Abstract
Although variation in male fertilization efficiency has been shown to have a genetic basis in several species, the genes responsible for the effect are generally unknown. Here, we show a strong association between the fertilization success of males and their phosphogluconate dehydrogenase (Pgdh) genotype in the bulb mite Rhizoglyphus robini. Males homozygous for the slow (S) allele fathered a significantly greater proportion of offspring when competing with males homozygous for the fast (F) allele. There was no evidence that female fecundity was influenced by their Pgdh genotype. The fecundity of FF females did not differ significantly from the fecundity of SS females but female fecundity was significantly influenced by the genotype of their mate. Females paired with SS males laid significantly fewer eggs than females paired with FF males. Altogether these data show a trade-off, with the male SS genotype associated with their higher fertilization efficiency but at the cost of a negative impact on the fecundity of females mating with them.
Keywords: sexual selection, sperm competition, inter-sexual conflict, enzyme polymorphism
1. Introduction
Because the females of many sexually reproducing organisms mate with several males (Birkhead & Møller 1998), the reproductive success of males is commonly influenced by processes occurring after insemination has taken place. Variation in fertilization efficiency between males can result from the difference in one or several of the four following characteristics (Keller & Reeve 1995): (i) the quantity of sperm they produce, (ii) the success of their sperm in reaching and fertilizing an egg, (iii) their ability to displace the sperm that females stored during previous matings and (iv) their ability to prevent any other male from subsequently introducing sperm (e.g. differential efficiency of mating plugs).
Experimental studies have revealed consistent differences among males in fertilization efficiency (Lewis & Austad 1990; Cordero & Miller 1992; Radwan 1996) and there is increasing evidence that this variation has an important genetic component. For example, in various species males of different strains exhibit variation in fertilization success (Napier 1961; Wooley & Beatty 1967; Krzanowska et al. 1991; Kirby & Froman 1991). Many phenotypic traits presumably influencing fertilization efficiency have also been shown to have additive genetic variance and exhibit a significant heritability (reviews in Keller & Reeve 1995; Pizzari & Birkhead 2002), and the heritability of male sperm competition success has been demonstrated (Radwan 1998; Konior et al. in press). Finally, selection experiments have revealed that the fertilization efficiency of males is increased in selection lines where females mate with several males compared to monogamous lines (Bernasconi & Keller 2001; Hosken et al. 2001; Pitnick et al. 2001), implying its heritability.
Although the fertilization efficiency of males has been shown to have a strong genetic basis in many species, the genes responsible for the effect have been identified in only a couple of instances. In Drosophila melanogaster particular alleles at four accessory gland protein (Acp) genes were significantly associated with males' ability to resist sperm-displacement by sperm from males that subsequently mated with the same females (Clark et al. 1995). By contrast, no clear association was found between the genotype at Acp and males' ability to displace sperm that females stored during previous matings (Clark et al. 1995). In the yellow dung fly Scatophaga stercoraria, the male genotype at the Pgm locus was found to interact with the female genotype and the environment in which she oviposited, in affecting male paternity (Ward 2000). This effect was attributed to cryptic female choice evolved to produce optimal progeny genotypes in a given environment (Ward 2000).
Here, we investigated the association between the enzyme-encoding gene phosphogluconate dehydrogenase (Pgdh) genotype and male fertilization efficiency in the bulb mite Rhizoglyphus robini. The study was prompted by the reanalysis of data obtained by Kołodziejczyk et al. (2002) in a survey of enzyme polymorphisms in mites of the genus Rhizoglyphus. The reanalysis (reported below) indicated that a male's genotype may be associated with his sperm competition success. This result was confirmed in a new set of experiments where we mated females with males of different Pgdh genotypes. We also conducted experiments to determine whether a female's genotype at Pgdh was associated with differences in fecundity. Finally, we tested whether the fecundity of females was influenced by the genotype of males. These experiments were prompted by previous studies indicating the presence of sexually antagonistic genes having a positive effect on male reproductive success while decreasing the fitness of females mating with males harbouring such genes (Rice 1996; Friberg & Arnqvist, 2003).
