Abstract
Several species use the number of young produced as public information (PI) to assess breeding site quality. PI is inaccessible for synchronously breeding birds because nests are empty by the time the young can collect this information. We investigate if location cues are the next best source of inadvertent social information (ISI) used by young prospectors during breeding site choice. We experimentally deployed ISI as decoys and song playbacks of breeding males in suitable and sub-optimal habitats during pre- and post-breeding periods, and monitored territory establishment during the subsequent breeding season for a social, bobolink (Dolichonyx oryzivorus), and a more solitary species, Nelson's sharp-tailed sparrow (Ammodramus nelsoni). The sparrows did not respond to treatments, but bobolinks responded strongly to post-breeding location cues, irrespective of habitat quality. The following year, 17/20 sub-optimal plots to which bobolink males were recruited were defended for at least two weeks, indicating that song heard the previous year could exert a ‘carry-over attraction’ effect on conspecifics the following year. Sixteen recruited males were natal dispersers, as expected when animals have little opportunity to directly sample their natal habitat quality. We suggest that differences in breeding synchronicity may induce an equivalent clinal distribution of ISI use.
Keywords: conspecific attraction, dispersal, habitat selection, prospecting, public information, inadvertent social information
1. Introduction
Animals are rarely uniformly distributed (Sutherland 1983; Stamps 1988; Reed & Dobson 1993); they aggregate in patterns sometimes different from the clumpiness of their resources (Shields et al. 1988; Greene & Stamps 2001; Tarof & Ratcliffe 2004). The way distribution patterns arise have been the subject of theories such as isoleg models (Rosenzweig 1981; Abramsky et al. 1991), species assembly models (Fox 1987; Brown et al. 2000a) and a family of ‘ideal’ distribution models: ideal free (Fretwell & Lucas 1970), ideal despotic (Fretwell 1972), ideal pre-emptive (Pulliam 1988) and ideal interference (Sutherland 1983; Lessells 1995) that are perhaps the best known among site-dependent theories (Rodenhouse et al. 2003).
Behavioural studies of habitat selection are often based on ideal free-type models and offer animals alternatives at a spatial scale that corresponds more to choice among resource clumps than habitats (review in Giraldeau & Caraco 2000). Although the studies generally support ideal free models, quantitative discrepancies between predictions and observations are common. The mechanisms invoked to account for these discrepancies, such as asymmetries in priority of access to a point food source (Harper 1982), the need for individuals to sample alternative resource clumps (Milinski 1984) and the switching between clumps that ensues (Regelmann 1984) easily apply at small spatial scales but are unlikely to account for discrepancies at large spatial scales such as choice of breeding habitats (Stamps 2001; Shochat et al. 2002). The behavioural mechanisms that could account for such large-scale discrepancies remain relatively unexplored because studies conducted at large spatial scales, generally, ignore behaviour altogether and focus instead on broad environmental correlates of animal distributions. Here, we explore behavioural processes that may account for the large-scale distribution patterns of breeding birds among habitats.
When habitats vary in profitability, the quality of an animal's choice depends on the completeness and accuracy of the information available. With perfect information, animals can always select the best option, but without any information, one guess is as good as another. Information about a habitat's quality can come from an individual's own sampling of alternatives (e.g. searching for structural cues; Smith & Shugart 1987) or from cues derived from the behaviour or presence of other animals: inadvertent social information (ISI; Danchin et al. 2004). At large spatial scales, sampling all the alternatives to obtain personal information may become costly (Boulinier & Danchin 1997), making ISI a more profitable source of information (Forbes & Kaiser 1994; Valone & Benkman 1999; Danchin et al. 2001).
