Abstract
Sex pheromones are chemical signals frequently required for mate choice, but their reciprocal role on mate preference has rarely been shown in both sexes. In Drosophila melanogaster flies, the predominant cuticular hydrocarbons (CHs) are sexually dimorphic: only females produce 7,11-dienes, whereas 7-tricosene (7-T) is the principal male CH. Males generally prefer females with 7,11-dienes, but the role of 7-T on female behaviour remains unclear. With perfumed males, control females mated faster and more often with males carrying increased levels of 7-T showing that this CH acts as a chemical stimulant for D. melanogaster females. Control females—but not antenna-less females—could detect small variation of 7-T. Finally, our finding that desat1 mutant female showed altered response towards 7-T provides an additional role for this gene which affects the production and the perception of pheromones involved in mate choice, in both sexes.
Keywords: male pheromone, 7-tricosene, female receptivity, antenna, desat1, Drosophila
1. Introduction
The multiple sensory signals exchanged during courtship serve to precisely inform potential sex partners about their reciprocal reproductive status. Many studies involving both vertebrates and invertebrates have shown a clear relationship between mating success and the quality of various sex-specific characters, presumably subject to sexual selection (Andersson 1994). Among the sexually dimorphic signals exchanged during courtship, sex pheromones are frequently used for mate discrimination and choice (Wyatt 2003). However, in only a very few examples have the sex pheromones that reciprocally influence the mating response in both sexes been identified (Andersson 1994). In insects, and more specifically in moths, pheromones are multicomponent blends of chemicals, some of which tend to stimulate partner attraction while other components can induce repulsion (Linn & Roelofs 1989; Mustaparta 1996).
In Drosophila species, cuticular hydrocarbons (CHs) can differ between the sexes and be involved in mate recognition and preference (Ferveur 2005). In D. melanogaster, the predominant CHs of wild-type females, 7,11-dienes, tend (i) to increase male intraspecific courtship and mating and (ii) to inhibit interspecific male sexual activity (Antony & Jallon 1982; Coyne & Oyama 1995; Savarit et al. 1999). With conspecific males, 7,11-diene-rich females were preferred by males but produced less daughters (Marcillac & Ferveur 2004).
The male principal CH, 7-tricosene (7-T), is the most efficient pheromone able to prevent or reduce male homosexual courtship (Ferveur & Sureau 1996; Sureau & Ferveur 1999; Svetec & Ferveur 2005), but its role on female mating behaviour remains elusive. Studies, based on the comparison between D. melanogaster males that largely varied for their hydrocarbon profile but also for their genetic background (Averhoff & Richardson 1974; Jallon 1984; Scott 1994; Cobb & Ferveur 1996a; Coyne 1996), made it impossible to reveal the identity of the substance(s) involved. For example, to verify the hypothesis (Jallon 1984) that 7-T enhances female sexual receptivity, Scott (1994) compared the response of canton-S (Cs) and Tai females to each male (Cs=7-T rich; Tai=7-T-poor) with/out synthetic 7-T added on a piece of a filter paper placed in the mating chamber. However, the role that 7-T was playing in female receptivity was debated especially because (i) of the limited number of male types used (Cs and Tai) and (ii) male sexual activity was not measured (Cobb & Ferveur 1996b). The possibility of transferring specific CHs by rub-off from donors (Coyne et al. 1994) onto receiver flies partially or totally depleted for these CHs (Savarit et al. 1999; Marcillac & Ferveur 2004) is a powerful method which overcomes some of these limitations and allows to measure the behavioural effect of CHs carried by flies of a similar genotype.
Drosophila female discrimination and receptivity to male courtship is genetically based (Pineiro et al. 1993; Doi et al. 2001), and partially depends on the antenna and on a dorso-anterior brain region (Mayr 1950; Petit 1958; Manning 1967; Tompkins & Hall 1983). The distal part of the female antenna (consisting of the arista attached to the third antennal segment) is clearly involved in the perception of male acoustic signals (‘love song’) and changes in female receptivity (Bennet-Clark & Ewing 1967; Kyriacou & Hall 1982; Göpfert & Robert 2002; Tauber & Eberl 2003). The few studies suggesting a relationship between female receptivity and chemical signal(s), have been either based (i) on mutant females with defective sensory perception (Tompkins et al. 1982; Gailey et al. 1986), or (ii) on males with abnormal CH profiles (Ferveur & Jallon 1993b; Rybak et al. 2002). But in all these cases, the multiple effects caused by mutant genes and/or by the experimental treatment equally came to no conclusion in this respect.
Here, we tested the effect of male pheromones, and especially that of 7-T which is the principal male-specific component, on female mating activity. First, we used three genetically related males producing radically different levels of 7-T and compared female receptivity to these males. Then, sibling males genetically depleted for 7-T and other unsaturated CHs were perfumed with a slightly variable quantity of 7-T to evaluate whether females could detect smaller variations in male pheromones. The comparison of male courtship towards decapitated and intact females was carried out to assess the respective activities of the male and the female during courtship. We also measured the response of females homozygous for the desat1 mutation previously shown to alter male perception of sex pheromones (Marcillac et al. 2005a). Finally, different segments of female antenna were surgically removed to determine their role in male pheromone perception.
2. Material and methods
(a) Drosophila melanogaster strains and crosses
All D. melanogaster strains were raised on yeast/cornmeal/agar medium and kept at 24±0.5 °C with 65±5% humidity on a 12 : 12 h light/dark cycle.
We chose homozygous mutant desat11573-1 males (desat1) as tester males because they were drastically depleted for 7-T, while they still show a strong courtship (Marcillac et al. 2005a). Mutant desat1 females were also used. These flies contain a transposable PGal4 element inserted in the desat1 gene.
