Abstract
Because of differential investment in gametes between sexes, females tend to be the more selective sex. Based on this concept, we investigate mate selection in a large carnivore: the brown bear (Ursus arctos). We hypothesize that, in this species with sexually selected infanticide (SSI), females may be faced with a dilemma: either select a high-quality partner based on phenotypic criteria, as suggested by theories of mate choice, or rather mate with future potentially infanticidal males as a counter-strategy to SSI. We evaluated which male characteristics were important in paternity assignment. Among males available in the vicinity of the females, the largest, most heterozygous and less inbred and also the geographically closest males were more often the fathers of the female's next litter. We suggest that female brown bears may select the closest males as a counter-strategy to infanticide and exercise a post-copulatory cryptic choice, based on physical attributes, such as a large body size, reflecting male genetic quality. However, male–male competition either in the form of fighting before copulation or during the post-copulatory phase, in the form of sperm competition, cannot entirely be ruled out.
Keywords: female choice, infanticide, mating system, microsatellites, parentage analysis, Ursus arctos
1. Introduction
Mate selection is defined as the process leading to the tendency of members of one sex to mate non-randomly with respect to one or more varying traits in members of the other sex (Heisler et al. 1987). It is a component of the intersexual conflict and an evolutionary force driving mating systems (Darwin 1871; Andersson 1994). Females are usually the more selective sex in mate selection, because of the higher reproductive investment of females than males (Darwin 1871; Clutton-Brock 1989). Females may gain direct benefits (increased fecundity or amelioration of a cost) and/or indirect benefits (increased fitness of their offspring) by choosing a high-quality reproductive partner (e.g. Kokko et al. 2003). However, female choice is rarely obvious and can even be very subtle or cryptic, occurring during or even after mating (Birkhead & Møller 1993; Eberhardt 1996). Why and how females select their partners and how mating preferences have evolved remains under debate among evolutionary biologists and understanding these mechanisms is one of the greatest tasks in behavioural ecology (for a review see Cordero & Eberhard 2003).
Several surrogate measures of male quality have been used to evaluate female choice. Morphological traits, such as body size, weaponry and intense signals of fighting ability, are essential in male–male competition and are expected to be important cues in female choice (Andersson 1994), as is male age (viability selection theory; Trivers 1972), provided that survival rates are not age-dependent (Beck & Powell 2000). Females may also gain genetic benefits by selecting the most heterozygous males (the ‘good genes’ hypothesis; Brown 1997). Assuming a correlation between heterozygosity and fitness-associated traits (Hansson & Westerberg 2002), females may base their choice on traits directly reflecting heterozygosity at key loci or at many loci, such as the expression of vigour, symmetry or condition-sensitive ornaments. Also, by choosing mates based on compatible genes such as the Major Histocompatibility Complex (MHC), females may enhance their offspring viability and performance (Penn & Potts 1999; Trezenga & Wedell 2000). Finally, mate selection directed towards less related individuals has been suggested as an efficient mechanism for inbreeding avoidance (Blouin & Blouin 1998). Generally, these surrogate measures of male quality are tested separately. Limited by the availability of field or genetic data, and also by appropriate statistical models, very few studies have included several of these factors in the same model to determine the extent each factor influences female mate selection.
Female choice may also be context-dependent. For example, female choice may differ for species in which young are vulnerable to sexually selected infanticide (SSI), i.e. where males kill dependent offspring, but not their own progeny, to gain access to breeding opportunities with the mother (Hrdy 1979). This phenomenon is rarely of benefit to females, and may lead to a dilemma: select a high-quality mating partner or prioritize mating strategies to counter infanticide. A potential counterstrategy to SSI is multiple mating, or ‘promiscuity’, in which the female attempts to confuse paternity. This idea has received much support in recent decades, and paternity uncertainty has been hypothesized as a major factor explaining multi-male mating by female mammals (Wolff & Macdonald 2004). Based on this hypothesis, females would tend to mate with any males they are likely to meet in the future, while accompanied by their dependent young, rather than trying to select a high-quality partner. Wolff & Macdonald (2004) pointed out that future studies should quantify the role of female choice to elucidate the evolutionary significance of multi-male mating in female mammals.
The mating system of bears, including mate selection, is poorly known. To our knowledge, only a few studies have examined this question in brown bears (Ursus arctos; Craighead et al. 1995a, 1998) and American black bears (Ursus americanus; Schenk & Kovacs 1995; Kovach & Powell 2003), all with a limited number of genetic samples and field observations. Female bears are induced ovulators, i.e. eggs are released after behavioural, hormonal or physical stimulation (Craighead et al. 1995b; Boone et al. 1998). This may allow females to evaluate male quality inside the reproductive tract and may provide them with more control over the paternity of their offspring than with spontaneous ovulation (Larivière & Fergusson 2003).
Based on the assumption that female bears may be choosy, we investigated female mate selection in two subpopulations of Scandinavian brown bears that have been studied for about 20 years and for which good field and genetic data are available. SSI has been documented in these subpopulations (Swenson et al. 1997; Swenson 2003), and it has been shown that infanticidal males were not related to the cubs they killed (Bellemain et al. 2005a). Males seem to be able to differentiate their own cubs from unrelated cubs, perhaps by recognizing the females they mated with the year before.
Based on paternity assignment of the female's litter, we tested the following predictions:
females select males based on morphological, age or genetic criteria to maximize their reproductive output or inclusive fitness (direct or indirect benefits). Based on theory and the literature review described above, we predicted that paternity assignment would be positively correlated with male age, body size, and negatively correlated with a male's internal relatedness (an index reflecting both heterozygosity and inbreeding; see §2) and his relatedness to the female; and
females use a strategy to minimize the risk of SSI by confounding paternity, i.e. mating with the geographically closest males, which have the highest potential to kill their future cubs.