ISI can provide either ‘public information’ (PI; Valone 1989; Danchin et al. 2004), a performance-based type of information that provides an indication of local resource quality, or ‘location cues’ that indicate a resource is present (Danchin et al. 2004). Either type of ISI is easily acquired but provides varying levels of reliability (Clark & Mangel 1984; Mangel 1990). The use of ISI has been reported in selection of novel food (Galef & Allen 1995), patch quality assessment (Templeton & Giraldeau 1995, 1996), and daily breeding territory location (Warner 1988, 1990). It has also been reported in the selection of breeding sites or colonies in several bird groups, e.g. raptors (Serrano & Tella 2003; Alonso et al. 2004; Serrano et al. 2004), swallows (Shields et al. 1988; Brown et al. 2000b) and colonial seabirds (Cadiou et al. 1994; Forbes & Kaiser 1994; Boulinier et al. 1996; Danchin et al. 1998; Danchin & Cam 2001). One of the most detailed studies of PI use in avian breeding aggregations involves the collared flycatcher (Ficedula albicollis), a socially breeding land bird in which non-breeding or failed-breeding adult birds use conspecifics' reproductive success in their future settlement decisions (Doligez et al. 1999, 2002, 2003, 2004; Pärt & Doligez 2003). PI, however, is not always available at breeding group locations. In highly synchronous breeding species, for instance, by the time juveniles are ready to prospect and gather PI from other nests, most of them are empty and provide little information. In such cases, location cues may provide the next best form of ISI for immature birds. Adult bobolinks (Dolichonyx oryzivorus), for example, can use local reproductive success (PI) in site selection and deciding whether to return to those sites in subsequent seasons (Bollinger & Gavin 1989). However, because they are such synchronous breeders, the question remains whether the breeding site selection mechanism of natal dispersers involves the use of location cues. Here, we investigate the use of location cues in the breeding site choice of first-time breeding animals in two species of synchronously breeding grassland birds with common resource requirements but different social systems, the bobolink and Nelson's sharp-tailed sparrow (Ammodramus nelsoni subvirgatus). We examine habitat from the standpoint of important vegetative features and provide false ISI about the quality of an otherwise sub-optimal breeding habitat. We then determine whether settlement decisions were subsequently affected by the false information on habitat quality and which class of bird is most likely to be affected by it.
2. Material and methods
(a) Study species
Bobolinks are polygynous and gregarious and, in the agricultural environment in which we studied them, they arrive in large groups by mid-May, complete their nests in early June and fledge their young three weeks later (Nocera et al. 2005). The full breeding cycle, therefore, is accomplished in just six weeks or less and by late July, they initiate their southern migration.
Nelson's sharp-tailed sparrows are polygynandrous breeders. They are less social than the bobolink and males arrive at the breeding grounds singly or in small groups in early June. Females arrive more than a week later, initiate their nests in mid-June and fledging typically begins in mid-July (Nocera et al. 2005). Nelson's sharp-tailed sparrows depart on their southern migration in September (5–6 weeks later than bobolinks).
(b) Study sites
Our study was conducted in the western Annapolis Valley of Nova Scotia, Canada (44°45′N, 65°31′W) at four different agricultural sites: Belleisle Marsh Wildlife Management Area (hereafter ‘Belleisle’; 210 ha), Upper Belleisle (116 ha), Queen Anne (180 ha) and Pea Round (142 ha). Hayfields at Queen Anne (totalling 84 ha), Belleisle (77 ha) and Upper Belleisle (43 ha) are mixtures of timothy (Phleum pratense L.), meadow fox-tail (Alopecurus pratensis L.), bluegrass (Poa spp.) and reed canary grass (Phalaris arundinacea L.). The same grass mixtures are planted across Pea Round, with the addition of several homogenous swards of alfalfa (Medicago sativa L.). Several fields at Queen Anne, Belleisle, and Upper Belleisle are fallow (96, 82 and 40 ha, respectively) and offer ‘rough cover’ vegetation that does not support breeding of either of our study species in this, or other, regions (Wittenberger 1980; Greenlaw & Rising 1995). Rough cover is dominated by goldenrods (Solidago spp.), meadowsweet (Spirea latifolia W. Aiton), sedges (Carex spp.), elders (Sambucus canadensis L. and S. pubens Michx.), wild rose (Rosa virginiana Mill.) and young birch trees (Betula papyrifera Marsh.).
(c) Baseline sampling
To identify individuals for future re-observations, and determine their age, individual birds were captured primarily in May and June using 36 mm nylon mesh mist-nets, and banded with a numeric metal United States Fish and Wildlife Service leg band and a unique combination of coloured celluloid bands. Plumage criteria for ageing bobolinks in-hand (Pyle 1997; Nocera 2005) determined if a bird was hatch-year (fledgling), second-year, probable-second-year, or after-second-year. No definitive plumage criteria exist for ageing Nelson's sharp-tailed sparrow beyond hatch year and after-hatch-year.
We established 52 sampling plots in hayfields across our four study sites. The plots were randomly located in each study site, with a minimum 200 m separation between plots. Belleisle had 22 plots, Queen Anne had 12, Pea Round had 10, and Upper Belleisle had eight.
We conducted point counts (Hutto et al. 1986) in these plots to determine whether a plot had a territory owner, how many females were paired to that male, and the number of fledglings produced. We sampled birds at these plots 10–12 times per season (29 May–8 August 2002–2004) between 30 min after sunrise and 10.00 AST (only if winds were less than 25 kph, with no precipitation) by counting all individuals seen or heard within a 50 m radius from the observer for a 5 min period. Whenever possible, all visually censused birds were coarsely classified as either fledgling or adult based on appearance (finer measurements are only possible when birds are in-hand). They were also sexed either by appearance (bobolinks are sexually dichromatic) or by song (only males sing in both species). During all observations, individuals were closely followed to avoid double counting. There was no substantial local population increase of either focal species during our study.