Homozygous desat11573-N2 (N2) male and female flies were also used. N2 is an allele resulting from the complete and precise excision of the transposable PGal4 element, and is the best available control genotype to assess the effect of the desat11573-1 mutation. Indeed, N2 males showed a fully rescued production and male perception of sex pheromones (Marcillac et al. 2005a, in press). In preliminary experiments, N2 females paired with control males showed a mating pattern that resembled those shown by control females.
EP males result from the cross between desat1 virgin females and E(p)-10164 males. The E(p)-10164 strain (a gift of Toshiro Aigaki, Tokyo Metropolitan University) contains a transposable element inserted in desat1 that can deregulate the expression of this gene under the conditional activation of Gal4 (Rörth 1996). We used EP males because they produced a very high amount of 7-tricosene (7-T). Therefore, all tester males are genetically related: desat1 and N2 males are similar, except at the desat1 locus; EP and desat1 males share the same X chromosome and half of their autosomal genes.
(b) Characterization of cuticular hydrocarbons
CHs were analysed from 5 day old intact individual flies by gas chromatography following hexane extraction according to standard procedures (Antony & Jallon 1982; Ferveur 1991). Analyses were performed with a Varian CP3380 chromatograph, equipped with a Cp-sil 25 m capillary column with hydrogen as the carrier gas. All the D. melanogaster predominant CHs have already been identified and characterized (Pechine et al. 1985). Twenty-four CHs were systematically detected in female flies, and 14 in male flies, both with a chain length ranging from 23 to 29 carbons (see Marcillac et al. in press for the complete list of compounds analysed in male and female flies). For the sake of clarity, the only amounts shown here are for 7-tricosene (7-T, 23C), 7-pentacosene (7-P, 25C), n-tricosane (23Lin), n-pentacosane (25Lin), and the sum of all CHs (SumCHs).
(c) Hydrocarbon transfer
We adapted the procedure described by Coyne et al. (1994). Briefly, one hundred and fifty 4–7 day old donor males were instantly killed by immersion in liquid nitrogen and placed, after thawing, in a fresh food vial to be confined overnight with fifteen 4 day old tester desat1 males. The plug of the vial was pushed down to reduce the volume to 2 cm3, and the vial was held upside down to increase the contact between donors and testers for a period of 14–16 h. Immediately before the test, the plug was pulled up, and each tester male was aspirated without anaesthesia. Tester desat1 males to be perfumed received the CHs of one of three donor genotypes: desat1 (shown as *desat1* in the text), N2 (*N2*) and EP (*EP*). The comparison between three perfumed males allowed thus to eliminate any stressful effect caused by the transfer procedure. We do not know whether or not the transferred CHs were deposited homogeneously on the body.
(d) Female antennal surgery
One day old N2 females were anaesthetized with carbon dioxide (CO2) and their antennae removed under a binocular microscope: both aristae or both funiculi were removed with a pair of dissecting forceps (MC32, Moria, Paris, France). As the arista is attached to the funiculus, the removal of funiculi implies that aristae were also eliminated. Control females were treated with the same experimental procedure, but their antennae were not removed.
(e) Behavioural experiments
Two kinds of experiments were carried out: (i) with decapitated females to measure male sexual activity and (ii) with intact females to estimate female willingness to mate. Decapitated females were alive but did not respond to male courtship: this allowed us to assess male heterosexual activity independently of female response. Our results are mostly based on the observation that the frequency and the latency to mate did not follow the propensity to court decapitated females, between male genotypes, and this may reflect the active involvement of the female and her preference to mate.
All flies were isolated under light CO2 anaesthesia 0–4 h after eclosion. Tester male flies (i.e. those whose sexual responses to target flies were measured) were held individually in fresh glass food vials for 4 days (in case of hydrocarbon transfer) or for 5 days, before testing. Donor males (i.e. those whose hydrocarbons were transferred) were kept in vials in groups of 20 until 4 days old, and females were kept for 5 days in groups of five.
All experiments, carried out with a heterosexual pair of 5 day old flies, were performed in a room at 24±0.5 °C with 65±5% humidity. Tests were performed simultaneously over several days for the tester males of each type and always took place 1–4 h after lights on. Tester males were individually aspirated (without anaesthesia) under a watch glass used as an observation chamber (1.6 cm3). After 10 min, a virgin female was introduced.
Male courtship index (CI) was measured during a 10 minute period with a single decapitated virgin. CI is the proportion of time that the male spends in active courtship (tapping, wing vibration, licking and attempting copulation). Prior to the test, anaesthetized females were decapitated with a razor blade cleaned with ethanol between each treated genotype. They were kept in groups of 10 in food vials and allowed at least 15 min to recover. Only standing headless females were used for the test.
For the mating experiment, each tester male was paired with an intact or antenna-less (and non-anaesthetized) virgin female, for 1 h. We measured the latency to copulate (time in min from the introduction of the female into the chamber until copulation), the duration of copulation (time in min from the copulation onset until disengagement), as well as the overall frequency of copulating pairs for each treatment. We measured the CH profile of males that were, or were not perfumed.
(f) Statistics
All tests compare flies bred and used during the same period of time. To compare the levels of CHs, of CI, of copulation latency and duration between male genotypes or between perfumed males (separately for each female genotype), and between different genotypes or females which had been operated on (with either control or perfumed males), we used an ANOVA with Bonferroni tests (or Fisher's PLSD post hoc test for CH levels). Data for copulation latency were log-transformed to normalize their distribution before the ANOVA test. A chi-square homogeneity test was used to compare copulation frequency between two or three samples.
3. Results
(a) Mating of control females is enhanced with 7-T-rich males
N2, desat1 and EP males largely differ for their level of 7-tricosene (7-T; table 1a). EP males produced much more 7-T (1294 ng) than N2 males (450 ng), and both genotypes showed much higher levels of 7-T than desat1 males (63 ng). EP and N2 showed similar amounts for all other unsaturated CHs, including 7-pentacosene (7-P) that was much abundant than in desat1 males (p<0.0001). Conversely, desat1 males produced more saturated linear CHs (including n-tricosane=23Lin and n-pentacosane=25Lin) than EP and N2 males which had similar levels. Finally, the global amount of CHs (SumCHs) was higher in EP and desat1 males than in N2 males.
