Abstract
Social spiders are unusual among social organisms in being highly inbred—males and females mature within their natal nest and mate with each other to produce successive generations. Several lines of evidence suggest that in spiders inbred social species originated from outbred subsocial ancestors, a transition expected to have been hindered by inbreeding depression. As a window into this transition, we examined the fitness consequences of artificially imposed inbreeding in the naturally outbred subsocial spider Anelosimus cf. jucundus. Subsocial spiders alternate periods of solitary and social living and are thought to resemble the ancestral system from which the inbred social species originated. We found that inbreeding depression in this subsocial spider only becomes evident in spiders raised individually following the end of their social phase and that ecological and demographic factors such as eclosion date, number of siblings in the group and mother's persistence are more powerful determinants of fitness during the social phase. A potential explanation for this pattern is that maternal care and group living provide a buffer against inbreeding depression, a possibility that may help explain the repeated origin of inbred social systems in spiders and shed light on the origin of other systems involving regular inbreeding.
Keywords: inbreeding depression, maternal effects, social effects, sociality, fitness, Anelosimus
1. Introduction
Most social organisms avoid inbreeding by the pre-mating dispersal of members of one or both sexes. A handful of organisms, however, have evolved social systems that involve regular inbreeding (Sherman et al. 1991; Kirkendall 1993; Riechert & Roeloffs 1993; Avilés 1997). Notable among them are the social spiders, where males and females mature within their natal nest and mate with each other to produce successive generations (Riechert & Roeloffs 1993; Avilés 1997). Because the offspring of close relatives are expected to suffer inbreeding depression—i.e. reduced fitness due to loss of heterosis and/or the expression of deleterious recessive alleles inherited simultaneously from both parents (Charlesworth & Charlesworth 1987, 1999; Pusey & Wolf 1996; Crnokrak & Roff 1999; Keller & Waller 2002)—the origin of such inbred systems from presumably outbred ancestral ones remains a mystery. As a window into this transition, we examined the fitness consequences of artificially imposed inbreeding in the naturally outbred subsocial spider Anelosimus cf. jucundus (Araneae: Theridiidae).
Taxonomic and phylogenetic evidence (Kraus & Kraus 1989; Avilés 1997; Agnarsson 2004, in press) suggests that in spiders inbred social species originated from outbred subsocial ancestors. In subsocial spiders, new nests are established by single females who raise their offspring without the aid of others (reviewed in Krafft 1979; Buskirk 1981; D'Andrea 1987). Following their mother's death, clutchmates remain together for variable periods of time helping each other maintain the nest and acquire food. Nestmates disperse prior to mating (e.g. Avilés & Gelsey 1998; Bukowski & Avilés 2002; Powers & Avilés 2003; Bilde et al. 2005). In social spider species, in contrast, nestmates remain together throughout their lives and mate and reproduce within the natal nest from generation to generation (reviewed in Avilés 1997). In addition to cooperating in web building, prey capture and feeding, females help each other in brood care (reviewed in Avilés 1997).
One hypothesis for the origin of inbred social species suggests a transition from outbred subsocial ancestors via suppression of the pre-mating dispersal phase (Buskirk 1981; Wickler & Seibt 1993; Avilés 1997). Given the switch from an outbred to an inbred breeding system, this transition is expected to have been hindered, at least during its early stages, by inbreeding depression (Charlesworth & Charlesworth 1987, 1999; Keller & Waller 2002). Despite this expected negative effect of inbreeding, inbred social systems in spiders have arisen repeatedly in several spider genera (Avilés 1997) requiring, at the latest count, no less than 21 independent origins to explain sociality in 26 spider species (I. Agnarsson et al., unpublished).