2. Material and methods
(a) Study species, study areas and sampling
During the mating season, male and female brown bears remain together for a few hours to several days, or even several weeks (Craighead et al. 1995b), and both males and females mate promiscuously, with females mating with up to eight males in a mating season (Craighead et al. 1995b). Both sexes roam to mate, increasing their home range during the early May to mid-July mating season (Dahle & Swenson 2003a). Implantation is delayed until November (Renfree & Calaby 1981). After 6–8 weeks of effective gestation, females give birth to 1–4 small cubs in January, while still hibernating in dens (Pasitschniak-Arts 1993). Young bears receive extended maternal care, staying with the mother for 1.5–2.5 years in the studied populations (Dahle & Swenson 2003b). Females do not mate while caring for their young (Schwartz et al. 2003) and there is no paternal investment in rearing of the offspring. A previous study (Bellemain et al. 2005a) showed that multiple paternities were frequent in this population, occurring in 14.5% of 69 litters with greater than or equal to two young and 28% of 32 litters with greater than or equal to three young. Scandinavian brown bears exhibit a sex ratio close to 50 : 50 (Bellemain et al. 2005b). Cub mortality averages 35% annually in the southern study area and 4% in the northern study area (Swenson et al. 2001). A study examining nutritional, social (SSI), and den disturbance factors found that the patterns of cub mortality were best explained by social factors in both populations (Swenson et al. 2001).
The study areas are located in southcentral Sweden (49 000 km2) and northern Sweden (8000 km2) and are described by Bjärvall & Sandegren (1987). The two subpopulations located in each study area differed in mortality regimes and in their male age structure. Bear hunting was, generally, allowed during the autumn in both areas, but the northern area included three national parks, where bear hunting was illegal during the study period, although there was evidence of intensive poaching (Swenson & Sandegren 1999). There were few large adult males in the northern study area and a more evenly distributed male age structure in the southern study area (Swenson et al. 2001).
We used radio-telemetry for long-term monitoring of adult bears. Between 1984 and 2003, brown bears, including females accompanied by their yearling offspring, were immobilized in the spring and received radio-transmitters. Home ranges of radio-marked bears were estimated using 95% Minimum Convex Polygon as described by Dahle & Swenson (2003a). In addition, we obtained teeth for age determination and location of death from all killed bears (legally hunted or traffic-killed) in Sweden. Tissue samples were collected from both marked and killed bears and stored in 95% alcohol until extraction for genetic typing.
(b) DNA extraction and typing
Our genetic database contained 977 bear genotypes, of which 396 were from marked animals. The amplification and analysis of microsatellites were carried out following the protocol described by Waits et al. (2000). The following 18 microsatellite loci were used: G1A, G1D, G10B, G10C, G10L, G10P, G10X, G10H, G10O, G10J (Paetkau & Strobeck 1994; Paetkau et al. 1995) and Mu05, Mu10, Mu15, Mu23, Mu50, Mu51, Mu59, Mu61 (Taberlet et al. 1997).
(c) Parentage analysis
Based on the multilocus genotypes of mothers, offspring and males, we analysed parentage using the software PARENTE (Cercueil et al. 2003, available at http://www2.ujf-grenoble.fr/leca/membres/manel.html). One allelic incompatibility of 18 loci was allowed in the comparison of the parent–offspring genotypes to account for possible genotyping errors or mutations. We assessed the proportion of individuals for which parentage was assigned in the population (table 1). The results were checked with observational field data and for geographical consistency.
Table 1.
Number and percentage of brown bears for whom the mother and/or father have been determined genetically or verified (from prior field observations) with a parentage probability greater than 80%, in two study areas in Scandinavia.
| determined paternities | maternities | ||
|---|---|---|---|
| genetically determined | verified from field data | ||
| north study area (n=148) | 96 (64.8%) | 12 (8.1%) | 113 (76.3%) |
| south study area (n=248) | 146 (58.9%) | 31 (12.5%) | 160 (64.5%) |
| total (n=396) | 242 (61%) | 41 (10.4%) | 273 (68.9%) |
(d) Evaluation of female choice
We evaluated selection of reproductive partners by radio-marked oestrous females based on the comparison of characteristics of males that became fathers of the subsequent litter and other males in the vicinity of the female that did not become fathers. We proceeded in two steps:
we considered geographical information (radio-telemetry data for marked bears or kill location for unmarked bears) of all males in the vicinity of each radio-marked oestrous female as potential reproductive partners (hereafter referred to as ‘large dataset’); and
to evaluate further if females actually had the opportunity to choose among males, we considered only observed (visually or radio-telemetry) cases of female–male encounters during the mating season (hereafter referred to as ‘behavioural dataset’).
The data selection and evaluation of female mate selection is detailed below for each of the datasets.
(i) Data selection for the large dataset
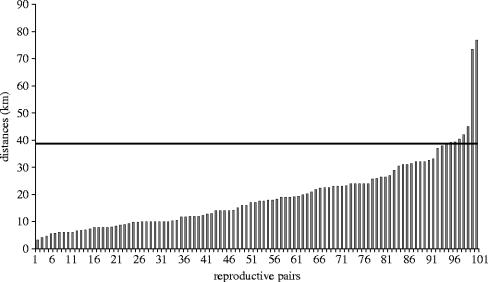
A male was considered available for potential reproduction with a given female if three criteria were fulfilled: (i) he was at least 3 years old (age of sexual maturity in male Scandinavian brown bears; our unpublished data) during the year the female was in oestrus; (ii) he was known to be alive during the female oestrous year (based on radio-tracking or killed-bear data); (iii) his home range centre (or kill location for unmarked males) was located within 40 km of the home range centre of the oestrous female. This 40 km distance corresponds to the 95% distribution of the distances between all reproductive pairs, known from parentage analysis (i.e. distance between the centres of the respective home ranges or kill locations; figure 1). This distance was chosen rather than the maximum distance between reproductive pairs to avoid overestimation of the number of males available in the vicinity of the female. Also, this distance seems reasonable based on behavioural data from both males and oestrous females during the mating season; oestrous females travelled a mean of 5.25±0.47 km per day (range 0–24.9 km) and males travelled a mean of 13.25±1.05 km per day (range 0.1–42.4 km) in our southern study area (Kristoffersen 2002).
Figure 1.
Geographical distances between the centres of the home ranges (or kill location) of 102 reproductive pairs (determined from parentage analysis) of brown bears in two populations in Scandinavia. The horizontal black line represents the distance corresponding to the 95% of the distribution of the distance between reproductive pairs (40 km).
