Abstract
Seasonal disease dynamics are common in nature, but their causes are often unknown. Our case study provides insight into the cyclic prevalence pattern of the horizontally and vertically transmitted microsporidium Octosporea bayeri in its Daphnia magna host. Data from several populations over a four year period revealed a regular prevalence increase during summer and a decrease over winter when hosts underwent diapause. Prevalence also decreased after summer diapause indicating that the decline is causally linked to diapause rather than to winter conditions. Experiments showed that host diapause itself can explain a certain proportion of the decline. The decline further depends on the environmental conditions during diapause: infected resting eggs suffered from higher mortality under experimental winter than under experimental summer diapause conditions. Investigating the mechanisms of prevalence increase after diapause, the parasite was found to survive winter outside its host, enabling horizontal infection of susceptible hosts in the following growing season. Allowing for horizontal transmission in experimental host populations resulted in a steep prevalence increase, while excluding it led to a pronounced decline. Thus, the apparent seasonality in O. bayeri prevalence is characterized by a decline during host diapause followed by horizontal spread of the parasite during the host's asexual growth phase.
Keywords: experimental epidemiology, Daphnia magna, diapause, Octosporea bayeri, transmission, seasonal dynamics
1. Introduction
Cyclic prevalence dynamics are commonly observed phenomena in a number of diseases, among which measles may be one of the best known examples (Bartlett 1957; Anderson & May 1991). Such dynamics can be driven by external (environmental) factors and/or result from processes that are intrinsic to the host–parasite system under study (May & Anderson 1979; Grenfell & Bjornstad 2005). Among the external factors are climatic conditions (Kelly et al. 2002), food conditions (Yan & Larsson 1988) and host behaviour (Hosseini et al. 2004), whereas intrinsic factors result from the dynamic feedback between host and parasite populations and include host immunity (Hosseini et al. 2004; Grassly et al. 2005), parasite virulence (Ebert et al. 2000) and parasite transmission modes (Lipsitch et al. 1995). Genetic interactions between host and parasite, causing for example differential susceptibility of different host genotypes (e.g. Carius et al. 2001) or differential effects of immune response on different parasite genotypes, may further drive cyclic disease dynamics.
Prevalence often varies on a seasonal basis. In plankton, seasonality of parasite occurrence has been observed in numerous systems (Canter & Lund 1948; Green 1974; Miracle 1977; Ruttner-Kolisko 1977; Brambilla 1983; Yan & Larsson 1988) and seems the rule rather than the exception. Several of these parasite dynamics correlate with patterns in temperature and thus, temperature is often considered to play a major role in creating seasonality (Miracle 1977; Ruttner-Kolisko 1977). This could be caused by temperature effects on parasite transmission, which can be impaired at low temperatures in horizontally (Ebert 1995) and vertically transmitted parasites (Kelly et al. 2002). However, seasonal variation in disease may correlate with but not be caused by climatic conditions (Anderson & May 1991). Other factors such as host size, host nutritional status and host population density underlie seasonal variation and have been proposed to drive prevalence dynamics (Ruttner-Kolisko 1977; Yan & Larsson 1988). Yet the underlying mechanisms are not resolved for any of the cyclic prevalence patterns in planktonic host–parasite systems and no conclusions have been reached.
Most studies on prevalence cycles in plankton concentrate on observations in natural host populations (Miracle 1977; Ruttner-Kolisko 1977; Brambilla 1983; Yan & Larsson 1988). In the field, however, several factors may interact or depend on each other making it impossible to disentangle the effects of single factors on parasite dynamics. In a study of a microsporidian parasite in rotifer hosts, for example, prevalence increase correlated with a rise in temperature and a concurrent increase in host population density (Ruttner-Kolisko 1977). Thus, observational studies do not allow for a determination of the mechanisms of parasite dynamics. Experimental testing of separate factors is needed. Furthermore, most studies on parasite dynamics in natural plankton populations are limited to investigations of one or two populations (Miracle 1977; Ruttner-Kolisko 1977; Brambilla 1983; Yan & Larsson 1988), and thus the generality of the observed patterns remains unknown.
The prevalence pattern in the microsporidium Octosporea bayeri Jírovec 1936 in populations of its host Daphnia magna Straus 1820 provides a case study of cyclic prevalence patterns suitable to elucidate the underlying mechanisms. Preliminary observations indicated pronounced seasonality in parasite prevalence and the host–parasite system allows both observational investigation in natural populations and experimental manipulation. Octosporea is by far the most abundant parasite in a D. magna metapopulation in the Tvärminne archipelago in Southern Finland and is present in 44.9% of all host populations, whereas none of the other seven endoparasites identified reached an abundance of more than 8% (Ebert et al. 2001). This high abundance of Octosporea makes the simultaneous investigation of parasite dynamics in numerous host populations possible. The parasite utilizes a combination of horizontal and vertical transmission (Vizoso et al. 2005) and can maintain 100% prevalence in laboratory populations of its host for several years (D. B. Vizoso and S. Lass, unpublished data). Given the efficient combination of horizontal and vertical transmission in this parasite, its cyclic prevalence pattern, especially the recurring decrease in prevalence, as observed in natural host populations, is particularly puzzling. Here, we set out to understand parasite seasonality by investigating the role of both external and intrinsic factors in driving prevalence dynamics in the microsporidium O. bayeri.