To gain insights into the factors involved in this transition, we investigated the potential costs of inbreeding depression in the subsocial spider A. cf. jucundus (see Avilés & Gelsey 1998; Bukowski & Avilés 2002; Powers & Avilés 2003; Klein et al. in press), a naturally outbred subsocial species belonging to the spider genus known to have the largest number of inbred social species (Avilés 1997; Agnarsson in press). We found that life-history traits expected to influence fitness, such as spider size, development rate and survival probability, only show evidence of inbreeding depression during the solitary phase of the spider's life cycle. Instead, demographic and ecological factors such as number of siblings in the group, mother's persistence and eclosion date were much more powerful determinants of fitness during the social phases. We suggest several hypotheses to explain a lack of inbreeding effects during the early social phases in this and another subsocial spider studied in parallel by Bilde et al. (2005). In particular, we suggest the possibility that maternal care and group living may buffer against severe inbreeding depression, a possibility that may help explain the repeated origin of inbred social systems in spiders and shed light on the origin of other systems of regular inbreeding.
2. Material and methods
The species we studied, so far referred to as A. cf. jucundus (Bukowski & Avilés 2002; Powers & Avilés 2003) or simply A. jucundus (Avilés & Gelsey 1998), will be shortly described as Anelosimus arizona, following an upcoming revision of the genus by Agnarsson (in press). The species occurs in southern Arizona where it is a univoltine species found exclusively in riparian habitats near permanent water (Avilés & Gelsey 1998; Bukowski & Avilés 2002). At Garden Canyon (31.47° N, 110.35° W, 1630 m), where we conducted our study, nests occur within 25–30 m of a permanent creek and contain, as is typical of subsocial spiders, the offspring of a single female (Avilés & Gelsey 1998; Bukowski & Avilés 2002). In early March 1999, 8–10 weeks prior to the dispersal season, we collected 35 nests from two locations separated by 3.2 km. We raised the spiders to maturity in the laboratory, separating them in individual containers prior to their final molt to insure virginity. We mated the spiders once, either with a member of their own sibship (full or half-sibs) to produce inbred offspring or with spiders from the other locality (unrelated) to produce outbred offspring. In late July, we returned to the field 43 inbred- and 43 outbred-inseminated females, placing them singly on individual tree branches for nest re-establishment. To control for potential site and family effects, at each site we paired an inbred-inseminated with an outbred-inseminated female from the same family. Females in the two treatment categories did not differ in size (F1,58=0.51, p=0.48), egg-laying date (F1,74=0.40, p=0.53), the size of the clutch they laid (number of spiderlings eclosing from the eggsac, F1,73=0.06; p=0.81; observed range 9–64, median 63, n=75), or their persistence time (number of days the mother lived with her clutch following its eclosion from the eggsac; F1,74=0.03, p=0.86). During the nest establishment and sac guarding periods, we enclosed within netting each incipient individual nest to protect females and their clutch from predation and other accidents. After the offspring eclosed from the eggsac (starting in mid-August), we removed the netting, leaving the offspring and their mothers exposed to all contingencies of their natural habitat.
During the egg laying and emergence periods we monitored the nests every other day to measure development time within the eggsac. Following emergence from the sac, we monitored the nests every other week for presence/absence of the mother and approximate count of offspring number. We collected all nests nine weeks after the offspring they contained had emerged from the eggsac (early-October to late-November, depending on the nest), at a time when over 80% of the mothers had died or disappeared. After processing the spiders in the laboratory (see below), we re-established the sibling groups (but not their mothers) in the field at the same location from which we had removed them and allowed them to overwinter in the field for a period of 12 weeks. After this period, we collected the sibships for a second time. Following their second collection, we processed the spiders as before (see below) and then raised them individually to maturity in the laboratory, placing them in individual containers unmarked with respect to treatment category. We treated all spiders identically, sprinkling them daily with water and feeding them ad libitum with houseflies. All monitoring in the field and spider processing in the lab was done blind with respect to treatment category.