2. Material and methods
(a) PGDH allozymes
The fast (F) and slow (S) phosphogluconate dehydrogenase (PGDH, EC 1.1.1.44) electromorphs were separated by cellulose acetate electrophoresis. Cellulose acetate plates (Malta Chemetron, Milano, Italy) were soaked for 20 min in 10% TME 7.4 (0.1 M Tris, 0.1 M maleic acid, 0.01 M diosodium EDTA, 0.01 M MgCl2·6H2O, adjusted to pH 7 with KOH). Before samples were applied, the plates were gently dried on filter paper. Individual mites were homogenized in 3 μl of distilled water containing NADP (5 mg/100 ml) and immediately transferred onto the plate. The plate was loaded with seven samples, and the eighth lane contained a mixture of FF and SS homozygotes from pre-selected sub-populations (see below) and served as a reference. The plate was placed in a horizontal tank and run in 10% TME 7.4 buffer at 170–250 V, ca 4 mA for 20–30 min at room temperature. Plates were stained with 5 ml 0.2 M Tris HCl pH 8.90, 0.1 ml MgCl2, 10 mg 6-phosphogluconic acid, 0.1 ml NADP, 0.1 ml PMS and 0.1 ml MTT.
(b) Re-analysis of previous data
As a part of a survey of enzymatic polymorphism in the genus Rhizoglyphus. Kołodziejczyk et al. (2002) conducted a preliminary study to determine whether Pgdh could be used as a marker of paternity in R. robini (Kołodziejczyk et al. 2002). In that study females from a population made homozygous for the S allele were mated with two males randomly selected from a stock population. The males were then genotyped, and paternity was analysed for the double-matings that involved males homozygous for the two alternative alleles (i.e. five double-matings in which a female mated first with a SS male and 14 matings where the female mated first with a FF male, see Kołodziejczyk et al. 2002 for further details), but the test comparing P2 (proportion of eggs fertilized by the second male) between these two mating orders was not reported. Here, we present a reanalysis of that data in which we compare fertilization success of males homozygous for alternative Pgdh alleles.
(c) The mites
The mites came from a founding population of about 100 mites obtained from onions collected in 2001 near Kraków in Poland and, after population expansion, maintained as a large stock culture (further referred to as 2001 stock culture) containing over 500 individuals. The mites were maintained in desiccators at room temperature and >90% relative humidity. Individuals were fed ad libitum with a 3 : 1 mixture of powdered yeast and wheat germ. Three weeks after the mites were brought to the laboratory, i.e. when the first lab-reared generation achieved maturity, a sample of 42 females was genotyped to determine whether the population was polymorphic at Pgdh (we used females because they are larger and easier to genotype). Another 40 females were genotyped in 2004 to determine whether the frequency of the two Pgdh alleles had changed after 3 years of laboratory rearing (i.e. after about 60 generations).
(d) Homozygous sub-populations
After two months of laboratory rearing we established two sub-populations homozygous for Pgdh (i.e. the SS and FF sub-populations). The SS sub-population was established by mixing the progeny of 20 pairs of parents homozygous for the S allele (4 offspring per pair). Both parents were genotyped after they produced progeny. Because the F allele is uncommon it was not possible to use the same procedure to establish an FF population in a single generation. The FF sub-population was established over two generations. In the first generation, we mated females with one male and selected 4–8 offspring in families where each parent had at least one copy of the F allele (parents were genotyped after offspring were produced). In the next generation we coupled these families at random and for each couple we paired males from one family with females from another family and vice versa (i.e. 4–8 pairs per couple of families). Subsequent genotyping of these pairs revealed 13 couples of families containing at least one pair of mates both homozygous for the F allele. These 13 pairs of individuals were unrelated within pairs and across pairs, and we used 4 offspring from each pair to establish the FF sub-population.
The sub-populations were then allowed to expand for about three months. During that time, each sub-population was maintained in a 2.5 cm diameter tube under identical regimes. All the experiments described below were completed within six months after the end of the expansion period. Before conducting the sperm competition and male harmfulness experiments we isolated larvae in 0.8 cm diameter glass tubes (2 cm high) with Plaster of Paris bases soaked with water. All these tubes were placed in the same desiccator throughout larval development (about 6 days). After adult males emerged, they remained in the same tubes and were supplied with a female from the stock population. They remained paired for 2 days before being used for experiments. This was done to mimic the natural situation of this colonial species where males have constant access to females. Additionally, this helped to standardize reserves of semen between males (Radwan 1997).