2. Material and methods
(a) Host–parasite system
The host D. magna is a planktonic freshwater microcrustacean that reproduces by cyclical parthenogenesis. Under favourable conditions, reproduction is clonal and females produce genetically identical offspring. When environmental conditions deteriorate males are produced and sexual reproduction takes place. Sexual reproduction results in the formation of resting eggs enclosed by a so-called ephippial case (part of the mother's carapace). Together, resting eggs and ephippial case form an ephippium and can outlast unfavourable conditions, such as frost and drought. When environmental conditions once more become favourable females hatch from these resting eggs and start clonal reproduction.
The parasite O. bayeri is a microsporidium specific to D. magna, causing chronic infections. O. bayeri has a direct life-cycle (transmitting from infected Daphnia to susceptible Daphnia hosts) and transmits horizontally (via spores from dead decaying hosts) and vertically (from mothers to their offspring; Vizoso et al. 2005). The parasite transmits to asexual eggs with an estimated efficiency of 100% whereas vertical transmission to sexual, resting eggs is less efficient (Vizoso et al. 2005).
The metapopulation, in which we study dynamics of O. bayeri, consists of D. magna populations inhabiting rainwater-filled depressions in the rock of several islands of the Tvärminne archipelago along the southern coast of Finland (Ebert et al. 2001). There, D. magna typically hatch from resting eggs in spring (April–May), reproduce asexually and sexually during summer and autumn (June–October) and undergo diapause during winter. Small rock pools may fall dry during summer, forcing their D. magna populations to undergo an additional summer diapause.
(b) Prevalence estimation
Infection with O. bayeri can be assessed via detection of the typical spores, which become microscopically detectable (phase contrast, 400× magnification) about 8–12 days after infection (Vizoso & Ebert 2004). Therefore, we kept samples alive for 10–14 days under laboratory conditions (in artificial medium (Klüttgen et al. 1994; modified after Ebert et al. (1998), without adding any well water), regular food supply, constant temperature) to ensure sufficient spore development. To prevent new infections we removed dead hosts from populations daily (in field observations and field experiment) or kept each host in a separate vial (in laboratory experiments). Wet-mount preparations of all Daphnia were checked for parasite presence.
(c) Field observation 1: prevalence in natural host populations
Prevalence of O. bayeri in field populations of D. magna was studied early (April/May) and late (August/September) in the seasons from 2001–2005. Seven of these populations were chosen arbitrarily. In order to investigate prevalence dynamics in recently infected host populations, we included a further four populations, which were parasite-free in 2001 and early 2002 but were invaded by O. bayeri during the summer of 2002. To include populations harbouring old infections, we studied seven persistent populations in which O. bayeri was known to occur in 1998 (see Ebert et al. 2001) and was likely to have been there ever since.
Daphnia population samples were brought to the laboratory and prevalence was determined in approximately 50 randomly chosen Daphnia from each population. In order to test if prevalence changed during the season, we compared prevalence in spring versus summer for each year (paired t-tests). In these tests we did not include those populations that had fallen dry in this season (see §2d). To test if prevalences change across winter, we compared summer samples with spring samples of the following year.
(d) Field observation 2: parasite loss during summer diapause
During the observation of prevalence in field populations some of the rock pools dried up in summer 2002, 2003 and/or 2005 forcing the Daphnia populations into summer diapause before rain filled the pools again. Prevalence was estimated in these rock pools before and after the summer drought, using a paired t-test, pairing the last prevalence before (within seven weeks) and after (within eight weeks) the drought.
(e) Field experiment: parasite loss during winter diapause
In August 2003, we started six new Daphnia populations in previously unoccupied natural rock pools. We chose well-spaced pools to avoid cross contamination and introduced different monoclonal populations of D. magna, each of them with 100% O. bayeri prevalence. After the following winter, in May 2004, we sampled hatchlings from each population and assessed prevalence in an average number of 133 Daphnia per pool.
(f) Laboratory experiment 1: effect of winter and summer diapause
In order to investigate the effect of environmental conditions during diapause on O. bayeri prevalence, we sampled sediments from two rock pools (A and B) on two islands during a summer drought in 2003. O. bayeri prevalence was >90% in both pools before they had naturally dried up. These sediment samples were split evenly among 20 plastic tubes for each population. Ten tubes with sediment from each pool were exposed to two different diapause conditions mimicking either summer diapause (dry, 16 h light: 8 h dark rhythm, eight weeks at 20 °C) or winter diapause (emerged in artificial medium, 24 h dark, cooled down to 4 °C for 3 days, kept frozen for six weeks at −1 °C, thereafter at −15 °C for one week and then thawed at 4 °C for 3 days).
To induce hatching, sediments of all tubes were placed in twenty 400 ml jars filled with artificial medium and exposed to 16 h light : 8 h dark at 20 °C. We checked for Daphnia hatchlings daily for 33 days. Hatchlings were counted and prevalence was assessed as described above.
Prevalence and number of hatchlings were analysed in two 2-way ANOVAs with pool as random and treatment as fixed factor. Prior to analysis, prevalence data were arcsine-transformed and number of hatchlings log-transformed to meet the requirements of normal distribution and homogeneity of variance. Since the interaction terms in both ANOVAs were not significant, we pooled the interaction sum of squares with the error sum of squares before testing the mean squares of the main factors (Sokal & Rohlf 1995).
(g) Laboratory experiment 2: infection from sediments
To test whether O. bayeri survive the winter outside its hosts, we sampled water and sediments from four populations known to harbour D. magna and O. bayeri towards the end of winter diapause in March 2004. At the time of sampling, all of the rock pools were covered with ice and snow and sediments of rock pools B and C were completely frozen, whereas the sediments of rock pools A and D were not frozen but contained highly saline water.