In addition to assessing the eclosion and development time of eggsacs containing inbred and outbred clutches, we tested for possible inbreeding depression effects at three stages of the spider's life cycle—the end of the period of maternal care (the first collection date), the end of the period of sibling cohabitation (the second collection date) and at maturity. At each of these three stages we examined traits expected to be associated with overall fitness—spider size, development rate and survival rate. Spider size was assessed from the length of the tibia plus patella of leg pair I (7PI), measured to the nearest 0.1 mm using an SZH Olympus dissecting stereo microscope. Development rate was assessed from the median instar found in the nests at the first and second collection dates and from the date at which the spiders moulted to maturity (with the day at which the first spider moulted to maturity set as day 0). Survival rate was estimated from the proportion of offspring in a clutch that survived to the first and second collection dates and to maturity. A total of 2425 spiders, belonging to 36 inbred and 39 outbred clutches, was processed at the first collection date; 796, belonging to 36 inbred and 37 outbred clutches, at the second collection; and 232, belonging to 34 inbred and 32 outbred clutches, at maturity.
Early during the experiment it became obvious that some uncontrolled variables varied across sibships, including eclosion date of the eggsac, number of offspring in the sibship and persistence time of the mother. Also, due to limited availability of nest site locations, we could not completely eliminate variability of distance to the creek. We have included these variables as covariates in all our analyses. In our data, the size of the mother did not have a significant effect on the variables analysed and was thus not included as a covariate after the initial analyses. All statistical tests were performed on family means, using sibship as the experimental unit.
3. Results
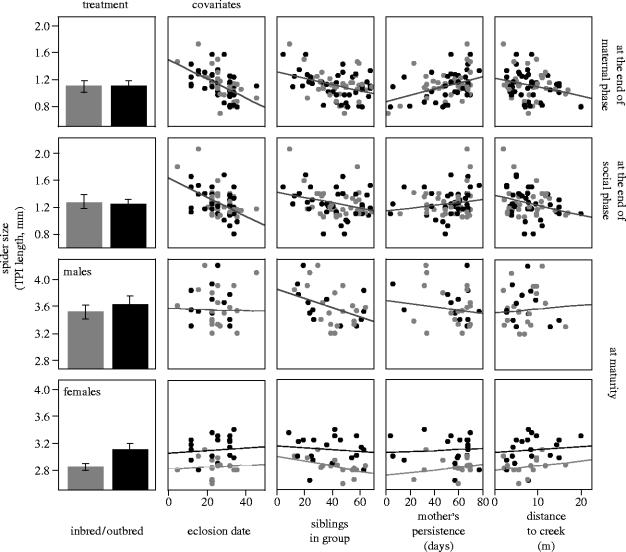
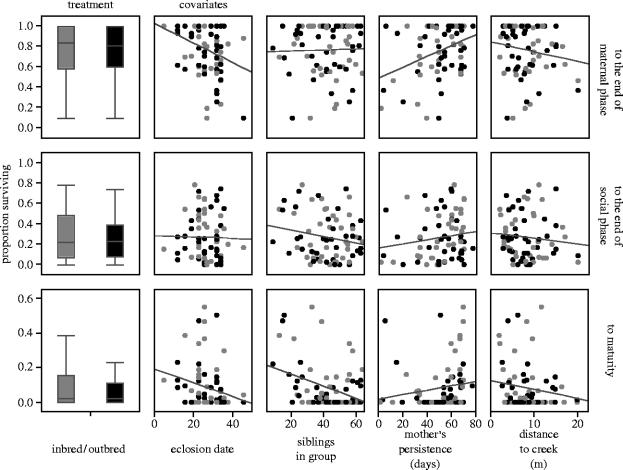
We obtained evidence of inbreeding depression, in the form of smaller size and longer development time, but not survival probability, among spiders that reached maturity following a period of solitary living (table 1). Inbred females were significantly smaller as adults than were outbred females (TPI length 2.8 versus 3.1 mm) and matured at a later date (68.0 versus 53.7 days); adult males exhibited non-significant trends in the same direction (inbred versus outbred males, TPI length: 3.5 versus 3.6 mm, maturation date: 57.2 versus 55.3 days; see table 1 for significance tests). Earlier in the life cycle, there was no difference in the proportion of inbred versus outbred clutches that eclosed from the eggsac (40 out of 41 outbred, 36 out of 40 inbred clutches laid; likelihood ratio chi-square=2.1, p=0.14) and only a nearly significant trend for inbred clutches to take slightly longer to develop (25.4 versus 24.3 days to emerge from the sac, F1,72=3.9, p=0.051, in a model including sac laying date as a covariate and sac laying date×treatment interaction, which were both significant). At the end of the periods of maternal care and of group living, inbred and outbred spiders did not differ in size, development rate (median instar), or survival probability (table 1, figures 1 and 2).
