., Rickoll,W. L, Fessler,L.I., Prout,M., White,R.A. and Fristrom,J.W. (2002) Talin is essential for integrin function in Drosophila. Dev. Cell, 3, 569–579. [DOI] [PubMed] [Google Scholar]
- Calderwood D.A., Huttenlocher,A., Kiosses,W.B., Rose,D.M., Woodside,D.G., Schwartz,M.A. and Ginsberg,M.H. (2001) Increased filamin binding to β-integrin cytoplasmic domains inhibits cell migration. Nat. Cell Biol., 3, 1060–1068. [DOI] [PubMed] [Google Scholar]
- Chang D.D., Wong,C., Smith,H. and Liu,J. (1997) ICAP-1, a novel β1 integrin cytoplasmic domain-associated protein, binds to a conserved and functionally important NPXY sequence motif of β1 integrin. J. Cell Biol., 138, 1149–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.C., Appeddu,P.A., Parsons,J.T., Hildebrand,J.D., Schaller,M.D. and Guan,J.L. (1995) Interaction of focal adhesion kinase with cytoskeletal protein talin. J. Biol. Chem., 270, 16995–16999. [DOI] [PubMed] [Google Scholar]
- Chen L.M., Bailey,D. and Fernandez-Valle,C. (2000) Association of β1 integrin with focal adhesion kinase and paxillin in differentiating Schwann cells. J. Neurosci., 20, 3776–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christerson L.B., Vanderbilt,C.A. and Cobb,M.H. (1999) MEKK1 interacts with α-actinin and localizes to stress fibers and focal adhesions. Cell Motil. Cytoskeleton, 43, 186–198. [DOI] [PubMed] [Google Scholar]
- Cohen P. and Frame S. (2001) The renaissance of GSK3. Nat. Rev. Mol. Cell Biol., 2, 769–776. [DOI] [PubMed] [Google Scholar]
- Crawford A.W. and Beckerle,M.C. (1991) Purification and characterization of zyxin, an 82,000-dalton component of adherens junctions. J. Biol. Chem., 266, 5847–5853. [PubMed] [Google Scholar]
- Crespo P., Schuebel,K.E., Ostrom,A.A., Gutkind,J.S. and Bustelo,X.R. (1997) Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature, 385, 169–172. [DOI] [PubMed] [Google Scholar]
- Das B., Shu,X., Day,G.J., Han,J., Krishna,U.M., Falck,J.R. and Broek,D. (2000) Control of intramolecular interactions between the pleckstrin homology and Dbl homology domains of Vav and Sos1 regulates Rac binding. J. Biol. Chem., 275, 15074–15081. [DOI] [PubMed] [Google Scholar]
- Degani S., Balzac,F., Brancaccio,M., Guazzone,S., Retta,S.F., Silengo,L., Eva,A. and Tarone,G. (2002) The integrin cytoplasmic domain-associated protein ICAP-1 binds and regulates Rho family GTPases during cell spreading. J. Cell Biol., 156, 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo M.A., Kiosses,W.B., Alderson,N.B., Meller,N., Hahn,K.M. and Schwartz,M.A. (2002) Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol., 4, 232–239. [DOI] [PubMed] [Google Scholar]
- Delcommenne M., Tan,C., Gray,V., Rue,L., Woodgett,J. and Dedhar,S. (1998) Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl Acad. Sci. USA, 95, 11211–11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali K.A., Barlow,C.A. and Burridge,K. (2002) Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J. Cell Biol., 159, 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G., Pellegrini,L., Letinic,K., Cestra,G., Zoncu,R., Voronov,S., Chang,S., Guo,J., Wenk,M.R. and De Camilli,P. (2002) Recruitment and regulation of phosphatidylinositol phosphate kinase type 1γ by the FERM domain of talin. Nature, 420, 85–89. [DOI] [PubMed] [Google Scholar]
- Eksioglu Y.Z. et al. (1996) Periventricular heterotopia: an X-linked dominant epilepsy locus causing aberrant cerebral cortical development. Neuron, 16, 77–87. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. and Hall,A. (2001) Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCζ. Cell, 106, 489–498. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. and Hall,A. (2002) Rho GTPases in cell biology. Nature, 420, 629–635. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. and Hall,A. (2003) Cdc42 regulates GSK-3β and adenomatous polyposis coli to control cell polarity. Nature, 421, 753–756. [DOI] [PubMed] [Google Scholar]