We sampled in rough cover with spotting scopes less frequently (minimum once a week) because neither study species maintained territories in that vegetation type (Nocera et al. 2005; Nocera unpublished data).
To define territory boundaries, we mapped the location, identity and date for any colour-banded bird that was re-observed. Resighting was initiated in early June each year. Re-observation sessions were conducted daily (weather permitting) for greater than 2 h on a rotation of sites and observers.
(d) ISI location cue experiments
ISI experiments consist of first providing location cues after the breeding season one year and observing its effect on settlement the next year. We randomly chose 19 (of the 52) point count plots in the hayfields for the ISI experiments during the post-breeding period (roughly the first three weeks of July) of both 2002 and 2003. Each plot was known to be occupied (held a territory owner; n=9) or vacant (without a territory owner; n=10) that year (table 1). The numbers of vacant and occupied plots were similar for both study species (table 1). We used the remaining vacant plots as controls. Another 19 plots were chosen in rough cover all of which were vacant for both species. We chose 12 of these plots (table 1) to receive experimental treatment (location cues) and the seven others served as controls.
Table 1.
Sample size summary of vacant plots used in ISI experiments and treatment response in subsequent breeding season. (Experimental plots with no territory holder in previous breeding season (i.e. vacant) are followed (in parentheses) by the number of those plots adopted as territories in breeding season after treatment. Grouped by study species, period of treatment (pre-breeding, late-May/early June; post-breeding, late July), and habitat type (hayfield, typical habitat; rough cover, sub-optimal habitat; controls, from either habitat but received no treatment).)
| bobolink | Nelson's sharp-tailed sparrow | |||
|---|---|---|---|---|
| pre-breeding | post-breeding | pre-breeding | post-breeding | |
| hayfield | 11 (1) | 10 (9) | 11 (1) | 10 (0) |
| rough cover | 9 (1) | 12 (11) | 9 (2) | 12 (4) |
| all controls | 26 (2) | 21 (2) | 23 (3) | 20 (1) |
Location cues were provided by simultaneously deploying a visual model and audio playbacks for 1 h while an observer noted behaviour from a vantage point beyond 50 m from the plot centre. The model consisted of a male museum specimen in breeding plumage placed on a stick taller than the surrounding vegetation in the plot centre. The playbacks were done using a portable compact disc player playing both male songs and contact calls of the model species being presented. Each day during a given treatment period, observers randomly chose plots to receive a trial and the species to be tested. Six trials were completed in a typical day (weather permitting: wind less than 25 kph, no precipitation). An experimental plot was usually tested 5–6 times for each species per period.
A different set of 19 point count plots were then used for experiments in the hayfields during the next year's pre-breeding period, in both 2003 and 2004. Pre-breeding period sampling (last two weeks of May, and first week of June) was initiated immediately after the arrival of male bobolinks and concurrent with arrival of male Nelson's sharp-tailed sparrows. Eleven of these 19 plots were vacant (table 1), and eight were occupied. We also established 14 new vacant plots in rough cover, nine of which were treatments (table 1) and five were controls. Response to all treatments and controls consisted of noting whether a territorial male had settled a plot (for at least two weeks) that was unoccupied the previous breeding season. All newly established settlers were scanned for colour bands to detect any within-site movement of known individuals.
(e) Statistical analyses
We modelled the binary response of settled/not settled following treatment using logistic regression (Sokal & Rohlf 1995, pp. 767–778). Main effect variables included were habitat type (hayfield versus rough cover), breeding period (pre- or post-breeding) and year (to assess inter-annual effects). We also included an interaction term between habitat type and breeding period to account for different stages of vegetative growth at those periods. Model variable importance was initially assessed using z-tests and we then used analysis of deviance (implementing an α-level of 0.05) to justify model reductions (Dalgaard 2002). We selected the best-fit model as that with the lowest Mallow's Cp statistic (Mallow 1973). All statistical tests were conducted using R v. 1.8.1 (R Development Core Team 2003).
3. Results
One or more individuals were observed during experiments in each plot at least once during each treatment period. All plots occupied in year one also were occupied in year two and so we consider only the fate of vacant plots (table 1).