Table 1.
Production of cuticular hydrocarbons in various male flies. (Data shown are mean absolute amounts (±s.e.m.; in ng) for 7-tricosene (7-T), 7-pentacosene (7-P), n-tricosane (23Lin), n-pentacosane (25Lin) and for the sum of all detected cuticular hydrocarbons (SumCHs). Five day old males had either (a) different genotypes: homozygous for the desat11573-1 mutant allele (desat1), homozygous for the rescued desat11573-N2 allele (N2), F1 sons of the cross between desat1 virgin females and 10164-EP males (EP), or (b) they shared the same desat1 genotype, but were perfumed by rub-off with the CHs of desat1 males (*desat1*), of N2 males (*N2*), or of EP males (*EP*). The value and significance of the ANOVA carried out for each type of male is shown below each CH parameter; the letters in brackets indicate the significant differences. (a) n=9–13; (b) 52–83.)
| male | cuticular hydrocarbons | |||||
|---|---|---|---|---|---|---|
| genotype | perfume | 7-T | 7-P | 23Lin | 25Lin | SumCHs |
| (a) plain genotypes | ||||||
| desat1 | — | 63 (7) (a) | 39 (2) (a) | 936 (172) (a) | 346 (45) (a) | 2432 (245) (a) |
| N2 | — | 450 (26) (b) | 176 (11) (b) | 144 (10) (b) | 43 (8) (b) | 1284 (63) (b) |
| Ep | — | 1294 (59) (c) | 156 (8) (b) | 57 (8) (b) | 11 (1) (b) | 2054 (57) (a) |
| ANOVA F | 263,50 | 57,76 | 30,40 | 61,71 | 21,12 | |
| (d.f.=29, 2) p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| (b) perfumed genotype | ||||||
| desat1 | *desat1* | 52 (3) (a) | 35 (2) (a) | 1223 (45) (a) | 361(12) (a) | 2580 (73) (a) |
| desat1 | *N2* | 68 (4) (b) | 50 (2) (b) | 935 (42) (b) | 280 (11) (b) | 2147 (72) (b) |
| desat1 | *Ep* | 90 (3) (c) | 31 (1) (c) | 1004 (41) (b) | 300 (11) (b) | 2281 (71) (b) |
| ANOVA F | 39,37 | 36,91 | 11,66 | 12,02 | 8,65 | |
| (d.f.=206, 2) p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
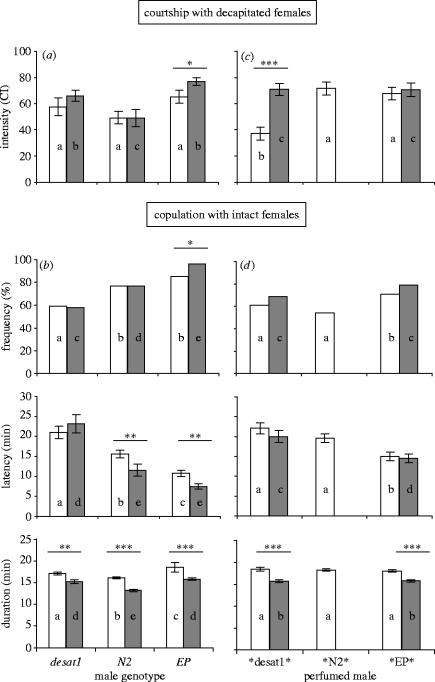
The courtship activity of these three males (CI) as measured to decapitated control N2 females, was similar (figure 1a, white bars). However, intact N2 females induced a different mating pattern in the three male genotypes: EP males mated faster than N2 males that mated faster than desat1 males (p<0.001; figure 1b). Also, EP and N2 males mated more frequently than desat1 males (p<0.01). The comparison with decapitated N2 females suggests that intact N2 females detect differences between male genotypes. Since the mating performance of N2 females was parallel to the quantitative variation of 7-T (EP>N2>desat1), but not that of other substantial male CHs, we tested the involvement of 7-T in female receptivity.
Figure 1.
Male courtship and female mating response with various males. Individual 5 day old tester males were either paired (a, c) with a single decapitated female and their courtship index (CI) was measured, for 10 min, or (b, d) with an intact female and the frequency of mating, the mean (±s.e.m.) for copulation latency (copulation onset) and for copulation duration (in min) were measured for 60 min. Females were either homozygous for the desat11573-N2 allele (N2; white bars), or the desat11573-1 allele (desat1; grey bars). Tester males either belonged to the desat1, N2, EP genotypes (a, b) or were desat1 males perfumed by donors of these three genotypes (*desat1*, *N2*, *EP*; c, d; see table 1). Statistical differences between different male types (genotype or perfume), measured separately for each female genotype, are shown by the letters inside the histogram bars. Significant differences between the two female genotypes, tested with ANOVA for the three male genotypes or for perfumed males, are indicated above bars as follows: ***p<0.001; **p<0.01; *p<0.05. (a) n=22–47; (b) 70–137; (c) 23–32; (d) 47–111.
(b) Female mating is enhanced by increased amounts of 7-tricosene
To measure the influence of male CHs—and try to rule out the contribution of other characters—on female mating, we exclusively used desat1 males and perfumed them by rub-off with the CHs provided by donor males of the three genotypes. As desat1 males are largely depleted for 7-T, CH transfer with either desat1, N2 or EP donors yielded perfumed males (respectively, noted *desat1*, *N2* and *EP*; see §2) with the same genotype but carrying different CHs. Rub-off induced significant differences between the three perfumed males for 7-T and 7-P (table 1b). Despite the fact that the variation of the absolute amounts of 7-T was moderate (20–40 ng), its relative increase was substantial: *EP* males carried 73% more 7-T than *desat1* and 32% more than *N2* males. The quantitative variation of 7-P was much smaller and followed a different pattern (*N2*>*desat1*>*EP*). Finally, *desat1* males showed higher amounts of linear alkanes (23Lin and 25Lin) than *EP* and *N2* males which had very similar levels. No significant difference was detected for any other CHs (not shown), and *desat1* males showed slightly increased SumCHs.