Sediment of each rock pool was sieved and rinsed with artificial medium to remove resting eggs. Sediment and supernatant each were split evenly over six 100 ml jars, which were then filled with artificial medium. In each of the six replicates, we placed five 3-day-old Daphnia from one clone, known to be susceptible to O. bayeri, and fed them regularly for 12 days. Thereafter, Daphnia were checked for O. bayeri infection.
(h) Laboratory experiment 3: role of horizontal transmission
To investigate the influence of horizontal transmission on the epidemiology of O. bayeri, we subjected experimental host populations to three different treatments: in the first treatment horizontal and vertical transmissions were possible, whereas in the second and third only vertical transmission was allowed. Horizontal transmission was made possible by leaving Daphnia after their death in the culture vessels, where they decomposed and released spores. To avoid horizontal transmission, dead Daphnia were removed daily. Previous investigations have shown that horizontal transmission does not take place within the first 2 days after host death (D. Ebert unpublished data). To take the removal of dead hosts into account, we replaced the removed D. magna with uninfected, smashed D. magna in one of the vertical-transmission-only treatments.
The experiment ran with two different starting prevalences: 5% and 50%. We chose one D. magna clone known to be susceptible to O. bayeri infection (the same clone as in laboratory experiment 2). Populations were started by mixing Daphnia from uninfected and infected cultures, the latter infected with O. bayeri isolates from different Finnish rock pools. There were no dead Daphnia at the start in the vessels. Each population was started with 40 Daphnia of mixed age in a 400 ml jar. Populations were kept at 20 °C and 16L : 8D. They were fed chemostat-grown Scenedesmus obliquus at a concentration of 30×106 cells per jar and the positions of the jars were changed daily. Adding fresh medium once a week compensated for medium evaporation. From all three treatments we sampled destructively (all Daphnia) replicates after three, six and nine weeks and assessed prevalence. In total, the experiment included 3 (treatments)×2 (starting prevalence)×3 (time points)×5 (replicates)=90 populations.
To test if prevalence changed in the treatments with and without horizontal transmission, we compared starting prevalence with prevalence after nine weeks (two paired t-tests).
3. Results
(a) Field observation 1: prevalence in natural host populations
During our study period, O. bayeri prevalence in D. magna populations was significantly higher in autumn than in spring (table 1, figure 1). This pattern was observed in recently infected host populations (figure 1b) and in populations that had been infected for several years (figure 1c). In contrast, prevalence decreased significantly during winter diapause (table 1, figure 1).
Table 1.
Results of paired t-tests comparing prevalence of the microsporidium Octosporea bayeri in natural populations of its host Daphnia magna in rock pools on islands of the Tvärminne archipelago, Southern Finland. (n, number of host populations; columns in the middle indicate mean prevalence change in %.)
| n | mean difference | t | p | |
|---|---|---|---|---|
| summer: prevalences in spring and summer were compared from 2001–2004 | ||||
| 2001 | 4 | 75.8 | 10.092 | 0.001 |
| 2002 | 7 | 62.6 | 7.934 | <0.001 |
| 2003 | 16 | 52.4 | 8.315 | <0.001 |
| 2004 | 17 | 31.9 | 6.301 | <0.001 |
| winter: prevalences before and after winter diapause were compared from 2001–2005 | ||||
| 2001/2002 | 4 | −53.3 | −3.715 | 0.017 |
| 2002/2003 | 11 | −44.4 | −5.356 | <0.001 |
| 2003/2004 | 18 | −17.9 | −3.007 | 0.004 |
| 2004/2005 | 14 | −33.3 | −5.385 | <0.001 |
Figure 1.
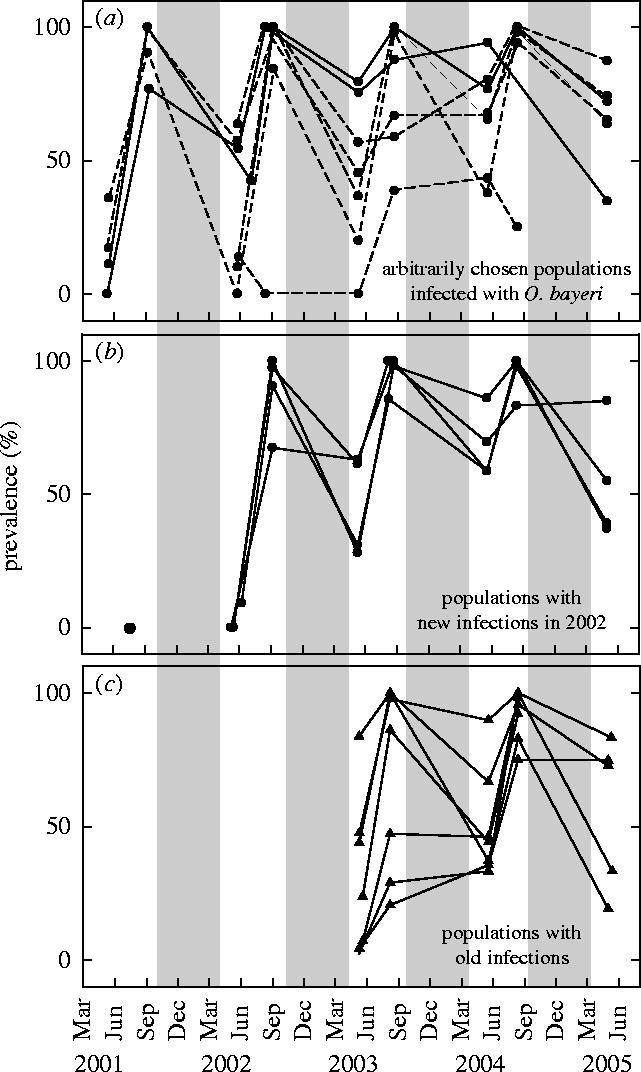
Prevalence of the microsporidium Octosporea bayeri in its host Daphnia magna in rock pools on islands of the Tvärminne archipelago, Southern Finland. Prevalences were estimated early and late in the season. Grey shading indicates winter diapause. (a) Prevalence in arbitrarily chosen host populations, some of which dried up during the study period, thereby experiencing a summer diapause (dashed lines). (b) Prevalence in recently infected host populations (uninfected in 2001). (c) Prevalence in host populations with infections at least since 1998.