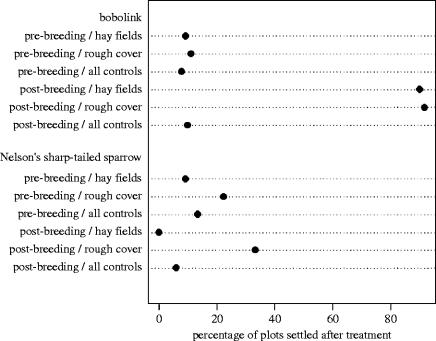
The best-fit model for Nelson's sharp-tailed sparrow settlement pattern retained only habitat type (z=0.81, p=0.03, Cp=41.8 versus intercept-only Cp=44.1); sparrow occupancy seemed to be most associated with presence of rough cover (figure 1), but this association is weak (i.e. ΔCp was only 2.3). Presence of Nelson's sharp-tailed sparrow showed no response (figure 1) to remaining predictors: breeding period (z=−0.24, p=0.93), year (z=0.21, p=0.82), and the interaction between habitat type and breeding period (z=0.27, p=0.20). The absence of a breeding period effect indicates that settlement rates were similar in experimental and control plots (figure 1). Although the period during which we supplied location cues was not a statistically significant predictor of Nelson's sharp-tailed sparrow settlement decision, four sparrows that settled on plots treated with location cues the previous year (table 1, figure 1) remained there for the entire breeding season.
Figure 1.
Effect of inadvertent social information experiments on settlement decisions in bobolink and Nelson's sharp-tailed sparrow. Response is shown as the percentage of trial plots with no previous territory holder (vacant) that were settled following treatments in pre- and post-breeding periods in two habitat types (hayfield, rough cover). Response in control plots per period trial type, with habitat types combined, is also presented. Significant responses are seen between pre- and post-breeding trials for bobolink (z=2.26, p=<0.0001, Cp=18.6 versus intercept-only Cp=44.05) and between habitat types for Nelson's sharp-tailed sparrow (z=0.81, p=0.03, Cp=41.8 versus intercept-only Cp=44.1).
The best-fit model for bobolink distributions retained only breeding period (z=2.26, p=<0.0001, Cp=18.6 versus intercept-only Cp=44.05), reflecting a strong treatment effect (figure 1). The presence of location cues resulted in only two vacant trial plots (10%) being settled the same year the treatment was applied, similar to the 7.8% settlement rate observed in control plots (both habitat types combined). The response to location cue, however, was significantly stronger in the next breeding season; 20 of 22 (91%) vacant plots harboured a territory owner the summer following treatment compared to the 9.9% settlement in control plots (table 1). Bobolink settlement responses were not statistically affected by predictive variables that were subsequently eliminated from the model set: habitat type (z=0.13, p=0.92), year (z=−0.28, p=0.78), and the interaction between habitat type and breeding period (z=−0.006, p=0.99).
Naive natal dispersers (second-year or probable-second-year birds) constituted 19 of 20 (95%) of the bobolinks that set up territories the year after treatment with location cues. There was one instance of a mature (an after-second-year) bird taking on a territory after treatment. Of the 20 bobolinks that set up territories, 17 abandoned them after two weeks and became floaters, the three others defended their territory beyond these two weeks, and in each case (two in rough cover, one in hayfield), a female was also present. We could not confirm breeding for any of these pairs, but no fledglings were observed in these areas until well into the post-breeding period of social flocking during early August.
4. Discussion
Our results show that the settlement decision of first-time breeders of a synchronously breeding species is influenced by location cues. Earlier studies have also shown that birds prospect for social information, typically at the end of a breeding season, when they can obtain PI from the fledging success of various nests within a site or colony (Smith 1978; Bollinger & Gavin 1989; Reed & Oring 1992; Cadiou et al. 1994; Boulinier et al. 1996; Boulinier & Danchin 1997; Danchin et al. 1998; Doligez et al. 2004). Our study, however, indicates that when this type of PI is inaccessible as a consequence of highly synchronous breeding; young birds can rely on the only ISI available to them: location cues. Young bobolinks prospect for next years' breeding sites in the period between the end of breeding and migration because only location cues that were provided during that time affected an individual's settlement decision (Boulinier & Danchin 1997; Danchin et al. 1998). Location cues provided in the period between spring arrival and the beginning of territory establishment had little effect on settlement decisions. This difference between cues obtained the previous year and those present just before breeding is not surprising given that location cues may vary according to the time of year. Cues from the previous year may indicate good quality habitat available for the following year, whereas location cues obtained prior to breeding may indicate the presence of a settled competitor that would be difficult to displace. Future studies could test this by providing false location cues during both treatment periods for some plots, and only during the post-breeding period for others. Birds will return to settle plots where ISI was observed in the post-breeding period, but if location cues are observed in the same plots during the spring arrival period the following year, birds should settle plots that received only post-breeding ISI and not settle those that received ISI in both periods.
The effect of location cues we report here may appear superficially similar to several experiments showing that animals are attracted to model conspecifics: conspecific attraction. However, evidence of conspecific attraction is usually limited to situations where a model of some sort exerts attraction on animals while the model is present. Our study goes beyond the usual results by showing that the models and playbacks attracted individuals to sites several months after they had been detected, perhaps a form of long-lived stimulus-independent conspecific attraction.