With decapitated N2 females, *desat1* males showed a very reduced CI (37.1±4.9) that was nearly half that shown by *EP* and *N2* (with similar CIs: mean=69.6±4.8; figure 1c). Intact N2 females showed an higher mating performance equal to 33% more matings and a copulation onset 8 min earlier—with *EP* males than with the two other perfumed males, which were not different (figure 1d). The comparison with decapitated females reveals that intact N2 females can distinguish *EP* and *N2* (which directed similar CIs to decapitated females), but not *N2* and *desat1* (which showed different CIs). When the mating response of N2 females obtained with the six male types (three genotypes and three perfumed males) was ranked by increasing efficiency (higher mating frequency combined with shorter latency: EP>N2>*EP*>*N2*≥*desat1*≥desat1), it followed the pattern for decreasing quantity of 7-T (table 1).
(c) desat1 is partially involved in female perception of male pheromones
We measured the effect of the desat1 mutation in homozygous females that were tested (similarly to N2 control females) with EP, N2 and desat1 males, and also with desat1 perfumed males (*EP* and *desat1*; figure 1, grey bars).
With decapitated desat1 females, males showed a CI pattern that resembled those directed to N2 decapitated females, and N2 males showed the weakest CI. However, if intact mutant females clearly distinguished desat1 males (58% pairs copulated after 23 min) from N2 and EP males, they showed only a slightly different response towards these two male genotypes: (i) their copulation latency was not different (and occurred 13 min earlier than with desat1 males and (ii) more mutant females mated with EP (96%) than with N2 males (78%), but this could reflect different male CI.
Then, we compared the attractivity of desat1 females and their response to *desat1* versus *EP* males (*N2* males which had the same influence as *desat1* males on N2 females were not tested). The two perfumed males directed similar CIs to decapitated desat1 mutant females, and were poorly distinguished by intact desat1 females: if mating was faster with *EP* males, both perfumed males induced similar mating frequencies. This result differs from that obtained with N2 females which showed both more frequent and faster matings with *EP* males than with *desat1* males.
N2 males had a shorter copulation duration than EP and desat1 males, but the CH transfer induced no difference. In all cases, copulation duration was shorter with mutant females than with N2 females, and this variation follows the CH difference between the two female genotypes (N2 are rich—and desat1 are poor—in 7,11-dienes; Marcillac et al. in press).
(d) The female antenna is necessary for perception of male pheromones
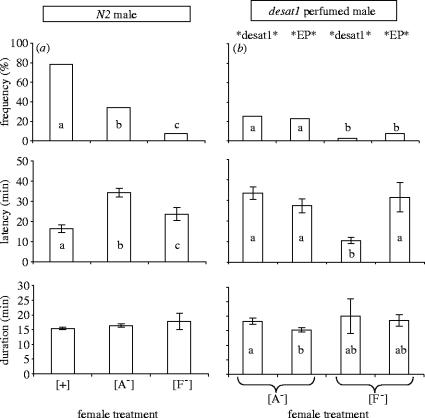
To determine the role of the female antenna in the perception of male courtship signals, the mating behaviour of control N2 females bilaterally deprived either of their aristae (A−) or of their third antennal segments, or funiculi (F−), was measured.
First, to measure the effect of antennal ablation on female behaviour, control, A−, and F− females were paired with control N2 males (figure 2a). The mating frequency (77%) and the copulation latency (16 min) of intact N2 females paired with homotypic N2 males were both very close to the values previously obtained with similar genotypes (figure 1b). The two types of antennal ablation drastically decreased mating frequency, and F− females mated less frequently than A− females (respectively, 8 and 34%). Although both operated females took more time to mate than intact ones, surprisingly, F− females mated faster than A− females.
Figure 2.
Effect of antennal ablation on female mating with control and perfumed males. Five day old females homozygous for the desat11573-N2 allele (N2) that were either intact ([+]), bilaterally ablated for their aristae ([A−]), or for their funiculi ([F−]) were either paired with (a) a same age N2 male or (b) with a desat1 male perfumed with the hydrocarbons of desat1 (*desat1*), or of EP males (*EP*). Data shown represent the frequency of mating, and the mean (±s.e.m.) for copulation latency and duration (in min) measured during a 1 h period. For genotypes, see table 1; for statistics, see figure 1. n=63–82 except for [+] females with N2 males (32).
To assess the role of the female antenna on the perception of male pheromone, N2 females which had been operated on were either paired with perfumed *EP* or *desat1* males, and their mating performance was compared (figure 2b). With both perfumed males, females which had been operated on showed mating frequencies very close to those obtained with N2 males: only 24% A− and 6% F− females mated. However, very few—if any—of the females which had been operated on distinguished the two perfumed males. This contrasts with the discriminatory response of intact N2 females paired with similarly perfumed males (figure 1d), and suggests that antennal ablation affected female perception of the male pheromone. As with N2 males (figure 2a), F− females mated faster than A− females with *desat1* males. Finally, antennal ablation had no or very little effect on copulation duration.
4. Discussion
Darwin (1874) postulated that if the most odoriferous males were the most successful in winning females, male odours should constitute a sexually selected trait. The sexual selection of a male scent was initially proposed to explain the ‘rare-male’ effect observed between strains of Drosophila pseudoobscura (Leonard et al. 1974; Leonard & Ehrman 1976). However, the active substance(s) was not identified, and the rare-male effect may be explained by an experimental artefact (Bryant et al. 1980; Partridge 1988). More recently, a functional coupling between acoustic and chemical signals obtained with transgenic males depleted for CHs and surgically deprived of wings was postulated (Rybak et al. 2002). Again, the nature of the chemical signal(s) was not revealed, but the variation of male behaviour could also be a by-product of the experimental treatment, which severely affected survival and behaviour in these flies (Savarit et al. 1999; Savarit & Ferveur 2002).