- Fisher P.R., Noegel,A.A., Fechheimer,M., Rivero,F., Prassler,J. and Gerisch,G. (1997) Photosensory and thermosensory responses in Dictyostelium slugs are specifically impaired by absence of the F-actin cross-linking gelation factor (ABP-120). Curr. Biol., 7, 889–892. [DOI] [PubMed] [Google Scholar]
- Fox J.W. et al. (1998) Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron, 21, 1315–1325. [DOI] [PubMed] [Google Scholar]
- Fukami K., Endo,T., Imamura,M. and Takenawa,T. (1994) α-actinin and vinculin are PIP2-binding proteins involved in signaling by tyrosine kinase. J. Biol. Chem., 269, 1518–1522. [PubMed] [Google Scholar]
- Fyrberg C., Ketchum,A., Ball,E. and Fyrberg,E. (1998) Characterization of lethal Drosophila melanogaster α-actinin mutants. Biochem. Genet., 36, 299–310. [DOI] [PubMed] [Google Scholar]
- Garcia-Alvarez B., de Pereda,J.M., Calderwood,D.A., Ulmer,T.S., Critchley,D., Campbell,I.D., Ginsberg,M.H. and Liddington,R.C. (2003) Structural determinants of integrin recognition by talin. Mol. Cell., 11, 49–58. [DOI] [PubMed] [Google Scholar]
- Gilmore A.P. and Burridge K. (1996) Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4-5-bisphosphate. Nature, 381, 531–535. [DOI] [PubMed] [Google Scholar]
- Grashoff C., Aszódi,A., Sakai,T., Hunziker,E.B. and Fässler,R. (2003) Integrin-linked kinase (ILK) regulates chondrocyte shape and proliferation. EMBO Rep., 4, 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood J.A., Theibert,A.B., Prestwich,G.D. and Murphy-Ullrich J.E. (2000) Restructuring of focal adhesion plaques by PI 3-kinase. Regulation by PtdIns (3,4,5)-p(3) binding to α-actinin. J. Cell Biol., 150, 627–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose R., Hutter,C., Bloch,W., Thorey,I., Watt,F.M., Fässler,R., Brakebusch,C. and Werner,S. (2002) A crucial role of β1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development, 129, 2303–2315. [DOI] [PubMed] [Google Scholar]
- Han D.C. and Guan,J.L. (1999) Association of focal adhesion kinase with Grb7 and its role in cell migration. J. Biol. Chem., 274, 24425–24430. [DOI] [PubMed] [Google Scholar]
- Han J., Das,B., Wei,W., Van Aelst,L., Mosteller,R.D., Khosravi-Far,R., Westwick,J.K., Der,C.J. and Broek,D. (1997) Lck regulates Vav activation of members of the Rho family of GTPases. Mol. Cell. Biol., 17, 1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan G.E., Leung-Hagesteijn,C., Fitz-Gibbon,L., Coppolino,M.G., Radeva,G., Filmus,J., Bell,J.C. and Dedhar,S. (1996) Regulation of cell adhesion and anchorage-dependent growth by a new β1-integrin-linked protein kinase. Nature, 379, 91–96. [DOI] [PubMed] [Google Scholar]
- Hemmings L., Rees,D.J., Ohanian,V., Bolton,S.J., Gilmore,A.P., Patel,B., Priddle,H., Trevithick,J.E., Hynes,R.O. and Critchley,D.R. (1996) Talin contains three actin-binding sites each of which is adjacent to a vinculin-binding site. J. Cell Sci., 109, 2715–2726. [DOI] [PubMed] [Google Scholar]
- Hildebrand J.D., Schaller,M.D. and Parsons,J.T. (1995) Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol. Biol. Cell., 6, 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M., Feng,J. and Hemmings,B. (2002) Identification of a plasma membrane Raft-associated PKB Ser473 kinase activity that is distinct from ILK and PDK1. Curr. Biol., 12, 1251–1255. [DOI] [PubMed] [Google Scholar]
- Honda K., Yamada,T., Endo,R., Ino,Y., Gotoh,M., Tsuda,H., Yamada,Y., Chiba,H. and Hirohashi,S. (1998) Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J. Cell Biol., 140, 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A., Duggan,K., Buck,C., Beckerle,M.C. and Burridge,K. (1986) Interaction of plasma membrane fibronectin receptor with talin—a transmembrane linkage. Nature, 320, 531–533. [DOI] [PubMed] [Google Scholar]