Female choice was evaluated in relation to the following explanatory variables: (i) study area, as a factor variable; (ii) number of males available around the oestrous female, i.e. the number of males at a distance less than or equal to 40 km from the centre of the female's home range; (iii) Male age, as determined from field data. For all bears that were not captured as yearlings of radio-marked females, we collected a first premolar for age estimation based on the cementum annuli in the tooth root (Matson et al. 1999); (iv) Male internal relatedness (IR). This IR index reflects a quantity measured between parental half-genotypes. It is an estimator of heterozygosity, giving more weight to homozygotes involving rare alleles. It is calculated as:
(Amos et al. 2001), where H represents the number of homozygous loci within an individual, N the number of loci genotyped and fi the frequency of the ith allele contained in the genotype; (v) Male body size, using head circumference (at the widest part of the skull using a tape measure) as a surrogate measurement of absolute size of an individual. To estimate absolute size in the years a male was not captured, we calculated the von Bertalanffy growth curve (von Bertalanffy 1938) for each subpopulation. The average deviation in size of an individual from the mean population growth curve was used to calculate an individual growth curve, from which we derived absolute body size at a given age (our unpublished data); (vi) Genetic relatedness between a female and her potential reproductive partners, pairwise relationship coefficients (‘r’ as defined by Wang 2002 and recommended in Blouin 2003) were calculated for any two individuals by comparing the shared alleles of these individuals with the allele frequencies in each subpopulation, using the software SPAGeDi (Hardy & Vekemans 2002, available at http://www.ulb.ac.be/sciences/lagev/spagedi.html); and (vii) Geographical distance between potential reproductive partners, calculated as the distance (in km) between the centres of the respective home ranges or kill sites.
Variables i and ii were used as control variables; female choice might differ between study areas, as those areas differed in their male age structure and mortality regimes, and may be influenced by the density of males available in the vicinity. Using variables iii–vi, we tested whether female choice was influenced by male quality (prediction 1) and, using variable vii, whether female choice was influenced by SSI (prediction 2).
(ii) Data selection for the behavioural dataset (see table 3)
Table 3.
Observations (visual or radio-telemetry) of radio-marked female brown bears for which the paternity of the next year's litter was determined.
| casea | femaleb | year | father(s)b,c | males observed together with the femaleb,d |
|---|---|---|---|---|
| 1 | BD01 | 1991 | BD06 | BD06 on May 16, 17; BD34 on June 2, 4 |
| 2 | BD01 | 1997 | BD59; 01BD02 | BD59 on May 29; both BD50 and BD38 on June 11 |
| 3* | BD01 | 2000 | unmarked | BD105 on June 8 |
| 4 | BD07 | 1993 | BD06; BD43 | BD43 on May 26, 27 and June 15 |
| 5 | BD07 | 1995 | BD35 | BD35 on May 16, 24, 28 and June 2; BD06 on June 8, 13 |
| 6* | BD10 | 1988 | unmarked | BD17 on May 25, 30 and June 1, 3 |
| 7* | BD104 | 2000 | BD06; BD38 | Both BD36 and BD73 on May 18; BD73 on May 22 |
| 8* | BD12 | 1990 | BD60 | BD32 on May 31 and June 3 |
| 9* | BD18 | 1988 | unmarked | BD09 on June 6, 7 |
| 10* | BD23 | 1994 | unmarked | BD35 on May 12, 16, 19, 25 |
| 11 | BD23 | 2001 | BD36 | BD36 on June 1; both BD36 and BD97 on June 4 |
| 12* | BD24 | 1992 | BD34 | BD40 on May 15, 18, 21; BD34 on May 20, 23, 25; BD06 on June 1 |
| 13 | BD27 | 1990 | BD06 | BD06 on May 17, 18, 21, 22, 23, 25; BD34 on June 5, 8 |
| 14 | BD37 | 1995 | BD36; BD61 | BD36 on June 2; BD38 on June 8 |
| 15* | BD47 | 1996 | BD06 | BD38 on May 28, 29 and June 3 |
| 16 | BD71 | 2000 | BD88 | BD88 on May 18, 22, 31; BD36 on June 8 |
| 17* | W8802 | 1988 | 03ZZ17; unmarked | W8801 on May 21 |
| 18* | W8808 | 1990 | W8503 | W8903 on July 4, 7 |
| 19* | W8808 | 1994 | W9011; unmarked | both W9301 and W8607 on May 20; W9301 on May 29; W9301 on June 16, 17 |
| 20* | W8904 | 1995 | 99X02 | W9202 on June 24 |
| 21* | W8906 | 1989 | W8607 | W8903 on May 21; W8503 on May 28 |
| 22* | W8906 | 1993 | W8607 | W9301 on May 18, 21; W8607 on May 24; W8607, W9301 and 2 unmarked males on May 28, 29; W8607 on May 30; W9301 on June 4, 5, 8 |
| 23* | W8906 | 1995 | unmarked | both W8607 and W9511on June 21; W8607 on June 23 |
| 24* | W8906 | 2001 | W0012 | W9301 on May 21 |
| 25* | W9003 | 1997 | W0108; unmarked | both W8807 and 1 unmarked male on May 21 |
| 26 | W9008 | 1998 | W9505 | W9505 on May 19 and June 12, 13; both W9311 and W9505 on May 24 |
| 27* | W9403 | 2000 | W0232; unmarked | W0016 on May 31 |
| 28* | W9615 | 2001 | W0233 | W9921 on June 6, 9 |
Asterisks indicate situations where the father, or one of the fathers, was not the first male observed with the mother during the mating season.
Identification numbers: BD, from the northern study area; W, from the southern study area; numerical, unmarked bears killed during the hunting season (their data was subsequently recorded).
Male(s) genetically identified as the father(s) of the female's next litter (cases 1, 4, 7, 14, 17, 19, 25, 27 represent cases of multiple paternity).
If the female was observed with an unmarked bear and this individual showed obvious mating behaviour (copulation, tending, fighting with marked males), then this bear is referred to as observed.
We selected visual or radio-telemetry observations of oestrous radio-marked females with at least one known male during a mating season and when the father of her next year's litter was genetically determined. Female choice was evaluated in relation to the following explanatory variables (as described above): male age; male IR; male absolute body size; genetic relatedness between the female and her potential reproductive partners. In this case, comparing the other variables described above (study area, number of males in the area, geographical distance) was meaningless.