(a) Female perception of male pheromones
Our data suggest that 7-tricosene (7-T) is a male-specific trait preferred by D. melanogaster females. A clear relationship was found between the amount of 7-T carried by the tester male (table 1) and his mating efficiency with control females, and this effect was not related to the variation of male courtship (with decapitated females; figure 1). The fact that control females could detect an increase of 20–40 ng of 7-T transferred to the cuticle of desat1 males suggests that 7-T was not masked by closely related unsaturated CHs like 7-P whose production was drastically decreased in desat1 males (Marcillac et al. in press). Indeed, the more successful types of male had generally a higher 7-T : 7-P ratio (2.90) than the less successful ones (1.42). If this is true, this means that Drosophila females can discriminate male pheromones based on the ratio between the principal component (7-T) and less abundant related molecules (7-P), similarly to moths (reviewed in Wyatt 2003).
With different tools, we impaired female discrimination of male pheromones: desat1 females mated faster (and more frequently) than genetically related control N2 females, whereas funiculi-less females mated faster (but less frequently) than aristae-less females. This shows that the female latency to copulate is, by itself, not a reliable indicator of receptivity. The effect of 7-T on female willingness to mate seems to be better reflected when both the latency and the mating frequency were examined together. With N2 females, 7-T seems to induce a dose-dependent effect above a 70–90 ng threshold: no behavioural variation was observed between males carrying less than 70 ng of 7-T (with desat1, *desat1* and *N2* males, 54–61% matings occurred after a 20–22 min latency), but with doses of 7-T increasing from 90 to 1300 ng, females proportionally enhanced their mating frequency (71–85% with *EP*, N2 and EP males) and decreased their mating latency (15–10 min). If the mating frequency of aristae-less females was close to the values obtained in previous studies (Petit 1958; Manning 1967), ‘our’ funiculi-less females mated less frequently than aristae-less females, and this difference was not previously reported. This indicates that the aristae and the funiculi both exert additive effects on female perception of male-specific signals. We believe that aristae-less females mated less frequently and showed a longer latency than control females, probably because they were much less receptive to—but still perceived—male signals. The faster matings observed with funiculi-less females indicate that in rare cases, males forced copulation with an unreceptive female. However, our results do not indicate whether other chemosensory structures are also involved in male pheromone perception.
Finally, the fact that *desat1* males—but not other perfumed or non-perfumed males—showed a much lower courtship of N2 females (but not of desat1 females) suggest that males can perceive the CHs transferred on their own cuticle and compare them to the CHs carried by the female.
(b) Evolution of pheromonal communication
Could the amount of 7-T be used by the Drosophila female as a indicator of the ‘good genetic quality’ of the male (Williams 1966) as shown for a male acoustic signal: the pulse song (PS)? This male trait, which provides an indication of male quality, was preferred by females: PS frequency was correlated with the survival rate of male's progeny in Drosophila montana (Hoikkala et al. 1998), and the amount of PS offered during courtship was positively correlated with increased mating in D. melanogaster (Talyn & Dowse 2004). We hypothesize that the high proportion of 7-T in males reflects their high degree of genetic and physiological homeostasis: 7-T was often decreased in males with altered genes or facing environmental stress (Ferveur & Jallon 1993a,b; Cobb & Ferveur 1996a; Sureau & Ferveur 1999; Savarit & Ferveur 2002).
If 7-T exerts such a marked effect on female preference, this substance should be abundant on the cuticle of all D. melanogaster males. This is not the case and males show an important quantitative variation for their 7-T/7-P ratio: in strains collected in temperate area, males have a much higher ratio (4.5 in Cs males) than males of tropical and equatorial strains (0.1 in Tai males; Sureau & Ferveur 1999). The persistence of 7-T-poor males could be explained if some females do not prefer males with higher doses of 7-T. This is consistent with the observation that Tai-like females were less accurate than Cs-like females in discriminating males with different CH profiles (Scott 1994; Haerty et al. 2002). However, as these strains are diverging for many genetic loci (Ferveur & Jallon 1996; Haerty et al. 2003), this pheromonal difference may not be related only to the behavioural variation, but could also reflects adaptation to variable climatic conditions (Rouault et al. 2004).
Males with low levels of 7-T (and high levels of 7-P) are mostly found in Africa, where the D. melanogaster ancestor is supposed to have arisen, 3–4 Myr ago (Lemeunier et al. 1986; David & Capy 1988). The expansion of this species to a colder environment could have relaxed the selection pressure that kept 7-T at a low level (in relation to its possible primary effect on desiccation; Gibbs 1998), subsequently allowing males of some populations to increase their 7-T production. In theory, a new variation of a signal could either reinforce a gradual divergence that already exists between two allopatric populations with postmating incompatibility (Dobzhansky 1937), or induce a rapid divergence if a high correlation between the emission and reception of the signal exists in a metapopulation (Lande 1981). In the latter case, a high positive assortative mating could reduce the gene flow with the principal population (Butlin 1987). This fits well with the effect of desat1: this gene changes (i) the amount of the principal female and male pheromones (7,11-dienes and 7-T), two critical signals for mate choice by conspecifics together with (ii) male and female perception of these sex pheromones (Marcillac et al. 2005a,b, in press). The present study also suggests that desat1 females perceived the difference between the three males less accurately than N2 females and supports the hypothesis that desat1 females have partially—but not totally—lost their ability to discriminate male differences including the variation of male CHs.