(b) Field observation 2: parasite loss during summer diapause
Prevalence could be studied before and after six summer drought events. Prevalence estimates before drought were significantly higher than prevalence of hatchlings after drought (d.f.=5, t=−2.759, p=0.019; figure 2).
Figure 2.
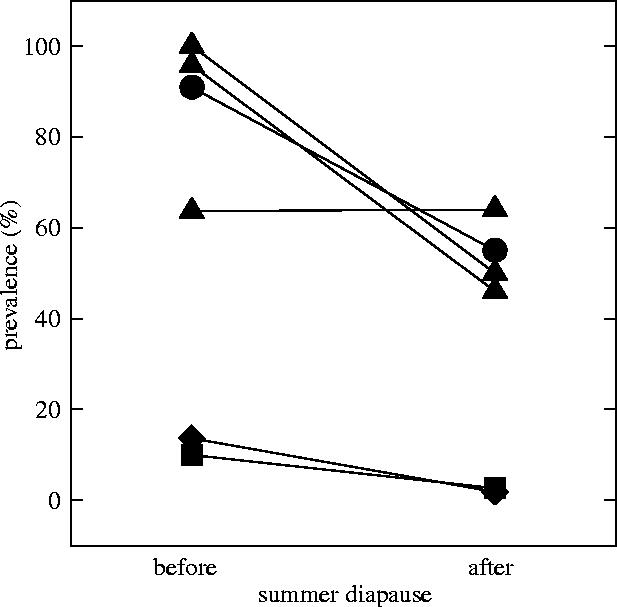
Prevalence of the microsporidium Octosporea bayeri in Daphnia magna in rock pools on islands of the Tvärminne archipelago, Southern Finland. Prevalence was estimated before and after the pools dried up in the summers of 2002, 2003 and 2005 thereby forcing D. magna into summer diapause. Different symbols indicate different host populations (circles indicate pool A and triangles pool B of laboratory experiment 2). One of the populations (pool B) dried up three times during the study period.
(c) Field experiment: parasite loss during winter diapause
In May 2004, five of the six experimental populations which were started in summer 2003 harboured a D. magna population. On average, prevalence had decreased from 100 to 94.4±1.8% (mean±95% CI).
(d) Laboratory experiment 1: effect of winter and summer diapause
In the field as well as in the laboratory, prevalences were much lower after the summer drought than before (figure 3a). Among the two diapause treatments, prevalences of hatchlings were significantly lower after the resting eggs had been exposed to winter conditions compared to those that experienced summer conditions (table 2, figure 3a). There was also a significant pool effect (table 2).
Figure 3.
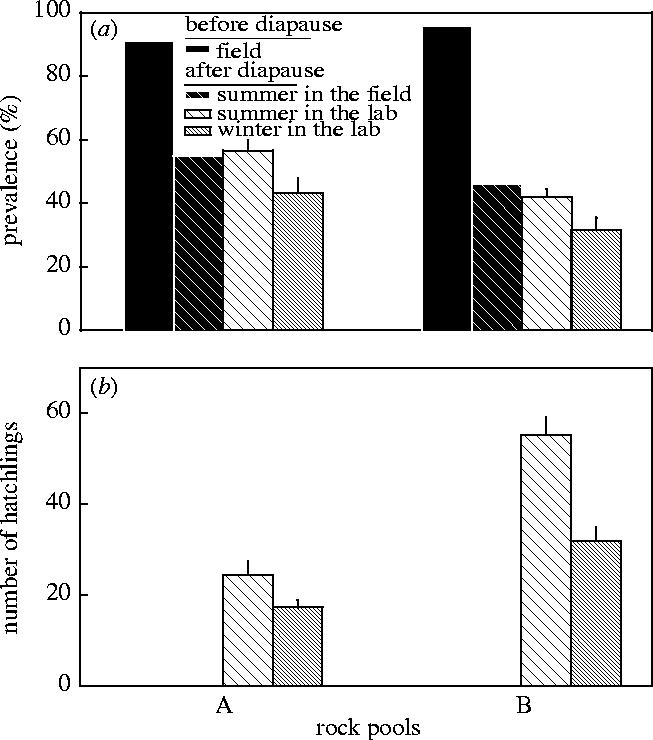
Results of laboratory experiment 1 investigating the effect of different diapause conditions on resting eggs from two rock pool populations. (a) Prevalence of the microsporidium Octosporea bayeri in Daphnia magna populations before and after summer drought and in hatchlings hatching from sediments that were sampled during the drought and exposed to two different diapause conditions in the laboratory. Conditions mimicked either summer diapause (warm, dry and light exposed) or winter diapause (cold, wet and dark). (b) Number of hatchlings from sediments from the two rock pools after exposure to different diapause conditions. Error bars represent +s.e.