(e) Statistical analysis
We evaluated female choice based on paternity assignment of the female's next litter, i.e. whether or not a particular male was the father of a female's cubs (binomial process). For the large dataset, we used a generalized linear mixed model (GLMM) with a logit link and binomial error distribution (McCullagh & Nelder 1989) to account for the effects of the explanatory variables on the probability of paternity assignment. The response variable (paternity assignment) was assumed to be binary (‘1’ for a male(s) genetically determined as a father(s) or ‘0’ for all other males within a 40 km radius), given random effects for female identity. Models were fitted using a penalized quasi likelihood method (Venables & Ripley 1999) in the statistical software R 1.9.1 (R Development Core Team 2004, http://www.R-project.org). After a stepwise exclusion of the least significant term (p≥0.05), the final model was revealed. Models were compared using the AIC criterion (Burnham & Anderson 1998).
For the behavioural dataset, we used pairwise t-tests to compare characteristics of males that were observed with oestrous females during the mating season with characteristics of the actual father(s) of the females' next litter. The software SPSS (SPSS 12.0.1, SPSS Inc., Chicago, IL) was used for those statistical analyses.
3. Results
(a) Parentage analysis
All mother–offspring combinations known from field observations (n=314) were genetically confirmed (table 1). In addition, we genetically determined the maternity for 41 marked bears with unknown pedigree (table 1). Paternity was genetically determined for 242 (61%) of the marked individuals; 6% of those fathers were unmarked.
(b) Female choice
The large dataset included 43 litters in the southern subpopulation (24 mothers) and 52 litters in the northern subpopulation (24 mothers). Totally, 107 different males were considered available (of which 20 were unmarked) for a total of 825 bear-years, and 102 reproductive pairs were considered (including 7 litters with multiple paternity with both fathers known). Two litters (ca 2%) resulted from incestuous matings (reproduction between the daughter and her father). The distance between reproductive pairs ranged from 3.3 to 76.8 km (figure 1). A minimum mean of 12.48±5.33 (s.e.) males (range 3–25) in the south and 7.82±3.81 (s.e.) males (range 2–16) in the north were known to be available in the vicinity of a given female (within a radius of less than or equal to 40 km) during her oestrous year.
Male age and male body size were highly correlated (Pearson correlation; r=0.657; P≤0.001). We therefore analysed these variables in separate models, including all other explanatory variables. The model including male body size was kept instead of the one including age, as it had a lower AIC value. The results of the final GLMM (table 2) showed that paternity assignment was positively correlated with male body size and negatively correlated with male IR, with geographical distance and with the number of males available within a 40 km radius. The explanatory variables ‘study area’ and ‘genetic relatedness’ did not significantly influence paternity assignment. All possible interactions making biological sense were tested in the model, but none of them were significant. The random effect of female identity was small (s.d.=0.0114) in our study, and it only slightly modified the estimates of the final model. All other models (results not shown) had a difference in the AIC value greater than 2 and were thus not considered in the discussion.
Table 2.
Summary of a generalized mixed linear model analysis of female brown bear choice in Scandinavia as a function of: number of males available (within a radius of 40 km around the female), study area, geographical distance between the home range centres of the male and female, male body size, male IR and relatedness between the male and female. (The response variable was assumed to be binomial, given random effect for female identity. After a successive exclusion of the least significant terms (p≥0.05), the significance values of the final model are shown in the table. Non-significant terms are presented with the values they were removed from the model with. d.f. is degrees of freedom, β is the logistic regression coefficient, s.d. is the standard deviation, s.e. is the standard error, t denotes the t-value and p the significance level. Number of observations, 837 and number of groups, 48.)
| explanatory variables | β | s.d. | s.e. | t | d.f. | P |
|---|---|---|---|---|---|---|
| fixed effects | ||||||
| area | −0.0238 | 0.3226 | 0.0738 | 46 | 0.9415 | |
| relatedness | 0.3182 | 0.5619 | 0.5662 | 773 | 0.5714 | |
| number of males available | −0.1375 | 0.0251 | −5.4823 | 773 | <0.0001 | |
| male IR | −2.426 | 0.7189 | −3.374 | 773 | 0.0008 | |
| male body size | 0.0485 | 0.0163 | 2.9699 | 773 | 0.0031 | |
| geographical distance | −0.0373 | 0.0112 | −3.3157 | 773 | 0.0010 | |
| intercept | −2.7080 | 1.2509 | −2.1648 | 773 | 0.0307 | |
| random effects | ||||||
| female identity | 0.0114 | |||||
| residual | 1.0219 |
The behavioural dataset (table 3) included 12 litters in the southern subpopulation (eight mothers) and 16 litters in the northern subpopulation (12 mothers). There was considerable variation in the observations of female–male encounters. In eight of 28 cases (28.5%), females were observed with two or more males at the same time; in all other cases, they encountered males sequentially. Thirteen females were observed with only one male, 13 females with two, one female with three males and one female with five different males during the mating season. Overall, 54% of the females were observed with more than one male during the course of a mating season. In 12 cases (43%), the female was observed with the father of her next years' litter. We did not observe any cases of females rejecting a male, nor have other observational studies of brown bear mating behaviour (Craighead et al. 1995b), however this is extremely difficult to document. We observed several instances of apparent long-term association (greater than 4 days) between a male and a female, without the male becoming a father (table 3). Most of the time (in 68% of the cases), the first male to be observed with the female was not the father of the subsequent litter. Females observed in more than one mating season did not always reproduce with the same male (e.g. BD07 reproduced with BD06 and BD43 in 1993 and with BD35 in 1995).
Pairwise t-tests showed that paternal males were significantly older (t20=3.36; p=0.003) and larger (t14=3.28; p=0.005) than males that did not become fathers. We did not find a difference in IR (t27=−0.25; p=0.808) nor relatedness to the female (t27=−0.58; p=0.568) between the fathers and other males.