Table 1.
Analyses of the effects of breeding treatment on life-history traits at different stages of the spider's life cycle. (Only covariates deemed significant in preliminary multivariate or univariate tests shown here and included in the final model. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.)
| source | Mancova whole model | mean offspring size | median offspring instar | proportion surviving |
|---|---|---|---|---|
| end of the maternal phase (Mancova Wilks' lambda=0.43, p<0.0001) | ||||
| breeding treatment | F3,68=0.5 | F1,70=0.6 | F1,70=0.0 | F1,70=0.3 |
| eclosion date | F3,68=9.9**** | F1,70=28.0**** | F1,70=17.8**** | F1,70=4.3* |
| siblings in group | F3,68=6.6*** | F1,70=16.1*** | F1,70=4.7* | F1,70=0.3 |
| mother's persistence | F3,68=6.1*** | F1,70=13.3*** | F1,70=7.8** | F1,70=7.4** |
| end of the social phase (Mancova Wilks' lambda=0.63, p=0.0005) | ||||
| breeding treatment | F3,63=0.7 | F1,65=1.0 | F1,65=1.6 | F1,65=0.6 |
| eclosion date | F3,63=7.8*** | F1,65=21.7**** | F1,65=22.1**** | F1,65=0.0 |
| siblings in group | F3,63=2.5 | F1,65=5.7* | F1,65=3.1 | F1,65=2.5 |
| sex | source | Mancova whole model | adult spider size | maturation date |
|---|---|---|---|---|
| maturity (females, whole model exact-F2,31=16.4, p<0.0001; males, Wilks' lambda=0.72, p=0.05) | ||||
| females | breeding treatment | F2,31=16.4**** | F1,32=31.2**** | F1,32=5.6* |
| males | breeding treatment | F2,28=0.13 | F1,29=0.3 | F1,29=0.1 |
| siblings in group | F2,28=4.4* | F1,29=7.9** | F1,29=0.0 |
| source | proportion surviving |
|---|---|
| for survival to maturity, sexes combined (whole model ANOVA F3,71=5.7, p=0.0015) | |
| breeding treatment | F1,71=0.9 |
| eclosion date | F1,71=6.7* |
| siblings in group | F1,71=8.9** |
Figure 1.
Effect of breeding treatment and four ecological and demographic factors (covariates) on the size of A. cf. jucundus inbred/outbred offspring at three stages of their life cycle. Leftmost panels show mean±95% CI, estimated across individual family means. Dots in the top two rows represent individual family means (inbred clutches, grey; outbred clutches, black); dots in the bottom two rows are means for male (third row) or female (bottom row) offspring in a family. Separate regression lines for inbred/outbred offspring shown only where there was a significant difference between treatments. None of the interactions between treatment and covariates were significant. See table 1 for significance tests and table 2 for the proportion of the variance accounted for by each of the factors at the end of the maternal and social phases.
Figure 2.
Effect of breeding treatment and various ecological and demographic factors on the proportion of A. cf. jucundus inbred/outbred offspring surviving to each of three stages of their life cycle. Boxplots show medians (line inside box), quartiles (box) and spread (whiskers) of the proportions surviving in different families. Dots in the panels to the right correspond to the proportion surviving in individual families (inbred clutches, grey; outbred clutches, black). Significance tests, performed on arcsine-transformed proportions, are shown in table 1.