Nordell & Valone (1998) predict that PI would most likely be useful to young, inexperienced individuals, especially when sampling information is costly to obtain personally. Our results suggest that the same argument can extend to the other form of ISI, location cues. We found that it was the young first-breeding male bobolinks that were most influenced by our false location cues; females and mature males were not. Females and mature males were probably using other sources of information, perhaps even PI, as suggested by Bollinger & Gavin (1989). Obtaining personal information of a breeding habitat's quality can be costly; young settlers required more than 2 weeks to overcome the false territory value provided by our location cues.
The importance of ISI in the settlement decision of new breeders is illustrated by the fact that our decoys and playbacks were sufficient to lead individuals many months later to defend territories in what was clearly a sub-optimal environment for this species. Our study plots designated as rough cover contained little of the structure usually present in bobolink territories (Wittenberger 1980). That we induced territory formation even in these sub-optimal sites suggests that choice of territorial location in these birds can have a strong cultural/traditional component (sensu Warner 1988, 1990; Danchin et al. 2004) and that it may even be subject to the dangers of informational cascades (Giraldeau et al. 2002).
Our results raise the question of the importance of tradition in many situations of habitat selection. Clearly, in the case of bobolinks we were not successful in initiating traditions as most birds tricked onto sub-optimal habitats switched to adopt a floater status after two weeks. However, this is not surprising given the extreme low quality of the habitats we selected to attract new settlers. Therefore, the perpetuation of a settlement tradition likely requires some minimum measure of adequate resources (e.g. mates, food, cover) beyond which traditions are unlikely to be maintained. It would be important for future work to investigate whether location cues can lead to the creation of sub-optimal settlement decisions (e.g. propagating an informational cascade). If such traditions exist, they carry an important conservation message and may provide an explanation for why established theoretical models often fail to predict species occurrence at large spatial scales.
If breeding synchrony is really the constraint that forces young birds to use location cues instead of more reliable PI, then we predict that ecological factors that favour synchronous breeding will also favour use of location cues over PI. This hypothesis could be tested at multiple scales and borne out across numerous systems that show different breeding synchrony levels. For instance, at a relatively small-scale, many frog species show higher synchrony when they breed in ponds and less synchrony when they breed in rivers (Vences et al. 2002). At a larger spatial and taxonomic scale, all pinnipeds are strikingly synchronous in breeding across their collective familial range except for the Australian sea lion (Neophoca cinerea) that exhibits unique asynchronous breeding both between and within colonies (Gales et al. 1994). Perhaps the broadest taxonomic and spatial scales that could be used to test our hypothesis would involve avian migration systems, where breeding synchrony tends to increase with latitude (Baker 1938; Spottiswoode & Møller 2004), usually because of increasingly short breeding seasons. If we are correct, then we predict that synchronously breeding species such as pond-breeding frogs, most pinnipeds, and high latitude migratory birds will be more likely not to use more reliable PI when choosing where to settle and will confine their use of ISI to location cues that are more open to error. Alternatively, animals with longer breeding seasons such as riverine frogs, the Australian sea lion, and equatorial birds may benefit from easier access to more reliable PI, but might also pay greater ecological costs. For instance, tropical birds that can breed several times per year run the risk of mismatching timing of breeding with local productivity when resources are seasonal (Wrege & Emlen 1991). As a consequence, nestling survival and recruitment might, therefore, be reduced (Smith 2004). Testing these predictions of clines in ISI and breeding synchronicity could generate challenging and rewarding future research regarding the evolution and adaptation of information use.
Acknowledgments
Our appreciation is extended to those who assisted with fieldwork: T. Fitzgerald, W. Fitzgerald, S. Glinz, C. Lawrie, S. LeMoine, Y. Luc, M. Peckford, J. Pennell, L. Penney, F. Rousseu and E. Whidden. We also thank the Annapolis Valley farmers who cooperated and/or supported this and companion studies. M. Betts, A. Breton, T. Fitzgerald, and N. Simon provided valuable comments on early manuscript drafts. The manuscript's final version was enhanced by critiques from W. Hill and three anonymous referees. JJN was supported by a Wildlife Habitat Canada doctoral scholarship (no. 2.37A.3S). We gratefully acknowledge financial and logistical assistance from the NS Habitat Conservation Fund, EJLB Foundation, Canadian Wildlife Federation, NS Dept. Natural Resources, Canadian Wildlife Service, and the Groupe de recherché en écologie comportementale et animale of the Université du Québec à Montréal.