4. Discussion
(a) Distinguishing between female choice and male–male competition in paternity assignment
Sexual selection predicts that the fundamental reproductive asymmetries between males and females give rise to a conflict between sexes (Darwin 1871). In mammals, females are typically choosy, as they invest the most into reproduction (Darwin 1871; Clutton-Brock 1989). Even if females do not choose their mate before mating, they may still have the post-mating opportunity to choose between the sperm of several males (cryptic female choice; Eberhardt 1996). However, male–male competition can also occur during the post-copulatory phase via sperm competition (Ginsberg & Huck 1989). It is extremely difficult to distinguish between those two aspects of sexual selection and to evaluate their relative importance. For instance, sperm selection by females (oocytes selecting sperm bearing compatible genes; e.g. Ehlers et al. 2000) can only be differentiated from sperm competition (the fittest sperm out-compete other sperm; Gomendio & Roldan 1993) under controlled conditions (e.g. Hugues et al. 1999).
In this paper, we chose to focus on the evaluation of female mate selection in brown bears, based on the background that, in mammals, females are the more selective sex, and, in species with induced ovulation, females might be able to control paternity. Our results are consistent with the female choice hypothesis; however they do not exclude a role of males in determining paternity. During the pre-copulatory phase, both sexes seem to play a role in paternity determination. Brown bears show large size dimorphism, with males being 1.2–2.2 times heavier than females (Stringham 1990), revealing the importance of intra-sexual selection, through male–male competition, for gaining access to females (Andersson 1994). Meanwhile, females roam extensively during the mating season (Dahle & Swenson 2003a), suggesting that they are actively searching for copulations, perhaps to confound paternities in the context of SSI. We did not observe females rejecting any males, which otherwise would argue against the SSI hypothesis. We documented that some highly reproductive males (our unpublished data), which, under a sperm competition hypothesis would have highly competitive sperm, were not always fathering the cubs after being observed with a female (e.g. table 3, cases 5 and 12 for male BD06). Thus, the sperm of dominant males does not always result in paternity, perhaps due to sperm selection by the female. Females encountered males simultaneously as well as sequentially. In the first case, females may be able to assess male quality by direct comparison. In the second case, selection inside the female reproductive tract via sperm selection or sexual stimulation may allow a female to gather information to compare several males simultaneously. We suggest that, by being promiscuous, females might mate with the geographically closest partners (as a counter-strategy to infanticide), and select a father for their offspring via post-copulatory choice. In the following section (§4b), we concentrate on mechanisms influencing female mate selection, although sperm competition cannot be ruled out.
(b) Factors influencing female choice
Optimal choosiness should be affected by at least three variables: distribution of mate quality, cost of searching for mates, and the chooser's quality (Gibson & Langen 1996). We were not able to evaluate the costs of searching for mates. However, the other two variables (distribution of mate quality and chooser's quality) were considered in our model. In our study, some observations were made of the same individual in different years and are consequently correlated, because both are modelled as a function of the same random effect, female identity. The GLMM considers the effects of ‘female identity’ (the chooser here) as a random factor (predicted as individual-specific deviations from a population mean once the independent effects of other variables are accounted for) and removes the effect of statistical dependence among repeated measures. As predicted, the number of males available around a given female had a significant effect on female choice. Therefore, by including this variable in the model, the effects of the other explanatory variables were corrected for.
Paternity assignment was negatively correlated with male's IR in the large dataset. This index reflects parental similarity better than commonly used heterozygosity indices (Amos et al. 2001). For example, negative values are suggestive of relatively outbred individuals, whereas high positive values suggest inbreeding. This negative correlation suggests that females would select both highly heterozygous and less inbred males, which will in turn favour the production of diverse and superior offspring (Brown 1997). Heterozygosity is probably linked to male quality via the functional overdominance hypothesis (Hansson & Westerberg 2002) and females might select their partners based on condition-sensitive traits in the male, such as body size, symmetry or other external features. This paternity bias toward more heterozygous and less inbred males may be explained by post-copulatory mechanisms inside the female reproductive tract. Either the most heterozygous sperm outcompete the rest by being the fittest (Ginsberg & Huck 1989), or a female is able to evaluate male sperm quality and select the most heterozygous sperm (Birkhead & Møller 1993). Nuclear heterozygosity might also be linked to polymorphism in MHC, with females obtaining indirect benefits from choosing males with the most compatible MHC genes (Penn & Potts 1999; Trezenga & Wedell 2000). MHC-based disassortative mating preferences would reduce homozygosity throughout the genome, and particularly within loci linked to the MHC. Progeny derived from such matings would have an increased fitness, because of reduced levels of inbreeding and increased resistance to infectious diseases arising from their increased MHC heterozygosity. This hypothesis has, to our knowledge, only been tested with laboratory animals (e.g. Yamazaki et al. 1978) and remains to be investigated in wild mammals. The finding of interactions between parental male and female genotypes calls for studying the physiological mechanisms involved (Bernasconi et al. 2004). The lack of significance of the IR factor in our behavioural dataset could be due to low power caused by small sample size.
The morphological factor ‘body size’ was positively correlated with paternity assignment both in the final model of the large dataset analysis and in the ‘behavioural data’ analysis. As previously suggested, large body size in males could reflect their genetic quality and females may select their reproductive partner based on this criteria. Body size is age-dependent in bears (male age and male body size were highly correlated in our data; see results), therefore females choosing the largest males also select for the oldest males, although the age variable was less important than body size in paternity assignment. In several mammals, age is correlated with dominance rank and it has been shown that dominant males obtain higher reproductive success (e.g. in red deer (Cervus elaphus); Clutton-Brock 1988). We have no data on dominance status of males in our subpopulations, however male bears do not defend exclusive territories but have overlapping home ranges (McLellan & Hovey 2001). Thus they may interact with each other throughout the year and a male dominance hierarchy may be established, as suggested in American black bears (Kovach & Powell 2003). Therefore, selection of older males by females might also reflect selection of dominant males.