- Hüttelmaier S., Harbeck,B., Steffens,O., Messerschmidt,T., Illenberger,S. and Jockusch,B.M. (1999) Characterization of the actin binding properties of the vasodilator-stimulated phosphoprotein VASP. FEBS Lett., 451, 68–74. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell, 110, 673–687. [DOI] [PubMed] [Google Scholar]
- Izaguirre G., Aguirre,L., Hu,Y.P., Lee,H.Y., Schlaepfer,D.D., Aneskievich,B.J. and Haimovich,B. (2001) The cytoskeletal/non-muscle isoform of α-actinin is phosphorylated on its actin-binding domain by the focal adhesion kinase. J. Biol. Chem., 276, 28676–28685. [DOI] [PubMed] [Google Scholar]
- Kiyokawa E., Hashimoto,Y., Kobayashi,S., Sugimura,H., Kurata,T. and Matsuda,M. (1998) Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev., 12, 3331–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukaitis C.M., Webb,D.J., Donais,K. and Horwitz,A.F. (2001) Differential dynamics of α5 integrin, paxillin and α-actinin during formation and disassembly of adhesions in migrating cells. J. Cell Biol., 153, 1427–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor M.A. and Alessi,D.R. (2001) PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci., 114, 2903–2910. [DOI] [PubMed] [Google Scholar]
- Leedman P.J., Faulkner-Jones,B., Cram,D.S., Harrison,P.J., West,J., O’Brien,E., Simpson,R., Coppel,R.L. and Harrison,L.C. (1993) Cloning from the thyroid of a protein related to actin binding protein that is recognized by Graves disease immunoglobulins. Proc. Natl Acad. Sci. USA, 90, 5994–5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinweber B.D., Leavis,P.C., Grabarek,Z., Wang,C.L. and Morgan,K.G. (1999) Extracellular regulated kinase (ERK) interaction with actin and the calponin homology (CH) domain of actin-binding proteins. Biochem. J., 344, 117–123. [PMC free article] [PubMed] [Google Scholar]
- Leptin M., Bogaert,T., Lehmann,R. and Wilcox,M. (1989) The function of PS integrins during Drosophila embryogenesis. Cell, 56, 401–408. [DOI] [PubMed] [Google Scholar]
- Li M.G., Serr,M., Edwards,K., Ludmann,S., Yamamoto,D., Tilney,L.G., Field,C.M. and Hays,T.S. (1999) Filamin is required for ring canal assembly and actin organization during Drosophila oogenesis. J. Cell Biol., 146, 1061–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Carbonetto,S., Fässler,R., Smyth,N., Edgar,D. and Yurchenco,P.D. (2002) Matrix assembly, regulation and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol., 157, 1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Edgar,D., Fässler,R. and Yurchenco,P.D. (2003) Basement membrane laminin assembly and the induction of cell polarity, apoptosis and cavitation. Dev. Cell, in press. [Google Scholar]
- Ling K., Doughman,R.L., Firestone,A.J., Bunce,M.W. and Anderson,R.A. (2002) Type Iγ phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature, 420, 89–93. [DOI] [PubMed] [Google Scholar]
- Loo D.T., Kanner,S.B. and Aruffo,A. (1998) Filamin binds to the cytoplasmic domain of the β1-integrin. Identification of amino acids responsible for this interaction. J. Biol. Chem., 273, 23304–23312. [DOI] [PubMed] [Google Scholar]
- Mackinnon A.C., Qadota,H., Norman,K.R., Moerman,D.G. and Williams,B.D. (2002) C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr. Biol., 12, 787–797. [DOI] [PubMed] [Google Scholar]
- Martel V., Racaud-Sultan,C., Dupe,S., Marie,C., Paulhe,F., Galmiche,A., Block,M.R. and Albiges-Rizo,C. (2001) Conformation, localization and integrin binding of talin depend on its interaction with phosphoinositides. J. Biol. Chem., 276, 21217–21227. [DOI] [PubMed] [Google Scholar]
- Marti A., Luo,Z., Cunningham,C., Ohta,Y., Hartwig,J., Stossel,T.P., Kyriakis,J.M. and Avruch,J. (1997) Actin-binding protein-280 binds the stress-activated protein kinase (SAPK) activator SEK-1 and is required for tumor necrosis factor-α activation of SAPK in melanoma cells. J. Biol. Chem., 272, 2620–2628. [DOI] [PubMed] [Google Scholar]