Rather than being affected by inbreeding depression, life-history traits during the group-living phase (periods of maternal care and sibling cohabitation) were influenced by a variety of ecological and demographic factors, including eclosion date, number of siblings in the group and mother's persistence (figures 1 and 2, table 1). A later eclosion date and a greater number of siblings had, for the most part, a negative effect on one or more of the traits measured, while the presence of the mother for a longer period of time had a generally positive effect on all three traits, in particular during the early phases of the spider's life cycle (figures 1 and 2, table 1). A greater distance to the creek had a negative, although non-significant, effect on all three traits (figures 1 and 2, table 1).
Sequential tests (Type I sums of squares (SS)) of the offspring size data (table 2) show that while breeding treatment accounted for, at most, 1% of the total variance during the maternal and group-living phases, the covariates collectively accounted for over 30 (at the end of the period of sibling cohabitation) to close to 50% (at the end of the maternal phase) of the total variance. A similar pattern held for the survival probability—at the end of the period of maternal care, for instance, R2=0.1% for breeding treatment, 16.7% for the covariates. Because after the end of the group-living phase the spiders were raised to maturity in the laboratory, it is not possible with our data to weigh the relative importance of intrinsic versus extrinsic factors in determining fitness during the solitary phase. The inbreeding depression effects detected, however, at least with respect to adult female size, appear dramatic enough to suggest an important role of inbreeding depression during the late life-history stages of this subsocial spider.
Table 2.
Sequential tests (Type I SS) showing the relative contribution of genetic, ecological and demographic factors to the explained variance in spider size at the end of the periods of maternal care and of group living.
| sourcea | end maternal phase | end social phase | ||
|---|---|---|---|---|
| SS | percentage of total | SSa | percentage of total | |
| distance to creek | 0.223 50 | 8.7 | 0.265 82 | 8.8 |
| eclosion date | 0.688 40 | 26.7 | 0.597 72 | 19.7 |
| siblings in group | 0.205 18 | 8.0 | 0.141 28 | 4.7 |
| mother's persistence | 0.137 44 | 5.3 | 0.007 50 | 0.2 |
| inbred/outbred | 0.022 45 | 0.9 | 0.030 33 | 1.0 |
| percentage explained (R2) | 49.5 | 34.3 | ||
Factors introduced in the order shown—covariates first, from earliest to latest acting; breeding treatment last, once the variance due to the covariates has been accounted for. Type I SS add up to the model SS, allowing the calculation of the percentage of variance accounted for by each of the factors.
Given the expected relationship between adult female size and fecundity (Gonzaga & Vasconcellos-Neto 2001), we can use the observed average sizes of inbred (TPI=2.8) and outbred (TPI=3.1) females to infer expected inbreeding depression based on female fecundity. Depending on the regression equation used (Appendix A), our estimates of inbreeding depression range from 0.1 to 0.39 (inbreeding depression=(fitness oubred−fitness inbred)/fitness outbred), corresponding to differences in offspring number between inbred and outbred females of 4–27, respectively (Appendix A). Although the fitness consequences of delayed maturation of inbred females are harder to pinpoint numerically, it is clear that late maturing spiders should be at a disadvantage locating mates (Matsumoto 1994; Henschel et al. 1995) and, as our results on the fitness effects of eclosion date show (figures 1 and 2), in raising their offspring.
4. Discussion
Two clear patterns emerge from our results: (i) the presence of inbreeding depression only during the solitary phases of this spider's life cycle and (ii) the predominance of extrinsic ecological and demographic factors, over intrinsic genetic factors, in determining fitness during its social phases. We found an almost complete absence of inbreeding depression during the phases of the A. cf. jucundus life cycle characterized by social interactions–period of maternal care and sibling cohabitation, but significant inbreeding depression, in the form of smaller spider size or longer developmental time, among females who reached maturity following a period of solitary living. Instead, during the social phases we found that extrinsic demographic and ecological factors, such as eclosion date, number of siblings in the group and mother's persistence, were much more powerful determinants of fitness than the intrinsic effects of inbreeding depression.