Table 2.
Results of two 2-way ANOVA on prevalence and number of hatchlings in laboratory experiment 1. (Sediment from two different pools was exposed to either summer or winter diapause conditions (treatment) prior to hatching. Treatment was included as fixed factor and pool as random factor. Prior to analysis prevalence data were arcsine-transformed and number of hatchlings log-transformed. The interaction terms in both ANOVAs were not significant, so the interaction sums of squares were pooled with the error sums of squares before testing the mean squares of the main factors.)
| source | d.f. | prevalence | number of hatchlings | ||||
|---|---|---|---|---|---|---|---|
| MS | F | p | MS | F | p | ||
| treatment | 1 | 0.159 | 9.701 | 0.004 | 1.946 | 17.502 | <0.001 |
| pool | 1 | 0.186 | 11.329 | 0.002 | 5.513 | 49.582 | <0.001 |
| error | 37 | 0.016 | 0.111 | ||||
Hatchling numbers were significantly lower after exposure to winter conditions than after exposure to summer conditions (table 2, figure 3b) and also differed between pools (table 2).
(e) Laboratory experiment 2: infection from sediments
Daphnia exposed to sediments collected from four frozen rock pools during late winter became infected. Three to six of six replicates were infected after exposure to sediment. Four to six of six replicates were infected after exposure to the supernatant.
(f) Laboratory experiment 3: role of horizontal transmission
Prevalence increased rapidly and significantly (d.f.=9, t-ratio=10.033, p<0.001) in all populations in which we allowed for both horizontal and vertical transmission irrespective of starting prevalence (figure 4). After nine weeks, populations that started with 5% had reached an average prevalence of 81% (figure 4a) and those that started with 50% prevalence had reached prevalences close to 100% (figure 4b). In contrast, prevalence decreased rapidly in all populations in which horizontal transmission was prevented (d.f.=19, t-ratio=−5.313, p>0.001; figure 4). Exclusion of horizontal transmission resulted in a complete disappearance of O. bayeri after six weeks from populations started with 5% prevalence (figure 4a). In populations started with 50% prevalence decreased to 11% at week 9 (figure 4b).
Figure 4.
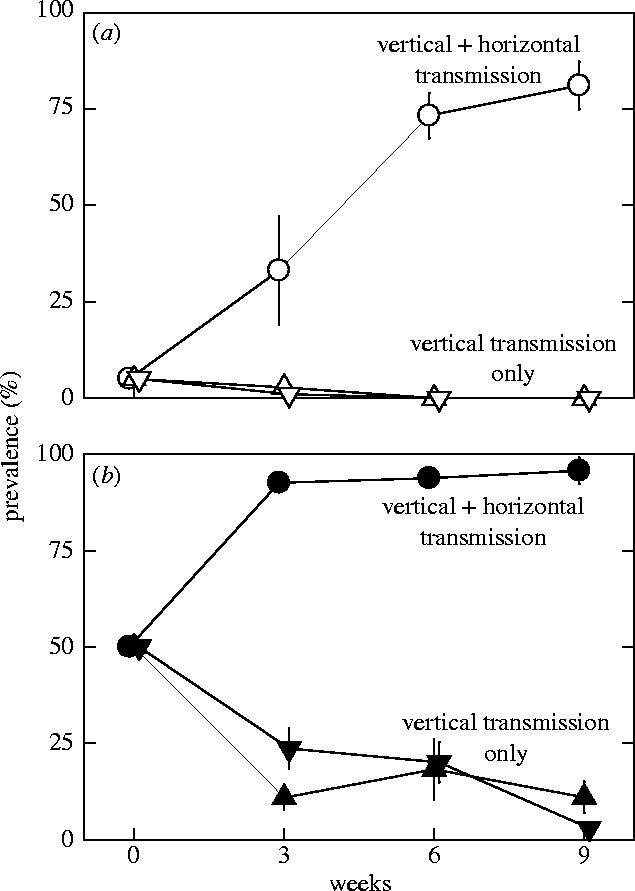
Prevalence of the microsporidium Octosporea bayeri in experimental populations of its host Daphnia magna. Populations were started with either (a) 5% or (b) 50% prevalence. Populations differed in the transmission routes that were allowed: circles indicate populations in which horizontal and vertical transmissions were allowed, triangles indicate populations in which only vertical transmission was allowed, whereas horizontal transmission was prevented (by removal of dead hosts). Additionally, dead infected hosts were replaced with uninfected Daphnia in populations indicated by downward triangles. Error bars represent ±s.e.
4. Discussion
(a) Diapause drives prevalence cycles
Cycles in parasite prevalence are commonly observed though rarely understood epidemiological phenomena (e.g. Green 1974; Miracle 1977; Ruttner-Kolisko 1977; Brambilla 1983; Hosseini et al. 2004). Here, we describe a regular pattern of disease dynamics, with a rise in O. bayeri prevalence during the host's growing season and a decrease associated with host diapause. This prevalence pattern is robust, as it is strikingly similar in host populations that differ in several attributes, such as size, exposure and the number of co-occurring organisms, e.g. additional parasites. The prevalence cycles further seem temporally stable as they do not only occur in host populations in which the parasite invaded recently, but also in populations that harbour the parasite for several years (figure 1). Yet, it has to be mentioned that having samples only from the beginning and the end of the season, we do not know the shape of the prevalence changes.