References
- Abramsky Z, Rosenzweig M.L, Pinshow B. The shape of a gerbil isocline: an experimental field study using principles of optimal habitat selection. Ecology. 1991;72:329–340. [Google Scholar]
- Alonso J.C, Martín C.A, Alonso J.A, Palacín C, Magaña M, Lane S.J. Distribution dynamics of a great bustard metapopulation throughout a decade: Influence of conspecific attraction and recruitment. Biodivers. Conserv. 2004;13:1659–1674. doi:10.1023/B:BIOC.0000029329.44373.47 [Google Scholar]
- Baker J.R. The relation between latitude and breeding season in birds. Proc. Zool. Soc. Lond. A. 1938;108:557–582. [Google Scholar]
- Bollinger E.K, Gavin T.A. The effects of site quality on breeding-site fidelity in Bobolinks. Auk. 1989;106:584–594. [Google Scholar]
- Boulinier T, Danchin E. The use of conspecific reproductive success for breeding patch selection in terrestrial migratory species. Evol. Ecol. 1997;11:505–517. doi:10.1007/s10682-997-1507-0 [Google Scholar]
- Boulinier T, Danchin E, Monnat J.-Y, Doutrelant C, Cadiou B. Timing of prospecting and the value of information in a colonial breeding bird. J. Avian Biol. 1996;27:252–256. [Google Scholar]
- Brown J.H, Fox B.J, Kelt D.A. Assembly rules: desert rodent communities are structured at scales from local to continental. Am. Nat. 2000a;156:314–321. doi: 10.1086/303385. doi:10.1086/303385 [DOI] [PubMed] [Google Scholar]
- Brown C.R, Brown M.B, Danchin E. Breeding habitat selection in cliff swallows: The effect of conspecific reproductive success on colony choice. J. Anim. Ecol. 2000b;69:133–142. doi:10.1046/j.1365-2656.2000.00382.x [Google Scholar]
- Cadiou B, Monnat J.-Y, Danchin E. Prospecting in the kittiwake, Rissa tridactyla: different behavioural patterns and the role of prospecting in recruitment. Anim. Behav. 1994;47:847–856. doi:10.1006/anbe.1994.1116 [Google Scholar]
- Clark C.W, Mangel M. Foraging and flocking strategies: information in an uncertain environment. Am. Nat. 1984;123:626–641. doi:10.1086/284228 [Google Scholar]
- Dalgaard P. Springer; Berlin: 2002. Introductory statistics with R. [Google Scholar]
- Danchin E, Cam E. Can non-breeding be a cost of breeding dispersal? Behav. Ecol. Sociobiol. 2001;51:153–163. doi:10.1007/s00265-001-0423-5 [Google Scholar]
- Danchin E, Boulinier T, Massot M. Conspecific reproductive success and breeding habitat selection: implications for the study of coloniality. Ecology. 1998;79:2415–2428. [Google Scholar]
- Danchin E, Heg D, Doligez B. Public information and breeding habitat selection. In: Clobert J, Danchin E, Dhondt A.A, Nichols J, editors. Dispersal. Oxford University Press; Oxford: 2001. pp. 243–258. [Google Scholar]
- Danchin E, Giraldeau L.-A, Valone T.J, Wagner R.H. Public information: from nosy neighbors to cultural evolution. Science. 2004;305:784–491. doi: 10.1126/science.1098254. doi:10.1126/science.1098254 [DOI] [PubMed] [Google Scholar]
- Doligez B, Danchin E, Clobert J, Gustafsson L. The use of conspecific reproductive success for breeding habitat selection in a non-colonial, hole-nesting species, the collared flycatcher. J. Anim. Ecol. 1999;68:1193–1206. doi:10.1046/j.1365-2656.1999.00362.x [Google Scholar]
- Doligez B, Danchin E, Clobert J. Public information and breeding habitat selection in a wild bird population. Science. 2002;297:1168–1170. doi: 10.1126/science.1072838. doi:10.1126/science.1072838 [DOI] [PubMed] [Google Scholar]
- Doligez B, Cadet C, Danchin E, Boulinier T. When to use public information for breeding habitat selection? The role of environmental predictability and density dependence. Anim. Behav. 2003;66:973–988. doi:10.1006/anbe.2002.2270 [Google Scholar]
- Doligez B, Pärt T, Danchin E. Prospecting in the collared flycatcher: gathering public information for future breeding habitat selection? Anim. Behav. 2004;67:457–466. doi:10.1016/j.anbehav.2003.03.010 [Google Scholar]
- Forbes L.S, Kaiser G.W. Habitat choice in breeding seabirds: when to cross the information barrier. Oikos. 1994;70:377–384. [Google Scholar]
- Fox B.J. Species assembly and the evolution of community structure. Evol. Ecol. 1987;1:201–213. doi:10.1007/BF02067551 [Google Scholar]