(e) Sperm competition
12 FF and 12 SS virgin females were sequentially presented with two males. Each male was paired for 2 h with the female. This time was sufficient for the majority of pairs to mate (in a similar setting, (Radwan et al. 2005) recorded about 90% pairs initiating copulation within 90 min, n=60), but not long enough for repeated matings to occur. In half of the experiments the female was presented first with a FF male then with a SS male while in the other half male order was reversed. After mating the females were allowed to lay eggs for 5 days, after which both parents were genotyped to confirm that they were both homozygous for the F or S alleles. After progeny matured, 20 individuals were randomly selected from each family to be genotyped at Pgdh.
(f) Female genotype and fecundity
To determine whether the genetic correlation between sperm competitiveness and fecundity uncovered in previous studies (Konior et al. 2001; Kozielska et al. 2004) was associated with the Pgdh locus, we paired 46 virgin SS females and 59 virgin FF females with males from the 2001 stock culture. The male was exchanged every day for the first 3 days, such that each female mated with three males. Three males were used to reduce the effects of occasional male infertility on female fecundity. On the fifth day, all eggs laid by each female were counted.
(g) Male harmfulness
To determine whether there was an association between male Pgdh genotype and the fecundity of their mates, virgin females from stock cultures were paired during 10 days with one SS (n=81) or FF (n=77) males. Males were replaced every 2 days with another male of the same genotype, so that each female was paired with five males in turns. Two days after the last male was removed, we counted the eggs laid by each female.
3. Results
(a) Re-analysis of previous data
The proportion of offspring fathered by the two males mating with the same female was influenced by their genotype. The proportion of offspring fathered by SS males that mated with a female previously mated with an FF male was 0.79±0.25 (mean±s.d., n=14) while this proportion was 0.33±0.41 (n=5) for FF males that mated with a female previously mated with an SS male. The difference in fertilization efficiency between the genotypes was significant (Mann–Whitney U=13, P=0.042).
(b) New data
The proportion of offspring fathered by the two males mating with the same female was also significantly influenced by their genotype, with SS males achieving a greater paternity than FF males (figure 1, F1,20=8.72, p=0.007). By contrast, the female genotype did not significantly affect the relative paternity of males of the two alternative Pgdh genotypes (F1,20=0.61, p=0.44). The interaction between male and female genotype was also not significant (F1,20=2.95, p=0.10).
Figure 1.
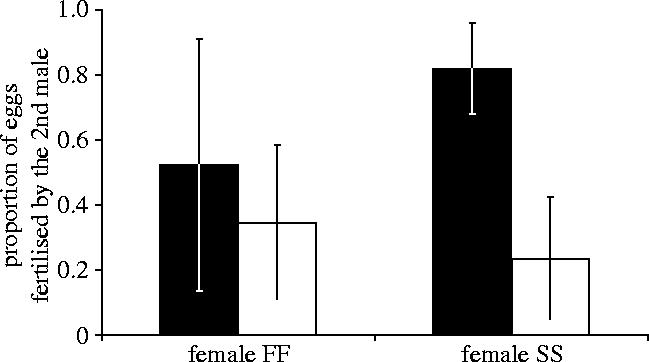
Proportion of eggs fertilized by the second male mating with the female; filled bars, SS males; open bars, FF males; error bars, standard deviation.
There was no evidence that female fecundity was influenced by their Pgdh genotype. The fecundity of FF females (mean±s.d.=129.6±64.9) was not significantly different (F1,103=12.37, p=0.13) from the fecundity of SS females (mean±s.d.=109.6±67.8). By contrast, the fecundity of females was influenced by the genotype of their mate. Females paired with a SS male laid 60.7±52.8 eggs, whereas females paired with a FF male laid 82.0±42.6 eggs (F1,156=7.76, p=0.006). Thus, males with the genotype associated with higher fertilization efficiency had a negative impact on the fecundity of their partners compared to males with the genotype associated with lowest fertilization efficiency.