Three hypotheses can explain the absence of inbreeding depression early in the life cycle of this spider (or near absence, depending on how the slight difference in eggsac emergence time is interpreted): (i) inbreeding effects tend to show up late in the life cycle of organisms; (ii) inbreeding effects show up late in the life cycle of this species, because low levels of inbreeding have already weeded out the most damaging deleterious recessive alleles, i.e. those with early life action (Husband & Schemske 1996); (iii) maternal care and group living provide a buffer against severe effects of inbreeding during the social phase of this spider's life cycle.
Husband & Schmeske (1996) examined the literature on inbreeding depression in plants and found that self-fertilizing species tended to express inbreeding depression late in life (growth and reproduction), while outcrossers expressed it both early (seed production) and late in the life cycle. Their findings, therefore, argue against late life cycle expression of inbreeding depression as a general feature of organisms. Their observations, on the other hand, are consistent with our second hypothesis which suggests that a history of inbreeding may have weeded out early-acting deleterious recessive alleles in our species (see also Koelewijn et al. 1999). Some level of inbreeding is in fact a possibility in A. cf. jucundus as its nests are patchily distributed (Avilés & Gelsey 1998), at least a fraction of both males and females disperse only short distances from their natal nests (Avilés & Gelsey 1998; Powers & Avilés 2003), and neither males nor females discriminate against kin as mates (T. C. Bukowski & L. Avilés, unpublished data). Other observations, however, suggest that inbreeding is only partial, at best, as there is strong asynchrony in the maturation times of male and female clutchmates (Bukowski & Avilés 2002), both males and females mate multiply (Klein et al. in press), and the proportion of males in the populations drops significantly—from 0.5 to 0.28—from pre- to the post-dispersal phases, as would be expected if a fraction of the males dispersed beyond the local area without being replaced by a similar number of incoming males due to mortality of migrating males (Avilés & Gelsey 1998). Finally, a strongly female-biased primary sex ratio, the signature of strong inbreeding and population subdivision in permanent social spiders (Avilés 1993, 1997), is absent in this species. In a parallel study, Bilde et al. (2005) have documented similar patterns in the subsocial spider Stegodyphus lineatus (Araneae: Eresidae) where inbreeding depression effects were only noticeable in offspring growth rates and adult body size. Supported by DNA fingerprinting data, Bilde et al. (2005) suggest that a history of mild inbreeding may have promoted inbreeding tolerance in S. lineatus.
Our third, non-mutually exclusive hypothesis suggests that maternal care and group living provide a buffer against severe inbreeding depression during phases of the life cycle characterized by social interactions. Moderating maternal effects have in fact been argued to be responsible for reduced inbreeding depression early in the life cycle of several plant species (Wolfe 1993; Helenurm & Schaal 1996). We suggest that group living and cooperation may play a similar role as, collectively, individuals may compensate for each other's deficiencies and succeed at obtaining food and maintaining the nest, somewhat in contrast to the way competition has been shown to exasperate the effects of inbreeding in some cases (Wolfe 1993; Meagher et al. 2000; Keller et al. 2002). The hypothesis that group living may provide a buffer against inbreeding depression is particularly appealing in light of the repeated origin of inbred social systems in spiders (Avilés 1997; Agnarsson et al. in press). With inbreeding depression effects dampened within a social context, extrinsic demographic and ecological factors selecting for group living and extreme philopatry may become much more powerful determinants of fitness than the intrinsic effects of inbreeding depression. Thus, the origin of inbred social systems in spiders from outbred (or mild to moderately inbred) subsocial ones may not have been as difficult as previously believed.
Acknowledgments
We acknowledge the assistance in the field and laboratory of graduate and undergraduate students from the University of Arizona, including Asher D. Cutter, Natalie Doerr, Sarah Kenyon, Felipe Perez and Kimberly S. Powers. Robin Richards kindly constructed the nets used in the field to protect the incipient spider nests. For comments on the manuscript, we thank Kimberly S. Powers, Patricio Salazar and two anonymous reviewers. This research was funded by the USA National Science Foundation NSF-DEB grant no. 9815938 to L.A.