Among all males available within a 40 km radius, paternity assignment was negatively correlated with geographical distance to potential reproductive partners. In these subpopulations, where SSI is prevalent and where infanticidal males are mostly residents (Swenson et al. 1997, 2001; Swenson 2003), it has been proposed that females use promiscuity as a counterstrategy to SSI (Swenson 2003; Bellemain et al. 2005a). In this study, we further suggested that female mating behaviour is influenced by the occurrence of SSI as the geographically closest males, i.e. potentially infanticidal males, were preferentially selected as fathers of the offspring. We suggest that females may chose to mate with as many close males as possible, which could be viewed as females making ‘the best of a bad job’ (Wolff & Macdonald 2004). In brown bears, both sexes roam to mate over large distances (Dahle & Swenson 2003a), thus individuals whose home range centres are separated by 40 km can easily meet. Therefore, we are confident that our results concerning the selection of geographically closest males is not due to bias, i.e. females would come into contact with close-living males more frequently than with more distant males. However, we cannot exclude the possibility that choosing the closest males also reduces the cost of searching for potential mates, and this may partly explain the effect of distance on female choice. Paternity assignment was not influenced by the factor study area, suggesting that females tended to use the same selection criterion in both subpopulations, independently of the male age structure or mortality regime of the area.
Paternity assignment was not influenced by relatedness between a female and her potential mates in both datasets, indicating that female bears neither prefer genetically distant nor close males. Following the ‘optimal outbreeding’ theory (Bateson 1983), females should preferentially select males with intermediate relatedness. Relatedness of fathers was not significantly different from non-fathers, either in the large dataset (Mann–Whitney U test; p=0.21; figure 2), nor in the behavioural dataset. This indicates a random mating scheme in relation to relatedness and suggests that mate choice is not a mechanism to avoid overall inbreeding or outbreeding. The spatial organization of bears may explain this pattern. Bears usually exhibit sex-biased natal dispersal: females are highly philopatric and establish their breeding home ranges in or near their natal areas, whereas males disperse from their mothers' home range and can move long distances (McLellan & Hovey 2001). In Scandinavia, about 36% of the females and 85% of the males have dispersed by 4 years of age (O. G. Støen, personal communication). Consequently, the probability of females mating with closely related males is low (except their father, which happened in ca 2% of the litters in our dataset) and they do not have to actively avoid inbreeding.
Figure 2.
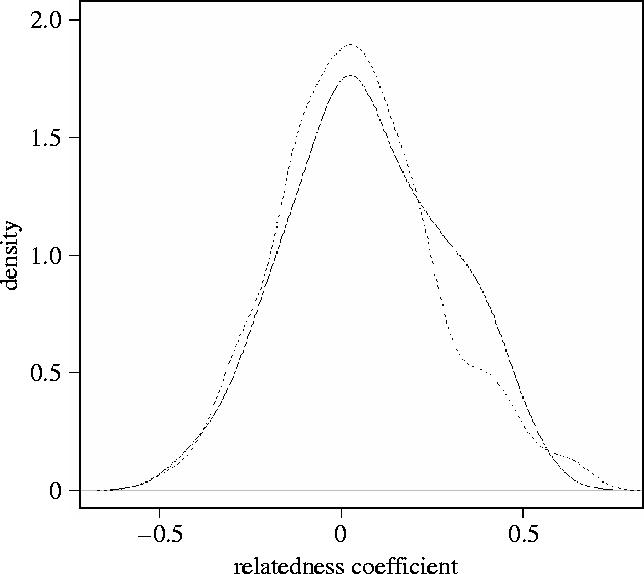
Distribution of relatedness coefficients between reproductive pairs (solid line) or potential pairs (dotted line) of brown bears in Scandinavia within a 40 km radius.
5. Conclusions
The high number of marked individuals and the large proportion of parentage assignments (table 1) allowed us to study behavioural characteristics in this brown bear population, and to improve our knowledge about female choice in this species. Due to the occurrence of SSI, the optimal strategy for female bears may be to mate with potentially future infanticidal males and exercise a post-copulatory cryptic choice of the father on her offspring. Our findings support this hypothesis, because not only geographical distance, but also male morphological, genetic, and age criteria were important in determining paternity. Females may be able to increase the survival of their offspring by choosing good genes in their reproductive partners (Brown 1997); we suggest that they use morphological traits such as age or body size and perhaps also dominance status as indicators for male genetic quality. Although our results might partly be explained by male intra-sexual competition, they are consistent with the female choice hypothesis in relation to SSI.
Acknowledgments
The Scandinavian Brown Bear Research Project was funded by the Swedish Environmental Protection Agency, the Norwegian Directorate for Nature Management, the Swedish Association for Hunting and Wildlife Management, WWF Sweden, the Research Council of Norway and the Norwegian Institute for Nature Research. We thank the research personnel in the Scandinavian Brown Bear Research Project for their assistance in the field, especially Sven Brunberg. We are grateful to Solve Sæbø for statistical advice. A.Z. was financially supported by the Austrian Science Fund, project P16236-B06.