- Fretwell S.D. Princeton University Press; Princeton, NJ: 1972. Populations in a seasonal environment. [PubMed] [Google Scholar]
- Fretwell S.D, Lucas H.L. On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor. 1970;19:16–36. doi:10.1007/BF01601953 [Google Scholar]
- Galef B.G, Allen C. A new model system for studying behavioural traditions in animals. Anim. Behav. 1995;50:705–717. doi:10.1016/0003-3472(95)80131-6 [Google Scholar]
- Gales N.J, Shaughnessy P.D, Dennis T.E. Distribution, abundance and breeding cycle of the Australian sea lion Neophoca cinerea (Mammalia, Pinnipedia) J. Zool. 1994;234:353–370. [Google Scholar]
- Giraldeau L.-A, Caraco T. Princeton University Press; Princeton, NJ: 2000. Social Foraging Theory. [Google Scholar]
- Giraldeau L.-A, Valone T.J, Templeton J.J. Potential disadvantages of using socially acquired information. Phil. Trans. R. Soc. B. 2002;357:1559–1566. doi: 10.1098/rstb.2002.1065. doi:10.1098/rstb.2002.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene C.M, Stamps J.A. Habitat selection at low population densities. Ecology. 2001;82:2091–2100. [Google Scholar]
- Greenlaw J.S, Rising J.D. Sharp-tailed sparrow. In: Poole A, Gill F, editors. The birds of North America, no. 112. The Academy of Natural Sciences; Washington, DC: The American Ornithologists' Union; Philadelphia: 1995. [Google Scholar]
- Harper D.G. C. Competitive foraging in mallards: ‘ideal free’ ducks. Anim. Behav. 1982;30:575–584. [Google Scholar]
- Hutto R.L, Pletschet S.M, Hendricks P. A fixed-radius point count method for nonbreeding and breeding season use. Auk. 1986;103:593–602. [Google Scholar]
- Lessells C.M. Putting resource dynamics into continuous input ideal free distribution models. Anim. Behav. 1995;49:487–494. doi:10.1006/anbe.1995.0063 [Google Scholar]
- Mallow C.L. Some comments on Cp. Technometrics. 1973;12:591–612. [Google Scholar]
- Mangel M. Dynamic information in uncertain and changing worlds. J. Theor. Biol. 1990;146:317–332. doi: 10.1016/s0022-5193(05)80742-8. [DOI] [PubMed] [Google Scholar]
- Milinski M. Competitive resource sharing: an experimental test of a learning rule for ESSs. Anim. Behav. 1984;32:233–242. [Google Scholar]
- Nocera J.J. A method to improve age determination of male Bobolinks in alternate plumage. N. Am. Bird Bander. 2005;30:1–5. [Google Scholar]
- Nocera J.J, Parsons G.J, Milton G.R, Fredeen A.H. Compatibility of delayed cutting regime with bird breeding and hay nutritional quality. Agr. Ecosyst. Environ. 2005;107:245–253. doi:10.1016/j.agee.2004.11.001 [Google Scholar]
- Nordell S.E, Valone T.J. Mate choice copying as public information. Ecol. Lett. 1998;1:74–76. doi:10.1046/j.1461-0248.1998.00025.x [Google Scholar]
- Pärt T, Doligez B. Gathering public information for habitat selection: prospecting birds cue on parental activity. Proc. R. Soc. B. 2003;270:1809–1813. doi: 10.1098/rspb.2003.2419. doi:10.1098/rspb.2003.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam H.R. Sources, sinks, and population regulation. Am. Nat. 1988;132:652–661. doi:10.1086/284880 [Google Scholar]
- Pyle P. Slate Creek Press; Bolinas, CA: 1997. Identification guide to North American Birds, Part 1. [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2003. A language environment for statistical computing. [Google Scholar]
- Reed J.M, Dobson A. Behavioral constraints and conservation biology: conspecific attraction and recruitment. Trends Ecol. Evol. 1993;8:253–256. doi: 10.1016/0169-5347(93)90201-Y. doi:10.1016/0169-5347(93)90201-Y [DOI] [PubMed] [Google Scholar]
- Reed J.M, Oring L.W. Reconnaissance for future breeding sites by spotted sandpipers. Behav. Ecol. 1992;3:310–317. [Google Scholar]
- Regelmann K. Competitive resource sharing: a simulation model. Anim. Behav. 1984;32:226–232. [Google Scholar]
- Rodenhouse N.L, Sillett T.S, Doran P.J, Holmes R.T. Multiple density-dependence mechanisms regulate a migratory bird population during the breeding season. Proc. R. Soc. B. 2003;270:2105–2110. doi: 10.1098/rspb.2003.2438. doi:10.1098/rspb.2003.2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig M.L. A theory of habitat selection. Ecology. 1981;62:327–335. [Google Scholar]