Appendix A
We can infer the eventual impact of small spider size on adult female fecundity by examining the relationship between female size and number of offspring in a clutch. Gonzaga & Vasconcellos-Neto (2001) examined this relationship in the congeneric subsocial spider, Anelosimus jabaquara. To apply their regression equation—clutch size=−79.879+57.715×carapace area—to our data, we convert our estimates of TPI length to carapace area using the following relationship: carapace area=−2.24+1.56×TPI length (derived from measurements of large and small spiders near the appropriate size range, assuming a linear relationship for the range explored). Applying the Gonzaga & Vasconcellos-Neto equation, we obtain a difference of ca 27 offspring between the average inbred (TPI=2.8 mm) and the average outbred female (TPI=3.1). Examining the female size–clutch size relationship among spiders of the maternal generation in our study, we find a non-significant trend (F1,58=1.6, p=0.2) for larger females to produce a larger number of offspring. Using the regression equation for this relationship—clutch size=−1.3+13.5 TPI length—the predicted difference between the average inbred and outbred female is four offspring. We estimate inbreeding depression (d=(fitness oubred−fitness inbred)/fitness outbred) from the predicted clutch sizes for inbred and outbred females based on these two estimates. With the Gonzaga & Vasconcellos-Neto (2001) equation we have d=(70.2−43.1)/70.2=0.39; with our relationship we have d=(40.6−36.5)/40.6=0.10.
References
- Agnarsson I. Morphological phylogeny of cobweb spiders and their relatives (Araneae, Araneoidea, Theridiidae) Zool. J. Linn. Soc. 2004;449:447–626. [Google Scholar]
- Agnarsson, I. In press A revision of the New World eximius lineage cf Anelosimus (Araneae, Theridiidae) and a phylogenetic analysis using worldwide exemplars. Zool. J. Linn. Soc.
- Avilés L. Interdemic selection and the sex ratio: a social spider perspective. Am. Nat. 1993;142:320–345. 10.1086/285540 [Google Scholar]
- Avilés L. Causes and consequences of cooperation and permanent sociality in spiders. In: Crespi B, editor. The evolution of social behavior in insects and arachnids. Cambridge University Press; Cambridge, UK: 1997. pp. 476–497. [Google Scholar]
- Avilés L, Gelsey G. Natal dispersal and demography of a subsocial Anelosimus species and its implications for the evolution of sociality in spiders. Can. J. Zool. 1998;76:2137–2147. [Google Scholar]
- Bilde T, Lubin Y, Smith D, Schneider J.M, Maklakov A.A. The transition to social inbred mating systems in spiders: role of inbreeding tolerance in a subsocial predecessor. Evolution. 2005;59:160–174. [PubMed] [Google Scholar]
- Bukowski T.C, Avilés L. Asynchronous maturation of the sexes may limit close inbreeding in a subsocial spider. Can. J. Zool. 2002;80:193–198. 10.1139/z01-220 [Google Scholar]
- Buskirk R.E. Sociality in the Arachnida. vol. 2. Academic Press; New York: 1981. pp. 281–367. [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 1987;18:237–268. 10.1146/annurev.es.18.110187.001321 [Google Scholar]
- Charlesworth B, Charlesworth D. The genetic basis of inbreeding depression. Genet. Res. 1999;74:329–340. doi: 10.1017/s0016672399004152. 10.1017/S0016672399004152 [DOI] [PubMed] [Google Scholar]
- Crnokrak P, Roff D.A. Inbreeding depression in the wild. Heredity. 1999;83:260–270. doi: 10.1038/sj.hdy.6885530. 10.1038/sj.hdy.6885530 [DOI] [PubMed] [Google Scholar]
- D'Andrea M. Social behaviour in spiders. Ital. J. Zool. (N.S.) Monogr. 1987;3:1–156. [Google Scholar]