References
- Amos W, Worthington Wilmer J, Fullard K, Burg T.M, Croxall J.P, Bloch D, Coulson T. The influence of parental relatedness on reproductive success. Proc. R. Soc. B. 2001;268:2021–2027. doi: 10.1098/rspb.2001.1751. doi:10.1098/rspb.2001.1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Bateson P. Optimal outbreeding. In: Bateson P, editor. Mate choice. Cambridge University Press; Cambridge: 1983. pp. 257–277. [Google Scholar]
- Beck C.W, Powell L. Evolution of female mate choice based on male age: are older males better mates? Evol. Ecol. Res. 2000;2:107–118. [Google Scholar]
- Bellemain E, Swenson J.E, Taberlet P. Mating strategies in relation to sexually selected infanticide in a nonsocial carnivore: the brown bear. Ethology. 2005a;111:1–14. doi:10.1111/j.1439-0310.2004.01057.x [Google Scholar]
- Bellemain E, Swenson J.E, Tallmon D, Brunberg S, Taberlet P. Estimating population size of elusive animals with DNA from hunter-collected faeces: comparing four methods for brown bears. Conserv. Biol. 2005b;19:150–161. doi:10.1111/j.1523-1739.2005.00549.x [Google Scholar]
- Bernasconi C, et al. Evolutionary ecology of the prezygotic stage. Science. 2004;303:971–975. doi: 10.1126/science.1092180. doi:10.1126/science.1092180 [DOI] [PubMed] [Google Scholar]
- Birkhead T.R, Møller A. Female control of paternity. Trends Ecol. Evol. 1993;8:100–104. doi: 10.1016/0169-5347(93)90060-3. doi:10.1016/0169-5347(93)90060-3 [DOI] [PubMed] [Google Scholar]
- Bjärvall A, Sandegren F. Early experiences with the first radio-marked brown bears in Sweden. Int. Conf. Bear Res. Manage. 1987;7:9–12. [Google Scholar]
- Blouin M.S. DNA-based methods for pedigree reconstruction and kinship analysis in natural populations. Trends Ecol. Evol. 2003;18:503–511. doi:10.1016/S0169-5347(03)00225-8 [Google Scholar]
- Blouin S.F, Blouin M. Inbreeding avoidance behaviors. Trends Ecol. Evol. 1998;3:230–233. doi: 10.1016/0169-5347(88)90164-4. doi:10.1016/0169-5347(88)90164-4 [DOI] [PubMed] [Google Scholar]
- Boone W.R, et al. Bears as induced ovulators: a preliminary study. Ursus. 1998;10:503–505. [Google Scholar]
- Brown J.L. A theory of mate choice based on heterozygosity. Behav. Ecol. 1997;8:60–65. [Google Scholar]
- Burnham K.P, Anderson D.R. Springer-Verlag; New York: 1998. Model selection and inference: a practical information-theoretic approach. [Google Scholar]
- Cercueil A, Bellemain E, Manel S. PARENTE: a software package for parentage analysis. J. Hered. 2003;93:458–459. doi: 10.1093/jhered/93.6.458. doi:10.1093/jhered/93.6.458 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H, editor. Reproductive success. studies of individual variation in contrasting breeding systems. The University of Chicago Press; Chicago: 1988. pp. 472–486. [Google Scholar]
- Clutton-Brock T.H. Mammalian mating systems. Proc. R. Soc. B. 1989;236:339–372. doi: 10.1098/rspb.1989.0027. [DOI] [PubMed] [Google Scholar]
- Cordero C, Eberhard W.G. Female choice of sexually antagonistic male adaptations: a critical review of some current research. J. Evol. Biol. 2003;16:1–6. doi: 10.1046/j.1420-9101.2003.00506.x. doi:10.1046/j.1420-9101.2003.00506.x [DOI] [PubMed] [Google Scholar]
- Craighead L, Paetkau D, Reynolds H.V, Vyse E.R, Strobeck C. Microsatellite analysis of paternity and reproduction in artic grizzly bears. J. Hered. 1995a;86:225–261. doi: 10.1093/oxfordjournals.jhered.a111578. [DOI] [PubMed] [Google Scholar]
- Craighead J.J, Sumner J.S, Mitchell J.A. Island Press; Washington, DC: 1995b. The grizzly bears of Yellowstone: their ecology in the Yellowstone Ecosystem; pp. 1959–1992. [Google Scholar]
- Craighead L, Reynolds H.V, Strobeck C, Vyse E.R. Use of microsatellite DNA analyses to infer breeding behavior and demographic processes in an artic grizzly bear population. Ursus. 1998;10:323–327. [Google Scholar]
- Dahle B, Swenson J.E. Seasonal range size in relation to reproductive strategies in brown bears Ursus arctos. J. Anim. Ecol. 2003a;72:660–667. doi: 10.1046/j.1365-2656.2003.00737.x. doi:10.1046/j.1365-2656.2003.00737.x [DOI] [PubMed] [Google Scholar]
- Dahle B, Swenson J.E. Factors influencing length of maternal care and its consequences for offspring in brown bears Ursus arctos. Behav. Ecol. Sociobiol. 2003b;54:352–358. doi:10.1007/s00265-003-0638-8 [Google Scholar]
- Darwin C. London; John Murray: 1871. The descent of man, and selection in relation to sex. (Reprinted by Princeton University Press 1981.) [Google Scholar]
- Eberhardt W.G. Princeton University Press; Princeton, NJ: 1996. Female control: sexual selection by cryptic female choice. [Google Scholar]
- Ehlers A, Beck S, Forbes S, Trowsdale J, Volz A, Younger R, Ziegler A. MHC-linked olfactory receptor loci exhibit polymorphism and contribute to extended HLA/OR haplotypes. Genome Res. 2000;10:1968–1978. doi: 10.1101/gr.10.12.1968. doi:10.1101/gr.10.12.1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R.M, Langen T.A. How do animals choose their mate? Trends Ecol. Evol. 1996;11:468–470. doi: 10.1016/0169-5347(96)10050-1. doi:10.1016/0169-5347(96)10050-1 [DOI] [PubMed] [Google Scholar]
- Ginsberg J.R, Huck U.W. Sperm competition in mammals. Trends Ecol. Evol. 1989;4:74–79. doi: 10.1016/0169-5347(89)90152-3. doi:10.1016/0169-5347(89)90152-3 [DOI] [PubMed] [Google Scholar]
- Gomendio M, Roldan E.R.S. Mechanisms of sperm competition: linking physiology and behavioural ecology. Trends Ecol. Evol. 1993;8:95–100. doi: 10.1016/0169-5347(93)90059-X. doi:10.1016/0169-5347(93)90059-X [DOI] [PubMed] [Google Scholar]
- Hansson B, Westerberg L. On the correlation between heterozygosity and fitness in natural populations. Mol. Ecol. 2002;11:2467–2474. doi: 10.1046/j.1365-294x.2002.01644.x. doi:10.1046/j.1365-294X.2002.01644.x [DOI] [PubMed] [Google Scholar]
- Hardy O.J, Vekemans X. SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes. 2002;2:618–620. doi:10.1046/j.1471-8286.2002.00305.x [Google Scholar]
- Heisler I.L, et al. The evolution of mating preferences and sexually selected traits: group report. In: Bradbury J.W, Andersson M.B, editors. Sexual selection: testing the alternatives. Wiley; Chichester, UK: 1987. pp. 96–118. [Google Scholar]
- Hrdy S.B. Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females. Ethol. Sociobiol. 1979;1:13–40. doi:10.1016/0162-3095(79)90004-9 [Google Scholar]
- Hugues K.A, Du L, Rodd F.H, Reznick D.N. Familiarity leads to female mate preference for novel males in the guppy, Poecilia reticulata. Anim. Behav. 1999;58:907–916. doi: 10.1006/anbe.1999.1225. doi:10.1006/anbe.1999.1225 [DOI] [PubMed] [Google Scholar]
- Kokko H, Brooks R, Jennions M.D. The evolution of mate choice and mating biases. Proc. R. Soc. B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. doi:10.1098/rspb.2002.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach A.I, Powell R.A. Effects of body size on male mating tactics and paternity in black bears Ursus americanus. Can. J. Zool. 2003;81:1257–1268. doi:10.1139/z03-111 [Google Scholar]
- Kristoffersen, S. 2002 Restricting daily movements as a counterstrategy against sexually selected infanticide in brown bears (Ursus arctos) Candidatus scientarum thesis, University of Oslo.