Cyclic patterns of disease may be driven by feedback mechanisms from within the host–parasite system itself, or follow external cyclic patterns such as temperature. Correlation with changes in environmental conditions may not necessarily reflect a causal connection. Prevalence patterns in measles, for instance, are seasonal but seasonality is not caused by climatic factors as shown by the fact that peaks in prevalence coincide with the recruitment of school children (Anderson & May 1991). Here, we obtained two lines of evidence that the decrease in Octosporea prevalence is not primarily caused by winter conditions, as the observed pattern suggests, but is rather associated with the host's life-cycle. First, O. bayeri prevalence in natural host populations did not decrease only after winter diapause but also after summer diapause (figure 2). Second, hatchlings from sediments which never experienced winter conditions still had a markedly reduced prevalence under laboratory conditions (figure 3a). Thus, host diapause explains at least part of the observed prevalence decline. External factors influencing the host's life-cycle drive prevalence dynamics in O. bayeri.
(b) Causes of prevalence decline
Prevalence decreases after both winter and summer diapause. Different environmental conditions during summer and winter diapause influence the magnitude of this decrease. Prevalence of hatchlings decreased more strongly after diapause under winter conditions than under summer conditions (figure 3a). This finding, together with a lower number of hatchlings after winter conditions than after summer conditions, suggests that under winter conditions mortality of infected resting eggs is higher than that of uninfected eggs. It is, however, possible that not only winter but also summer diapause conditions reduce survival of infected resting eggs. Such an effect would explain part of the prevalence decline associated with diapause in general (figure 3a). Natural diapause conditions are likely to be even more severe than conditions applied in the laboratory and cause an even higher mortality in infected resting eggs as they may include stronger temperature fluctuations and other stressors such as osmotic stress (salinity) and UV-radiation.
Higher mortality of infected resting eggs reduced prevalence after winter diapause in those host populations that produced both infected and uninfected resting eggs prior to diapause. Some populations in the metapopulation, however, may be founded by a single infected female and thus have 100% prevalence during the first growing season. We found that in such populations prevalence also decreased after winter diapause (field experiment). Since vertical transmission to asexual offspring has been shown to be 100% efficient (Vizoso et al. 2005), all resting eggs were most probably produced by infected females in these experimental populations. Apparently, the parasite was ‘lost’ in about 5% of the resting eggs, which supports results of an earlier laboratory study on prevalence of hatchlings from resting eggs (Vizoso et al. 2005). The underlying mechanism is not yet understood. Such a ‘loss’ may be caused by imperfect transmission or by parasite mortality during host diapause while the resting egg survives. Evidence for parasite mortality during host diapause has been found in a study on vertically transmitted bacteria (Wolbachia) infecting a wasp (Perrot-Minnot et al. 1996).
Survival of the parasite in Daphnia resting eggs may depend on the host genotype. Each resting egg represents a new genotype, given that they are the products of sexual reproduction. Since there is genetic variation in D. magna–O. bayeri interactions (Mucklow et al. 2004; Vizoso & Ebert 2005a, b) and local adaptation of parasites has been shown for other D. magna parasites (Ebert 1994; Ebert et al. 1998), some of these new genotypes may be more resistant to O. bayeri infection than their mothers, and thus uninfected. Since the genetic composition of host populations has been shown to influence prevalence dynamics (e.g. for plant–pathogen interactions; Garrett & Mundt 1999), the role of host genetics certainly deserves to be studied more intensively.
Several mechanisms may act together in decreasing prevalence associated with host diapause. These may either be mechanisms causing a decline in prevalence during diapause, such as winter diapause conditions increasing mortality of infected resting eggs, or mechanisms that affect prevalence of resting eggs already before diapause. As O. bayeri is known to reduce asexual fecundity of its host (Vizoso & Ebert 2005b), it may also reduce the production of sexual eggs. Such an effect would result in a prevalence decline after diapause in populations with infected and uninfected hosts, but this remains to be shown. Furthermore, if sexual reproduction starts early in the season when prevalence is still low, the number of uninfected eggs is likely to be larger than when all resting eggs are produced late in the season at high prevalences. Such mechanisms that reduce prevalence in resting eggs before actual diapause are the subject of a follow-up study.
(c) Causes of prevalence increase
During the asexual phase of host reproduction O. bayeri spreads rapidly in populations of its host (figures 1 and 4). The perfect vertical transmission of O. bayeri to parthenogenetic host offspring (Vizoso et al. 2005) ensures that maternal host lines remain infected during the asexual phase once they are infected. However, since O. bayeri reduces host fitness, its persistence and spread cannot be mediated by vertical transmission alone (figure 4), which is consistent with epidemiological models (Fine 1975; Lipsitch et al. 1995; Mangin et al. 1995). Theoretical models show that vertically transmitted virulent parasites require additional features, such as sex ratio distortion, biparental transmission and additional horizontal transmission, to remain in populations of their host (Fine 1975; Hurst 1993; Lipsitch et al. 1995).
Horizontal transmission of O. bayeri is mediated by spores released from dead decaying hosts (Vizoso et al. 2005). Here, we demonstrated that O. bayeri is able to survive winter outside its host and that this may contribute to prevalence increase during the growing season. An earlier study has further shown that O. bayeri spores survive drought (Vizoso et al. 2005). Long-lasting parasite spore banks have been found for other Daphnia microparasites and are likely to be important for disease dynamics (Ebert 1995; Ebert et al. 1997; Decastaecker et al. 2002, 2004).