- Meyer S.C., Sanan,D.A. and Fox,J.E. (1998) Role of actin-binding protein in insertion of adhesion receptors into the membrane. J. Biol. Chem., 273, 3013–3020. [DOI] [PubMed] [Google Scholar]
- Monkley S.J., Zhou,X.H., Kinston,S.J., Giblett,S.M., Hemmings,L., Priddle,H., Brown,J.E., Pritchard,C.A., Critchley,D.R. and Fässler,R. (2000) Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev. Dyn., 219, 560–574. [DOI] [PubMed] [Google Scholar]
- Monkley S.J., Pritchard,C.A. and Critchley,D.R. (2001) Analysis of the mammalian talin2 gene TLN2. Biochem. Biophys. Res. Commun., 286, 880–885. [DOI] [PubMed] [Google Scholar]
- Mukai H., Toshimori,M., Shibata,H., Takanaga,H., Kitagawa,M., Miyahara,M., Shimakawa,M. and Ono,Y. (1997) Interaction of PKN with α-actinin. J. Biol. Chem., 272, 4740–4746. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos S.N. and Turner,C.E. (2000) Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J. Cell Biol., 151, 1435–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos S.N. and Turner,C.E. (2001) Integrin-linked kinase (ILK) binding to paxillin LD1 motif regulates ILK localization to focal adhesions. J. Biol. Chem., 276, 23499–23505. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos S.N. and Turner,C.E. (2002) Molecular dissection of actopaxin-integrin-linked kinase–paxillin interactions and their role in subcellular localization. J. Biol. Chem., 277, 1568–1575. [DOI] [PubMed] [Google Scholar]
- Novak A., Hsu,S.C., Leung-Hagesteijn,C., Radeva,G., Papkoff,J., Montesano,R., Roskelley,C., Grosschedl,R. and Dedhar,S. (1998) Cell adhesion and the integrin-linked kinase regulate the LEF-1 and β-catenin signaling pathways. Proc. Natl Acad. Sci. USA, 95, 4374–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien L.E., Jou,T.S., Pollack,A.L, Zhang,Q., Hansen,S.H., Yurchenco,P. and Mostov,K.E. (2001) Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol., 3, 831–838. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Suzuki,N., Nakamura,S., Hartwig,J.H. and Stossel,T.P. (1999) The small GTPase RalA targets filamin to induce filopodia. Proc. Natl Acad. Sci. USA 96, 2122–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olski T.M., Noegel,A.A. and Korenbaum,E. (2001) Parvin, a 42 kDa focal adhesion protein, related to the α-actinin superfamily. J. Cell Sci., 114, 525–538. [DOI] [PubMed] [Google Scholar]
- Pavalko F.M. and Burridge,K. (1991) Disruption of the actin cytoskeleton after microinjection of proteolytic fragments of α-actinin. J. Cell Biol., 114, 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad S., Attwell,S., Gray,V., Mawji,N., Deng,J.T., Leung,D., Yan,J., Sanghera,J., Walsh,M.P. and Dedhar,S. (2001) Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J. Biol. Chem., 276, 27462–27469. [DOI] [PubMed] [Google Scholar]
- Peutz-Kootstra C.J., Hansen,K., De Heer,E., Abrass,C.K. and Bruijn,J.A. (2000) Differential expression of laminin chains and anti-laminin autoantibodies in experimental lupus nephritis. J. Pathol., 192, 404–412. [DOI] [PubMed] [Google Scholar]
- Priddle H., Hemmings,L., Monkley,S., Woods,A., Patel,B., Sutton,D., Dunn,G.A., Zicha,D. and Critchley,D.R. (1998) Disruption of the talin gene compromises focal adhesion assembly in undifferentiated but not differentiated embryonic stem cells. J. Cell Biol., 142, 1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajfur Z., Roy,P., Otey,C., Romer,L. and Jacobson,K. (2002) Dissecting the link between stress fibres and focal adhesions by CALI with EGFP fusion proteins. Nat. Cell Biol., 4, 286–293. [DOI] [PubMed] [Google Scholar]
- Reiske H.R., Kao,S.C., Cary,L.A., Guan,J.L., Lai,J.F. and Chen,H.C. (1999) Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J. Biol. Chem., 274, 12361–12366. [DOI] [PubMed] [Google Scholar]