We do not know whether the pleiotropic effect of desat1 is a unique example, but several aspects of the pheromonal communication system described in the D. melanogaster subgroup of species resembles those discovered in Drosophila serrata and Drosophila birchi. In these species, male and female flies use different CHs for mate recognition, and these pheromones are subject to directional sexual selection of a similar strength (Blows & Allan 1998; Chenoweth & Blows 2003). The genetic correlation between male and female components of the mate recognition system can rapidly coevolve suggesting that these traits are controlled by a small number of major genes (Blows 1999; Higgie et al. 2000). Therefore, subtle molecular changes in desat1, which links pheromonal emission and reception, could be related to examples of incipient speciation such as in the Zimbabwe area: Zimbabwean flies tend to mate in homogamy (Wu et al. 1995), and the gene(s) segregating with that behavioural variation have been mapped to a cytogenetic region that includes desat1 (Fang et al. 2002). Moreover, Zimbabwean flies also differ for the desat2 gene (flanking desat1) which affects cold adaptation in the fly (Greenberg et al. 2003).
In summary, we show that the principal Drosophila male cuticular pheromone (7-tricosene) can change female receptivity and mating behaviour. This finding should help us to better understand the implication of pheromonal communication in sexual selection and isolation.
Acknowledgments
The authors thank Matthew Cobb, Thierry Rigaud, Claude Everaerts and members of the UMR5548 for their comments on a previous version of the manuscript, Toshiro Aigaki for the generous gift of the E(P)-10164 strain. This work was partially funded by the Centre National de la Recherche Scientifique and by the Burgundy Regional Council (for L.D. and J.-F.F.).
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Antony C, Jallon J.M. The chemical basis for sex recognition in Drosophila melanogaster. J. Insect Physiol. 1982;28:873–880. doi:10.1016/0022-1910(82)90101-9 [Google Scholar]
- Averhoff W.W, Richardson R.H. Pheromonal control of mating patterns in Drosophila melanogaster. Behav. Genet. 1974;4:207–225. doi: 10.1007/BF01074155. doi:10.1007/BF01074155 [DOI] [PubMed] [Google Scholar]
- Bennet-Clark H.C. Stimuli provided by courtship of male Drosophila melanogaster. Nature. 1967;215:669–671. [Google Scholar]
- Blows M.W. Evolution of the genetic covariance between male and female components of mate recognition: an experimental test. Proc. R. Soc. B. 1999;266:2169–2174. doi: 10.1098/rspb.1999.0904. doi:10.1098/rspb.1999.0904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blows M.W, Allan R.A. Levels of mate recognition within and between two Drosophila species and their hybrids. Am. Nat. 1998;152:826–837. doi: 10.1086/286211. doi:10.1086/286211 [DOI] [PubMed] [Google Scholar]
- Bryant E.H, Kence A, Kimball K.T. A rare-male advantage in the housefly induced by wing clipping and some general considerations for Drosophila. Genetics. 1980;96:975–993. doi: 10.1093/genetics/96.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin B.J. Speciation by reinforcement. Trends Ecol. Evol. 1987;2:8–13. doi: 10.1016/0169-5347(87)90193-5. doi:10.1016/0169-5347(87)90193-5 [DOI] [PubMed] [Google Scholar]
- Chenoweth S.F, Blows M.W. Signal trait sexual dimorphism and mutual sexual selection in Drosophila serrata. Evolution. 2003;57:2326–2334. doi: 10.1111/j.0014-3820.2003.tb00244.x. [DOI] [PubMed] [Google Scholar]
- Cobb M, Ferveur J.F. Evolution and genetic control of mate recognition and stimulation in Drosophila. Behav. Process. 1996a;35:35–54. doi: 10.1016/0376-6357(95)00052-6. doi:10.1016/0376-6357(95)00052-6 [DOI] [PubMed] [Google Scholar]
- Cobb M, Ferveur J.F. Female mate discrimination or male responses to female stimulation? Evolution. 1996b;50:1719–1720. doi: 10.1111/j.1558-5646.1996.tb03944.x. [DOI] [PubMed] [Google Scholar]
- Coyne J.A. Genetics of a difference in male cuticular hydrocarbons between two sibling species, Drosophila simulans and D. sechellia. Genetics. 1996;143:1689–1698. doi: 10.1093/genetics/143.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J.A, Oyama R. Localization of pheromonal sexual dimorphism in Drosophila melanogaster and its effect on sexual isolation. Proc. Natl Acad. Sci. USA. 1995;92:9505–9509. doi: 10.1073/pnas.92.21.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J.A, Crittenden A.P, Mah K. Genetics of a pheromonal difference contributing to reproductive isolation in Drosophila. Science. 1994;265:1461–1464. doi: 10.1126/science.8073292. [DOI] [PubMed] [Google Scholar]
- Darwin C. 2nd edn. Murray; London: 1874. The descent of man and selection in relation to sex. [Google Scholar]
- David J.R, Capy P. Genetic variation of Drosophila melanogaster natural populations. Trends Genet. 1988;4:106–111. doi: 10.1016/0168-9525(88)90098-4. doi:10.1016/0168-9525(88)90098-4 [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. 2nd edn. Columbia University Press; New York: 1937. Genetics and the origin of species. [Google Scholar]