- Larivière S, Fergusson S. Induced ovulation in North American carnivores. J. Mammal. 2003;84:937–947. doi:10.1644/BME-003 [Google Scholar]
- Matson G.M, Casquilho-Gray H.E, Paynich J.D, Reynolds H.V, Swenson J.E. Cementum annuli are unreliable reproductive indicators in female brown bears. Ursus. 1999;11:275–280. [Google Scholar]
- McCullagh P, Nelder J.A. 2nd edn. Chapman & Hall; London: 1989. Generalized linear models. [Google Scholar]
- McLellan B.N, Hovey F.W. Natal dispersal of grizzly bears. Can. J. Zool. 2001;79:838–844. doi:10.1139/cjz-79-5-838 [Google Scholar]
- Paetkau D, Strobeck C. Microsatellite analysis of genetic variation in black bear populations. Mol. Ecol. 1994;3:489–495. doi: 10.1111/j.1365-294x.1994.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Paetkau D, Calvert W, Stirling I, Strobeck C. Microsatellite analysis structure of population structure in Canadian polar bears. Mol. Ecol. 1995;4:347–354. doi: 10.1111/j.1365-294x.1995.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Pasitschniak-Arts M. Ursus arctos. Mammal. Species. 1993;439:1–10. [Google Scholar]
- Penn D.J, Potts W.K. The evolution of mating preferences and major histocompatibility complex genes. Am. Nat. 1999;153:145–164. doi: 10.1086/303166. doi:10.1086/303166 [DOI] [PubMed] [Google Scholar]
- Renfree M.B, Calaby J.H. Background to delayed implantation and embryonic diapause. J. Reprod. Fertil. 1981;29(Suppl.):1–9. [PubMed] [Google Scholar]
- Schenk A, Kovacs K.M. Multiple mating between black bears revealed by DNA fingerprinting. Anim. Behav. 1995;50:1483–1490. doi:10.1016/0003-3472(95)80005-0 [Google Scholar]
- Schwartz C.C, Miller S.D, Haroldson M.A. Grizzly bear. In: Feldhamer G.A, et al., editors. Wild mammals of North America: biology, management, and conservation. The Johns Hopkins University Press; Baltimore, Maryland: 2003. pp. 556–586. [Google Scholar]
- Stringham S.F. Grizzly bear reproductive rate relative to body size. Int. Conf. Bear Res. Manage. 1990;8:433–443. [Google Scholar]
- Swenson J.E. Implication of sexually selected infanticide for the hunting of large carnivores. In: Festa-Bianchet M, Apollonio M, editors. Animal behavior and wildlife conservation. Island Press; Washington, DC: 2003. pp. 171–190. [Google Scholar]
- Swenson J.E, Sandegren F. Mistänkt illegal björnjakt i Sverige. Bilagor till Sammanhållen rovdjurspolitik; Slutbetänkande av Rovdjursurstredningen. Statens offentliga utredningar 1999, Stockholm, Sweden. 1999;146:201–206. [In Swedish.] [Google Scholar]
- Swenson J.E, Sandegren F, Söderberg A, Bjärvall A, Franzén R, Wabakken P. Infanticide caused by hunting of male bears. Nature. 1997;386:450–451. doi:10.1038/386450a0 [Google Scholar]
- Swenson J.E, Sandegren F, Brunberg S, Segerström P. Factors associated with loss of brown bear cubs in Sweden. Ursus. 2001;12:69–80. [Google Scholar]
- Taberlet P, Camarra J.J, Griffin S, Hanotte O, Waits L.P, Dubois-Paganon C, Burke T, Bouvet J. Noninvasive genetic tracking of the endangered Pyrenean brown bear population. Mol. Ecol. 1997;6:869–876. doi:10.1046/j.1365-294X.1997.00251.x [PubMed] [Google Scholar]
- Trezenga T, Wedell N. Genetic compatibility, mate choice and patterns of parentage: invited review. Mol. Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. doi:10.1046/j.1365-294x.2000.00964.x [DOI] [PubMed] [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B.G, editor. Sexual selection and the descent of man. Adline; Chicago: 1972. pp. 1871–1971. [Google Scholar]
- Venables W.N, Ripley B.D. Springer; Berlin: 1999. Modern applied statistics with S-Plus. [Google Scholar]
- von Bertalanffy L. A quantitative theory of organic growth (Inquiries on growth laws. II) Hum. Biol. 1938;10:181–213. [Google Scholar]
- Waits L.P, Taberlet P, Swenson J.E, Sandegren F. Nuclear DNA microsatellite analysis of genetic diversity and gene flow in the Scandinavian brown bear (Ursus arctos) Mol. Ecol. 2000;9:421–431. doi: 10.1046/j.1365-294x.2000.00892.x. doi:10.1046/j.1365-294x.2000.00892.x [DOI] [PubMed] [Google Scholar]
- Wang J. An estimator for pairwise relatedness using molecular markers. Genetics. 2002;160:1203–1215. doi: 10.1093/genetics/160.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J.O, Macdonald D.W. Promiscuous females protect their offspring. Trends Ecol. Evol. 2004;19:127–134. doi: 10.1016/j.tree.2003.12.009. doi:10.1016/j.tree.2003.12.009 [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Yamaguchi M, Andrews P.W, Peake B, Boyse A. Mating preferences of F2 segregants of crosses between MHC-congenic mouse strains. Immunogenetics. 1978;6:253–259. [Google Scholar]