- Serrano D, Tella J.L. Dispersal within a spatially structured population of lesser kestrels: the role of spatial isolation and conspecific attraction. J. Anim. Ecol. 2003;72:400–410. doi:10.1046/j.1365-2656.2003.00707.x [Google Scholar]
- Serrano D, Forero M.G, Donázar J.A, Tella J.L. Dispersal and social attraction affect colony selection and dynamics in lesser kestrels. Ecology. 2004;85:3438–3447. [Google Scholar]
- Shields W.M, Crook J.R, Hebblethwaite M.L, Wiles-Ehmann S.S. Ideal free coloniality in the swallows. In: Slobodchikoff C.N, editor. The ecology of social behavior. Academic Press; San Diego, CA: 1988. pp. 189–228. [Google Scholar]
- Shochat E, Abramsky Z, Pinshow B. Density-dependent habitat selection in migratory passerines during stopover: what causes the deviation from IFD? Evol. Ecol. 2002;16:469–488. doi:10.1023/A:1020851801732 [Google Scholar]
- Smith S.M. The ‘underworld’ in a territorial sparrow: adaptive strategies for floaters. Am. Nat. 1978;112:571–582. doi:10.1086/283298 [Google Scholar]
- Smith H.G. Selection for synchronous breeding in the European starling. Oikos. 2004;105:301–311. doi:10.1111/j.0030-1299.2004.10543.x [Google Scholar]
- Smith T.M, Shugart H.H. Territory size variation in the ovenbird: the role of habitat structure. Ecology. 1987;68:695–704. [Google Scholar]
- Sokal R.R, Rohlf F.J. W.H. Freeman and co.; San Francisco, CA: 1995. Biometry. [Google Scholar]
- Spottiswoode C, Møller A.P. Extrapair paternity, migration, and breeding synchrony in birds. Behav. Ecol. 2004;15:41–57. doi:10.1093/beheco/arg100 [Google Scholar]
- Stamps J. Conspecific attraction and aggregation in territorial species. Am. Nat. 1988;131:329–347. doi:10.1086/284793 [Google Scholar]
- Stamps J. Habitat selection by dispersers: integrating proximate and ultimate approaches. In: Clobert J, Danchin E, Dhondt A.A, Nichols J, editors. Dispersal. Oxford University Press; Oxford: 2001. pp. 230–242. [Google Scholar]
- Sutherland W.J. Aggregation and the ideal-free distribution. J. Anim. Ecol. 1983;52:821–828. [Google Scholar]
- Tarof S.A, Ratcliffe L.M. Habitat characteristics and nest predation do not explain clustered breeding in Least Flycatchers (Empidonax minimus) Auk. 2004;121:877–893. [Google Scholar]
- Templeton J.J, Giraldeau L.-A. Patch assessment of foraging flocks of European starlings: evidence for the use of public information. Behav. Ecol. 1995;6:65–72. [Google Scholar]
- Templeton J.J, Giraldeau L.-A. Vicarious sampling: the use of personal and public information by starlings foraging in a simple patchy environment. Behav. Ecol. Sociobiol. 1996;38:105–114. doi:10.1007/s002650050223 [Google Scholar]
- Valone T.J. Group foraging, public information, and patch estimation. Oikos. 1989;56:357–363. [Google Scholar]
- Valone T.J, Benkman C.W. Public information as a mechanism favouring social aggregation: A brief review of empirical evidence. In: Adams N.J, Slotow R.H, editors. Proc. 22 Int. Ornithol. Congr., Durban. BirdLife South Africa; Johannesburg: 1999. pp. 1328–1336. [Google Scholar]
- Vences M, Andreone F, Glaw F, Kosuch J, Meyer A, Schaefer H.C, Veith M. Exploring the potential of life-history key innovation: brook breeding in the radiation of the Malagasy treefrog genus Boophis. Mol. Ecol. 2002;11:1453–1463. doi: 10.1046/j.1365-294x.2002.01543.x. doi:10.1046/j.1365-294X.2002.01543.x [DOI] [PubMed] [Google Scholar]
- Warner R.R. Traditionality of mating-site preferences in a coral reef fish. Nature. 1988;335:719–721. doi:10.1038/335719a0 [Google Scholar]
- Warner R.R. Resource assessment vs. traditionality in mating site determination. Am. Nat. 1990;135:205–217. doi:10.1086/285039 [Google Scholar]
- Wittenberger J.F. Vegetation structure, food supply, and polygyny in bobolinks (Dolichonyx oryzivorus) Ecology. 1980;61:140–150. [Google Scholar]
- Wrege P.H, Emlen S.T. Breeding seasonality and reproductive success of white-fronted bee-eaters in Kenya. Auk. 1991;108:673–678. [Google Scholar]