- Gonzaga M.O, Vasconcellos-Neto J. Female body size, fecundity parameters and foundation of new colonies in Anelosimus jabaquara (Araneae, Theridiidae) Insectes Soc. 2001;48:94–100. [Google Scholar]
- Helenurm K, Schaal K. Genetic and maternal effects on offspring fitness in Lupinus texensis (Fabaceae) Am. J. Bot. 1996;83:1596–1608. [Google Scholar]
- Henschel J.R, Lubin Y.D, Schneider J. Sexual competition in an inbreeding social spider, Stegodyphus dumicola (Araneae: Eresidae) Insectes Soc. 1995;42:419–426. 10.1007/BF01242170 [Google Scholar]
- Husband B.C, Schemske D.W. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution. 1996;50:54–70. doi: 10.1111/j.1558-5646.1996.tb04472.x. [DOI] [PubMed] [Google Scholar]
- Keller L.F, Waller D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002;17:230–241. 10.1016/S0169-5347(02)02489-8 [Google Scholar]
- Keller L.F, Grant P.R, Grant B.R, Petren K. Environmental conditions affect the magnitude of inbreeding depression in survival of Darwin's finches. Evolution. 2002;56:1229–1239. doi: 10.1111/j.0014-3820.2002.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Kirkendall L.R. Ecology and evolution of biased sex ratios in bark and ambrosia beetles. In: Ebbert M.A, editor. Evolution and diversity of sex ratio in insects and mites. Chapman & Hall; New York: 1993. pp. 235–345. [Google Scholar]
- Klein, B., Bukowski, T. & Avilés, L. In press Male residency and mating patterns in a subsocial spider. J Arach.
- Koelewijn H.P, Koski V, Savolainen O. Magnitude and timing of inbreeding depression in Scots pine (Pinus sylvestris L.) Evolution. 1999;53:758–768. doi: 10.1111/j.1558-5646.1999.tb05370.x. [DOI] [PubMed] [Google Scholar]
- Krafft B. Organisation et evolution des sociétés d'araignées. Psychology. 1979;1:23–51. [Google Scholar]
- Kraus O, Kraus M. The genus Stegodyphus: systematics, biogeography, and sociality (Araneida, Eresidae) Acta Zool. Fenn. 1989;190:223–228. [Google Scholar]
- Matsumoto T. Male emergence timing and mating success in the funnel-web spider, Agelena limbata (Araneae: Agelenidae) J. Ethol. 1994;9:1–7. [Google Scholar]
- Meagher S, Penn D.J, Potts W.K. Male–male competition magnifies inbreeding depression in wild house mice. Proc. Natl Acad. Sci. USA. 2000;97:3324–3329. doi: 10.1073/pnas.060284797. 10.1073/pnas.060284797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers K.S, Avilés L. Natal dispersal patterns of a subsocial spider Anelosimus cf. jucundus (Theridiidae) Ethology. 2003;109:725–737. 10.1046/j.1439-0310.2003.00918.x [Google Scholar]
- Pusey A, Wolf M. Inbreeding avoidance in animals. Trends Ecol. Evol. 1996;11:201–206. doi: 10.1016/0169-5347(96)10028-8. 10.1016/0169-5347(96)10028-8 [DOI] [PubMed] [Google Scholar]
- Riechert S.E, Roeloffs R.M. Evidence for and consequences of inbreeding in the cooperative spiders. In: Thornhill N.W, editor. The natural history of inbreeding and outbreeding. The University of Chicago Press; Chicago, IL: 1993. pp. 283–303. [Google Scholar]
- Sherman P.W, Jarvis J.U.M, Alexander R.D, editors. The biology of the naked mole-rat. Monographs in behavior and ecology. Princeton University Press; Princeton, NJ: 1991. [Google Scholar]
- Wickler W, Seibt U. Pedogenetic sociogenesis via the “Sibling-route” and some consequences for Stegodyphus spiders. Ethology. 1993;95:1–18. [Google Scholar]
- Wolfe L.M. Inbreeding depression in Hydrophyllum appendiculatum: role of maternal effects, crowding, and parental mating history. Evolution. 1993;47:374–386. doi: 10.1111/j.1558-5646.1993.tb02100.x. [DOI] [PubMed] [Google Scholar]