- Rivero F., Furukawa,R., Fechheimer,M. and Noegel,A.A. (1999) Three actin cross-linking proteins, the 34 kDa actin-bundling protein, α-actinin and gelation factor (ABP-120), have both unique and redundant roles in the growth and development of Dictyostelium. J. Cell Sci., 112, 2737–2751. [DOI] [PubMed] [Google Scholar]
- Robertson S.P. et al. (2003) Localized mutations in the gene encoding the cytoskeletal protein filamin A cause diverse malformations in humans. Nat. Genet., 33, 487–491. [DOI] [PubMed] [Google Scholar]
- Roote C.E. and Zusman,S. (1995) Functions for PS integrins in tissue adhesion, migration and shape changes during early embryonic development in Drosophila. Dev. Biol., 169, 322–336. [DOI] [PubMed] [Google Scholar]
- Rosenberger G., Jantke,I., Galand,A. and Kutsche,K. (2003) Interaction of αPIX (ARHGEF6) with β-parvin (PARVB) suggests an involvement of αPIX in integrin-mediated signaling. Hum. Mol. Genet., 12, 155–167. [DOI] [PubMed] [Google Scholar]
- Sakai T., Li,S., Docheva,D., Grashoff,C., Sakai,K., Braun,A., Kostka,K., Pfeifer,A., Yurchenco,P.D. and Fässler,R. (2003) Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion and controlling actin accumulation. Genes Dev., 17, 926–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M.D. (2001) Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta, 1540, 1–21. [DOI] [PubMed] [Google Scholar]
- Schaller M.D., Otey,C.A., Hildebrand,J.D. and Parsons,J.T. (1995) Focal adhesion kinase and paxillin bind to peptides mimicking β integrin cytoplasmic domains. J. Cell Biol., 130, 1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer D.D. and Hunter,T. (1997) Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J. Biol. Chem., 272, 13189–13195. [DOI] [PubMed] [Google Scholar]
- Schöck F. and Perrimon,N. (2003) Retraction of the Drosophila germ band requires cell-matrix interaction. Genes Dev., 17, 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C.P., Ezzell,R.M. and Arnaout,M.A. (1995) Direct interaction of filamin (ABP-280) with the β2-integrin subunit CD18. J. Immunol., 154, 3461–3470. [PubMed] [Google Scholar]
- Shen Y. and Schaller,M.D. (1999) Focal adhesion targeting: the critical determinant of FAK regulation and substrate phosphorylation. Mol. Biol. Cell, 10, 2507–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki F., Fukami,K., Fukui,Y. and Takenawa,T. (1994) Phosphatidylinositol 3-kinase binds to α-actinin through the p85 subunit. Biochem. J., 302, 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol N.S. and Cooley,L. (1999) Drosophila filamin encoded by the cheerio locus is a component of ovarian ring canals. Curr. Biol., 9, 1221–1230. [DOI] [PubMed] [Google Scholar]
- Steimle P.A., Hoffert,J.D., Adey,N.B. and Craig,S.W. (1999) Polyphosphoinositides inhibit the interaction of vinculin with actin filaments. J. Biol. Chem., 274, 18414–18420. [DOI] [PubMed] [Google Scholar]
- Stossel T.P., Condeelis,J., Cooley,L., Hartwig,J.H., Noegel,A., Schleicher,M. and Shapiro,S.S. (2001) Filamins as integrators of cell mechanics and signaling. Nat. Rev. Mol. Cell Biol., 2, 138–145. [DOI] [PubMed] [Google Scholar]
- Tahiliani P.D., Singh,L., Auer,K.L. and LaFlamme,S.E. (1997) The role of conserved amino acid motifs within the integrin β3 cytoplasmic domain in triggering focal adhesion kinase phosphorylation. J. Biol. Chem., 272, 7892–7898. [DOI] [PubMed] [Google Scholar]
- Teramoto H., Salem,P., Robbins,K.C., Bustelo,X.R. and Gutkind,J.S. (1997) Tyrosine phosphorylation of the vav proto-oncogene product links FcεRI to the Rac1-JNK pathway. J. Biol. Chem., 272, 10751–10755. [DOI] [PubMed] [Google Scholar]
- Tu Y., Kucik,D.F. and Wu,C. (2001a) Identification and kinetic analysis of the interaction between Nck-2 and DOCK180. FEBS Lett., 491, 249–256. [DOI] [PubMed] [Google Scholar]