Our study demonstrates recurrent prevalence cycles in a parasite with a combination of horizontal and vertical transmission. Although parasites with such a combination of horizontal and vertical transmission seem in the minority, examples can be found among nematodes, fungi, microsporidia, bacteria, viruses of plants and animals and bacteriophages (Bull et al. 1991; Herre 1993; Kover et al. 1997; Dunn & Smith 2001; Kaltz & Koella 2003). Here, we show that the interplay between different transmission modes in combination with environmental conditions leads to cyclic prevalence dynamics. Whether these mechanisms are sufficient to explain the cycles or if there are further mechanisms involved, e.g. differential resting egg production in infected and uninfected hosts or differential susceptibility of different host genotypes, remains to be studied.
Acknowledgments
We thank Lusia Sygnarski for help in the lab, Jürgen Hottinger, Christoph Haag, Florian Altermatt and Thomas Zumbrunn for help in the field, Dita Vizoso, Marc Zbinden and Dominik Refardt for fruitful discussions, and Larry Weider and an anonymous reviewer for suggestions that improved the manuscript. This work was supported by a DFG (German Research Foundation) grant to S.L. and a grant from the Swiss National Science Foundation (SNF) to D.E. This work is part of project no. 97524006 at Tvärminne Zoological Station, Finland.
Footnotes
Present address: University of Basel, Zoological Institute, Evolutionary Biology, Vesalgasse 1, 4051 Basel, Switzerland.
References
- Anderson R.M, May R.M. 1st edn. Oxford University Press; Oxford, UK: 1991. Infectious diseases of humans. [Google Scholar]
- Bartlett M.S. Measles periodicity and community size. J. R. Stat. Soc. A. 1957;120:48–70. [Google Scholar]
- Brambilla D.J. Microsporidiosis in a Daphnia pulex population. Hydrobiologia. 1983;99:175–188. 10.1007/BF00008769 [Google Scholar]
- Bull J.J, Molineux I.J, Rice W.R. Selection of benevolence in a host parasite system. Evolution. 1991;45:875–882. doi: 10.1111/j.1558-5646.1991.tb04356.x. [DOI] [PubMed] [Google Scholar]
- Canter H.M, Lund J.W.G. Studies on plankton parasites. 1. Fluctuations in numbers of Asterionella formosa Hass. in relation to fungal epidemics. New Phytol. 1948;47:238–261. [Google Scholar]
- Carius H.J, Little T.J, Ebert D. Genetic variation in a host–parasite association: potential for coevolution and frequency-dependent selection. Evolution. 2001;55:1136–1145. doi: 10.1111/j.0014-3820.2001.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Decastaecker E, De Meester L, Ebert D. In deep trouble: habitat selection constrained by multiple enemies. Proc. Natl Acad. Sci. USA. 2002;99:5481–5485. doi: 10.1073/pnas.082543099. 10.1073/pnas.082543099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decastaecker E, Lefever C, De Meester L, Ebert D. Haunted by the past: evidence for dormant stage banks of microparasites and epibionts of Daphnia. Limnol. Oceanogr. 2004;49:1355–1364. [Google Scholar]
- Dunn A.M, Smith J.E. Microsporidian life cycles and diversity: the relationship between virulence and transmission. Microbes Infect. 2001;3:381–388. doi: 10.1016/s1286-4579(01)01394-6. 10.1016/S1286-4579(01)01394-6 [DOI] [PubMed] [Google Scholar]
- Ebert D. Virulence and local adaptation of a horizontally transmitted parasite. Science. 1994;265:1084–1086. doi: 10.1126/science.265.5175.1084. [DOI] [PubMed] [Google Scholar]
- Ebert D. The ecological interactions between a microsporidian parasite and its host Daphnia magna. J. Anim. Ecol. 1995;64:361–369. [Google Scholar]
- Ebert D, Payne R.J.H, Weisser W.W. The epidemiology of parasitic diseases in Daphnia. In: Dettner K, Bauer G, Völkl W, editors. Vertical food web interactions. vol. 130. Springer; Berlin: 1997. p. 390. [Google Scholar]
- Ebert D, Zschokke-Rohringer C.D, Carius H.J. Within- and between-population variation for resistance of Daphnia magna to the bacterial endoparasite Pasteuria ramosa. Proc. R. Soc. B. 1998;265:2127–2134. 10.1098/rspb.1998.0549 [Google Scholar]
- Ebert D, Lipsitch M, Mangin K.L. The effect of parasites on host population density and extinction: experimental epidemiology with Daphnia and six microparasites. Am. Nat. 2000;156:459–477. doi: 10.1086/303404. 10.1086/303404 [DOI] [PubMed] [Google Scholar]
- Ebert D, Hottinger J.W, Pajunen V.I. Temporal and spatial dynamics of parasite richness in a Daphnia metapopulation. Ecology. 2001;82:3417–3434. [Google Scholar]
- Fine P.E.M. Vectors and vertical transmission: an epidemiologic perspective. Ann. NY Acad. Sci. 1975;266:173–194. doi: 10.1111/j.1749-6632.1975.tb35099.x. [DOI] [PubMed] [Google Scholar]
- Garrett K.A, Mundt C.C. Epidemiology in mixed host populations. Phytopathology. 1999;89:984–990. doi: 10.1094/PHYTO.1999.89.11.984. [DOI] [PubMed] [Google Scholar]