- Doi M, Matsuda M, Tomaru M, Matsubayashi H, Oguma Y. A locus for female discrimination behavior causing sexual isolation in Drosophila. Proc. Natl Acad. Sci. USA. 2001;98:6714–6719. doi: 10.1073/pnas.091421598. doi:10.1073/pnas.091421598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Takahashi A, Wu C.I. A mutation in the promoter of desaturase 2 is correlated with sexual isolation between Drosophila behavioral races. Genetics. 2002;162:781–784. doi: 10.1093/genetics/162.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur J.F. Genetic control of pheromones in Drosophila simulans. I. Ngbo, a locus on the second chromosome. Genetics. 1991;128:293–301. doi: 10.1093/genetics/128.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur J.F. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav. Genet. 2005;35:279–295. doi: 10.1007/s10519-005-3220-5. doi:10.1007/s10519-005-3220-5 [DOI] [PubMed] [Google Scholar]
- Ferveur J.F, Jallon J.M. Genetic control of pheromones in Drosophila simulans. II. kete, a locus on the X chromosome. Genetics. 1993a;133:561–567. doi: 10.1093/genetics/133.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur J.F, Jallon J.M. Nerd, a locus on chromosome III, affects male reproductive behavior in Drosophila melanogaster. Naturwissenschaften. 1993b;80:474–475. doi: 10.1007/BF01136042. doi:10.1007/BF01136042 [DOI] [PubMed] [Google Scholar]
- Ferveur J.F, Jallon J.M. Genetic control of male cuticular hydrocarbons in Drosophila melanogaster. Genet. Res. 1996;67:211–218. doi: 10.1017/s0016672300033693. [DOI] [PubMed] [Google Scholar]
- Ferveur J.F, Sureau G. Simultaneous influence on male courtship of stimulatory and inhibitory pheromones produced by live sex-mosaic Drosophila melanogaster. Proc. R. Soc. B. 1996;263:967–973. doi: 10.1098/rspb.1996.0143. [DOI] [PubMed] [Google Scholar]
- Gailey D.A, Lacaillade R.C, Hall J.C. Chemosensory elements of courtship in normal and mutant, olfaction-deficient Drosophila melanogaster. Behav. Genet. 1986;16:375–405. doi: 10.1007/BF01071319. doi:10.1007/BF01071319 [DOI] [PubMed] [Google Scholar]
- Gibbs A.G. Water-proofing properties of cuticular lipids. Am. Zool. 1998;38:471–482. [Google Scholar]
- Göpfert M.C, Robert D. The mechanical basis of Drosophila audition. J. Exp. Biol. 2002;205:1199–1208. doi: 10.1242/jeb.205.9.1199. [DOI] [PubMed] [Google Scholar]
- Greenberg A.J, Moran J.R, Coyne J.A, Wu C.I. Ecological adaptation during incipient speciation revealed by precise gene replacement. Science. 2003;302:1754–1757. doi: 10.1126/science.1090432. doi:10.1126/science.1090432 [DOI] [PubMed] [Google Scholar]
- Haerty W, Jallon J.M, Rouault J, Bazin C, Capy P. Reproductive isolation in natural populations of Drosophila melanogaster from Brazzaville (Congo) Genetica. 2002;116:215–224. doi:10.1023/A:1021288527291 [PubMed] [Google Scholar]
- Haerty W, Gibert P, Capy P, Moreteau B, David J.R. Microspatial structure of Drosophila melanogaster populations in Brazzaville: evidence of natural selection acting on morphometrical traits. Heredity. 2003;91:440–447. doi: 10.1038/sj.hdy.6800305. doi:10.1038/sj.hdy.6800305 [DOI] [PubMed] [Google Scholar]
- Higgie M, Chenoweth S, Blows M.W. Natural selection and the reinforcement of mate recognition. Science. 2000;290:519–521. doi: 10.1126/science.290.5491.519. doi:10.1126/science.290.5491.519 [DOI] [PubMed] [Google Scholar]
- Hoikkala A, Aspi J, Suvanto L. Male courtship song frequency as an indicator of male genetic quality in an insect species, Drosophila montana. Proc. R. Soc. B. 1998;265:503–508. doi: 10.1098/rspb.1998.0323. doi:10.1098/rspb.1998.0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallon J.M. A few chemical words exchanged by Drosophila during courtship and mating. Behav. Genet. 1984;14:441–478. doi: 10.1007/BF01065444. doi:10.1007/BF01065444 [DOI] [PubMed] [Google Scholar]
- Kyriacou C.P, Hall J.C. The function of courtship song rhythms in Drosophila. Anim. Behav. 1982;30:784–801. doi: 10.1006/anbe.1998.0976. [DOI] [PubMed] [Google Scholar]
- Lande R. The minimum number of genes contributing to quantitative variation between and within populations. Genetics. 1981;99:541–553. doi: 10.1093/genetics/99.3-4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemeunier F, David J.R, Tsacas L, Ashburner M. The melanogaster species group. In: Ashburner M, Carson H.L, Thompson J.N, editors. The genetic and biology of Drosophila. vol. 3. Academic Press; London: 1986. pp. 147–256. [Google Scholar]
- Leonard J.E, Ehrman L. Recognition and sexual selection in Drosophila: classification, quantification, and identification. Science. 1976;193:693–695. doi: 10.1126/science.948745. [DOI] [PubMed] [Google Scholar]
- Leonard J.E, Ehrman L, Schorsch M. Bioassay of a Drosophila pheromone influencing sexual selection. Nature. 1974;250:261–262. doi: 10.1038/250261a0. doi:10.1038/250261a0 [DOI] [PubMed] [Google Scholar]
- Linn J.C.E, Roelofs W.L. Response specificity of male moths to different blends and dosages of sex pheromone. Chem. Senses. 1989;14:421–437. doi: 10.1007/BF01012203. [DOI] [PubMed] [Google Scholar]
- Manning A. Antennae and sexual receptivity in Drosophila melanogaster females. Science. 1967;158:136–137. doi: 10.1126/science.158.3797.136. [DOI] [PubMed] [Google Scholar]
- Marcillac F, Ferveur J.F. A set of female pheromones affects reproduction before, during and after mating in Drosophila. J. Exp. Biol. 2004;207:3927–3933. doi: 10.1242/jeb.01236. doi:10.1242/jeb.01236 [DOI] [PubMed] [Google Scholar]