- Tu Y., Huang,Y., Zhang,Y., Hua,Y. and Wu,C. (2001b) A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J. Cell Biol., 153, 585–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C.E., Brown,M.C., Perrotta,J.A., Riedy,M.C., Nikolopoulos,S.N., McDonald,A.R., Bagrodia,S., Thomas,S. and Leventhal,P. S (1999) Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J. Cell Biol., 145, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi R.K., Li,F., Adam,L., Nguyen,D., Ohta,Y., Stossel,T.P. and Kumar,R. (2002) Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat. Cell Biol., 4, 681–690. [DOI] [PubMed] [Google Scholar]
- van der Flier A. and Sonnenberg,A. (2001) Structural and functional aspects of filamins. Biochim. Biophys. Acta, 1538, 99–117. [DOI] [PubMed] [Google Scholar]
- Vinogradova O., Velyvis,A., Velyviene,A., Hu,B., Haas,T., Plow,E. and Qin,J. (2002) A structural mechanism of integrin αIIbβ3 ‘inside-out’ activation as regulated by its cytoplasmic face. Cell, 110, 587–597. [DOI] [PubMed] [Google Scholar]
- Wachsstock D.H., Wilkins,J.A. and Lin,S. (1987) Specific interaction of vinculin with α-actinin. Biochem. Biophys. Res. Commun., 146, 554–556. [DOI] [PubMed] [Google Scholar]
- White D.E., Cardiff,R.D., Dedhar,S. and Muller,W.J. (2001) Mammary epithelial-specific expression of the integrin-linked kinase (ILK) results in the induction of mammary gland hyperplasias and tumors in transgenic mice. Oncogene, 20, 7064–7072. [DOI] [PubMed] [Google Scholar]
- Witke W., Schleicher,M. and Noegel,A.A. (1992) Redundancy in the microfilament system: abnormal development of Dictyostelium cells lacking two F-actin cross-linking proteins. Cell, 68, 53–62. [DOI] [PubMed] [Google Scholar]
- Witke W., Hofmann,A., Koppel,B., Schleicher,M. and Noegel,A.A. (1993) The Ca(2+)-binding domains in non-muscle type α-actinin: biochemical and genetic analysis. J. Cell Biol., 121, 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. and Dedhar,S. (2001) Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J. Cell Biol., 155, 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji S., Suzuki,A., Sugiyama,Y., Koide,Y., Yoshida,M., Kanamori,H., Mohri,H., Ohno,S. and Ishigatsubo,Y. (2001) A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell–substrate interaction. J. Cell Biol., 153, 1251–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Sato,T. and Sugita,H. (1987) Antifilamin, antivinculin and antitropomyosin antibodies in myasthenia gravis. Neurology, 37, 1329–1333. [DOI] [PubMed] [Google Scholar]
- Yan B., Calderwood,D.A., Yaspan,B. and Ginsberg,M.H. (2001) Calpain cleavage promotes talin binding to the β3 integrin cytoplasmic domain. J. Biol. Chem., 276, 28164–28170. [DOI] [PubMed] [Google Scholar]
- Zervas C.G., Gregory,S.L. and Brown,N.H. (2001) Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J. Cell Biol., 152, 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.A. and Hemler,M.E. (1999) Interaction of the integrin β1 cytoplasmic domain with ICAP-1 protein. J. Biol. Chem., 274, 11–19. [DOI] [PubMed] [Google Scholar]
- Zhang X., Chattopadhyay,A., Ji,Q.S., Owen,J.D., Ruest,P.J., Carpenter,G. and Hanks,S.K. (1999) Focal adhesion kinase promotes phospholipase C-γ1 activity. Proc. Natl Acad. Sci. USA, 96, 9021–9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen,K., Guo,L. and Wu C. (2002a) Characterization of PINCH-2, a new focal adhesion protein that regulates the PINCH-1–ILK interaction, cell spreading and migration. J. Biol. Chem., 277, 38328–38338. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen,K., Tu,Y., Velyvis,A., Yang,Y., Qin,J. and Wu,C. (2002b) Assembly of the PINCH–ILK–CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J. Cell Sci., 115, 4777–4786. [DOI] [PubMed] [Google Scholar]
- Zhao Z.S., Manser,E., Loo,T.H. and Lim,L. (2000) Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol. Cell. Biol., 20, 6354–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]