- Grassly N.C, Fraser C, Garnett G.P. Host immunity and synchronized epidemics of syphilis across the United States. Nature. 2005;433:417–421. doi: 10.1038/nature03072. 10.1038/nature03072 [DOI] [PubMed] [Google Scholar]
- Green J. Parasites and epibionts of Cladocerans. Trans. Zool. Soc. Lond. 1974;32:417–515. [Google Scholar]
- Grenfell B.T, Bjornstad O. Sexually transmitted diseases: epidemic cycling and immunity. Nature. 2005;433:366–367. doi: 10.1038/433366a. 10.1038/433366a [DOI] [PubMed] [Google Scholar]
- Herre E.A. Population structure and the evolution of virulence in nematode parasites of fig wasps. Science. 1993;259:1442–1445. doi: 10.1126/science.259.5100.1442. [DOI] [PubMed] [Google Scholar]
- Hosseini P.R, Dhondt A.A, Dobson A. Seasonality and wildlife disease: how seasonal birth, aggregation and variation in immunity affect the dynamics of Mycoplasma gallisepticum in house finches. Proc. R. Soc. B. 2004;271:2569–2577. doi: 10.1098/rspb.2004.2938. 10.1098/rspb.2004.2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst L.D. The incidences, mechanisms and evolution of cytoplasmatic sex ratio distorters in animals. Biol. Rev. 1993;68:121–193. 10.1086/417973 [Google Scholar]
- Kaltz O, Koella J.C. Host growth conditions regulate the plasticity of horizontal and vertical transmission in Holospora undulata, a bacterial parasite of the protozoan Paramecium caudatum. Evolution. 2003;57:1535–1542. doi: 10.1111/j.0014-3820.2003.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Kelly A, Dunn A.M, Hatcher M.J. Incomplete feminisation by the microsporidian sex ratio distorter, Nosema granulosis, and reduced transmission and feminisation efficiency at low temperatures. Int. J. Parasitol. 2002;32:825–831. doi: 10.1016/s0020-7519(02)00019-x. 10.1016/S0020-7519(02)00019-X [DOI] [PubMed] [Google Scholar]
- Klüttgen B, Dulmer U, Engels M, Ratte H.T. ADaM, an artificial freshwater for the culture of zooplankton. Water Res. 1994;28:743–746. 10.1016/0043-1354(94)90157-0 [Google Scholar]
- Kover P.X, Dolan T.E, Clay K. Potential versus actual contribution of vertical transmission to pathogen fitness. Proc. R. Soc. B. 1997;264:903–909. 10.1098/rspb.1997.0125 [Google Scholar]
- Lipsitch M, Nowak M.A, Ebert D, May R.M. The population dynamics of vertically and horizontally transmitted parasites. Proc. R. Soc. B. 1995;260:321–327. doi: 10.1098/rspb.1995.0099. [DOI] [PubMed] [Google Scholar]
- Mangin K.L, Lipsitch M, Ebert D. Virulence and transmission modes of two microsporidia in Daphnia magna. Parasitology. 1995;111:133–142. [Google Scholar]
- May R.M, Anderson R.M. Population biology of infectious diseases: part II. Nature. 1979;280:455–461. doi: 10.1038/280455a0. 10.1038/280455a0 [DOI] [PubMed] [Google Scholar]
- Miracle M.R. Epidemiology in rotifers. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1977;8:138–141. [Google Scholar]
- Mucklow P.T, Vizoso D.B, Jensen K.H, Refardt D, Ebert D. Variation in phenoloxidase activity and its relation to parasite resistance within and between populations of Daphnia magna. Proc. R. Soc. B. 2004;271:1175–1183. doi: 10.1098/rspb.2004.2707. 10.1098/rspb.2004.2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Minnot M.-J, Guo L.R, Werren J.H. Single and double infections with Wolbachia in the parasitic wasp Nasonia vitripennis: effects on compatibility. Genetics. 1996;143:961–972. doi: 10.1093/genetics/143.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttner-Kolisko A. The effect of the microsporid Pleistophora asperospora on Conochilus unicornis in Lunzer Untersee (LUS) Arch. Hydrobiol. Beih. Ergebn. Limnol. 1977;8:135–137. [Google Scholar]
- Sokal R.R, Rohlf F.J. 3rd edn. W. H. Freeman and Co; San Francisco: 1995. Biometry. [Google Scholar]
- Vizoso D.B, Ebert D. Within-host dynamics of a microsporidium with horizontal and vertical transmission: Octosporea bayeri in Daphnia magna. Parasitology. 2004;128:31–38. doi: 10.1017/s0031182003004293. 10.1017/S0031182003004293 [DOI] [PubMed] [Google Scholar]
- Vizoso D.B, Ebert D. Mixed inoculations of a microsporidian parasite with horizontal and vertical infections. Oecologia. 2005a;143:157–166. doi: 10.1007/s00442-004-1771-4. 10.1007/s00442-004-1771-4 [DOI] [PubMed] [Google Scholar]
- Vizoso D.B, Ebert D. Phenotypic plasticity of host–parasite interactions in response to the route of infection. J. Evol. Biol. 2005b;18:911–921. doi: 10.1111/j.1420-9101.2005.00920.x. [DOI] [PubMed] [Google Scholar]
- Vizoso D.B, Lass S, Ebert D. Different mechanisms of transmission of the microsporidium Octosporea bayeri: a cocktail of solutions for the problem of parasite permanence. Parasitology. 2005;130:1–11. doi: 10.1017/s0031182004006699. 10.1017/S0031182004006699 [DOI] [PubMed] [Google Scholar]
- Yan N.D, Larsson J.I.R. Prevalence and inferred effects of microsporidia of Holopedium gibberum (Crustacea, Cladocera) in a Canadian Shield lake. J. Plankton Res. 1988;10:875–886. [Google Scholar]