- Marcillac F, Grosjean Y, Ferveur J.F. A single mutation alters production and discrimination of Drosophila sex pheromones. Proc. R. Soc. B. 2005a;272:303–309. doi: 10.1098/rspb.2004.2971. doi:10.1098/rspb.2004.2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcillac F, Houot B, Ferveur J.F. Female pheromone role revisited. Chem. Senses. 2005b;30:273–274. doi: 10.1093/chemse/bjh220. doi:10.1093/chemse/bjh220 [DOI] [PubMed] [Google Scholar]
- Marcillac F, Bousquet F, Alabouvette J, Savarit F, Ferveur J.F. A mutation with major effects on Drosophila melanogaster sex pheromones. Genetics. In press doi: 10.1534/genetics.104.033159. doi:10.1534/genetics [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr E. The role of the antennae in the mating behavior of female Drosophila. Evolution. 1950;4:149–154. [Google Scholar]
- Mustaparta H. Olfactory coding mechanisms for pheromone and interspecific signal information in related moth species. In: Cardé R.T, Minks A.K, editors. Insect pheromone research: new directions. Chapman & Hall; London: 1996. pp. 144–163. [Google Scholar]
- Partridge L. The rare-male effect: what is its evolutionary significance? Phil. Trans. R. Soc. B. 1988;319:525–539. doi: 10.1098/rstb.1988.0063. [DOI] [PubMed] [Google Scholar]
- Pechine J.M, Perez F, Antony C, Jallon J.M. A further characterization of Drosophila cuticular monoenes using a mass spectrometry method to localize double bonds in complex mixtures. Anal. Biochem. 1985;145:177–182. doi: 10.1016/0003-2697(85)90344-6. doi:10.1016/0003-2697(85)90344-6 [DOI] [PubMed] [Google Scholar]
- Petit C. Le déterminisme génétique et psychophysiologique de la compétition sexuelle chez Drosophila melanogaster. Bull. Biol. France Belg. 1958;92:248–329. [Google Scholar]
- Pineiro R, Carracedo M.C, Izquierdo J.I, Casares P. Bidirectional selection for female receptivity in Drosophila melanogaster. Behav. Genet. 1993;23:77–83. doi: 10.1007/BF01067556. doi:10.1007/BF01067556 [DOI] [PubMed] [Google Scholar]
- Rorth P. A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc. Natl Acad. Sci. USA. 1996;93:12 418–12 422. doi: 10.1073/pnas.93.22.12418. doi:10.1073/pnas.93.22.12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault J.D, Marican C, Wicker-Thomas C, Jallon J.M. Relations between cuticular hydrocarbon (HC) polymorphism, resistance against desiccation and breeding temperature; a model for HC evolution in D. melanogaster and D. simulans. Genetica. 2004;20:195–212. doi: 10.1023/b:gene.0000017641.75820.49. doi:10.1023/B:GENE.0000017641.75820.49 [DOI] [PubMed] [Google Scholar]
- Rybak F, Sureau G, Aubin T. Functional coupling of acoustic and chemical signals in the courtship behaviour of the male Drosophila melanogaster. Proc. R. Soc. B. 2002;269:695–701. doi: 10.1098/rspb.2001.1919. doi:10.1098/rspb.2001.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarit F, Ferveur J.F. Temperature affects the ontogeny of sexually dimorphic cuticular hydrocarbons in Drosophila melanogaster. J. Exp. Biol. 2002;205:3241–3249. doi: 10.1242/jeb.205.20.3241. [DOI] [PubMed] [Google Scholar]
- Savarit F, Sureau G, Cobb M, Ferveur J.F. Genetic elimination of known pheromones reveals the fundamental chemical bases of mating and isolation in Drosophila. Proc. Natl Acad. Sci. USA. 1999;96:9015–9020. doi: 10.1073/pnas.96.16.9015. doi:10.1073/pnas.96.16.9015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. Genetic variation for female mate discrimination in Drosophila melanogaster. Evolution. 1994;48:112–121. doi: 10.1111/j.1558-5646.1994.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Sureau G, Ferveur J.F. Co-adaptation of pheromone production and behavioural responses in Drosophila melanogaster males. Genet. Res. 1999;74:129–137. doi: 10.1017/s0016672399003936. doi:10.1017/S0016672399003936 [DOI] [PubMed] [Google Scholar]
- Svetec N, Ferveur J.F. Social experience and pheromonal perception can change male–male interactions in Drosophila melanogaster. J. Exp. Biol. 2005;208:891–898. doi: 10.1242/jeb.01454. doi:10.1242/jeb.01454 [DOI] [PubMed] [Google Scholar]
- Talyn B.C, Dowse H.B. The role of courtship song in sexual selection and species recognition by female Drosophila melanogaster. Anim. Behav. 2004;68:1165–1180. doi:10.1016/j.anbehav.2003.11.023 [Google Scholar]
- Tauber E, Eberl D.F. Acoustic communication in Drosophila. Behav. Process. 2003;64:197–210. doi:10.1016/S0376-6357(03)00135-9 [Google Scholar]
- Tompkins L, Hall J.C. Identification of brain sites controlling female receptivity in mosaics of Drosophila melanogaster. Genetics. 1983;103:179–195. doi: 10.1093/genetics/103.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins L, Gross A.C, Hall J.C, Gailey D.A, Siegel R.W. The role of female movement in the sexual behavior of Drosophila melanogaster. Behav. Genet. 1982;12:295–307. doi: 10.1007/BF01067849. doi:10.1007/BF01067849 [DOI] [PubMed] [Google Scholar]
- Williams G.C. Princeton University Press; Princeton, NJ: 1966. Adaptation and natural selection: a critique of some current evolutionary thought. [Google Scholar]
- Wu C.I, Hollocher H, Begun D.J, Aquadro C.F, Xu Y, Wu M.L. Sexual isolation in Drosophila melanogaster: a possible case of incipient speciation. Proc. Natl Acad. Sci. USA. 1995;92:2519–2523. doi: 10.1073/pnas.92.7.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt T.D. Cambridge University Press; Cambridge, UK: 2003. Pheromones and animal behaviour: communication by smell and taste. [Google Scholar]