The frequency of genotypes at the locus Pgdh changed significantly in the stock population over the 3 years of laboratory rearing. While the frequency of heterozygotes was 22% in 2001 (i.e. shortly after the population was brought to the laboratory; n=42 females, nine heterozygotes and 33 SS homozygotes), all females (n=40) genotyped in 2004 were homozygous for the S allele (Fisher exact: p=0.003).
4. Discussion
The results of our controlled mating experiments revealed a strong association between the fertilization success of males and their Pgdh genotype. Bulb mite males with the SS genotype at Pgdh fathered a significantly greater proportion of offspring than competing FF homozygote males. Two lines of evidence support the view that this difference in fertilization efficiency cannot be explained by a possible difference in inbreeding between the FF and SS sub-populations. First, and most importantly, the inbreeding associated with the founding of the two sub-populations was determined by their initial effective population size of 52 (2N individuals, as each of the founding pair contributed the same number of progeny) for the FF sub-population and 80 for the SS sub-populations. Since the sub-populations quickly expanded, the possible difference in inbreeding was mostly due to this initial bottleneck event, with a ΔF (an increase in inbreeding coefficient) of 1/52=0.02 for the FF sub-population and 1/80=0.012 SS subpopulation. Such a relatively low level of inbreeding is unlikely to significantly affect fertilization efficiency of males. Konior et al. (2005) reported a 53% decline in fertilization success after full-sib mating (F=0.25). Assuming that the decrease in fertilization efficiency is linearly associated with the level of inbreeding, the difference in inbreeding between the FF and SS sub-populations could account for roughly a 1.6% ((0.02−0.012)×212%) of the difference in fertilization efficiency. This value is an order of magnitude lower than the observed effect (i.e. 34% for matings with FF females, and 71% for matings with SS females).
The second piece of evidence that differences at the Pgdh locus are associated with differences in fertilization efficiency comes from our re-analysis of the data reported by Kołodziejczyk et al. (2002). In that study differences in fertilization efficiency between males of alternative genotype could not stem from differences in inbreeding because all males came from the same population, yet SS males had a ca 58% higher fertilization efficiency than FF males. Thus the competitive advantage of SS males was about the same in the two independent experiments reported here.
A potential caveat of our experiments is that difference in male reproductive success was measured at the adult, not egg, stage, making possible that genotypic differences in egg to adult survival could account for the different reproductive success of males. However, in another experiment where sperm competitiveness was measured at the egg stage using sterile male technique (e.g. Radwan & Siva-Jothy 1996), SS males were also found to have higher fertilization efficiency (P. Łukasik, M. Zygadlo & J. Radwan 2004, unpublished work).
The gene responsible for differences in male fertilization efficiency may be the Pgdh itself, or one or more genes in a very strong gametic disequilibrium with the Pgdh locus. The PGDH enzyme is involved in the pentose phosphate pathway of the oxidation of carbohydrates (Murray et al. 2003). Alternative Pgdh genotypes have general effects on metabolic activity (Cavener & Clegg 1981), with possible pleiotropic effect on a number of traits. This pathway also produces ribulose 5-phosphate, which is used for nucleotide biosynthesis. Thus, by controlling the rate of nucleic acid synthesis, the form of PGDH enzyme conceivably might affect the rate of cell division and spermatogenesis. That PGDH variants may indeed affect the phenotype is supported by several studies in plants and animals that reported an association between the Pgdh genotype and components of fitness such as the probability of survival and salinity resistance in the wild (Stockwell & Mulvey 1998; Conte et al. 2003). Similarly, differences in the expression of a number of metabolic enzymes have recently been shown to affect male competitive reproductive success in D. melanogaster (Drnevich et al. 2004). It would thus be of interest to investigate whether males with alternative Pgdh genotypes differ in weight, testis weight or sperm morphology.
The finding that males with the SS Pgdh genotype have higher fertilization efficiency raises the question of the selective forces maintaining a polymorphism at this locus (or the linked loci responsible for the difference in fertilization efficiency). Under natural condition, the frequency of the F allele is not rare (0.11 in the population used in this study and 0.32 in a population collected from the same location in 2003 (P. Łukasik, M. Zygadlo & J. Radwan 2004, unpublished work). There are several possible mechanisms that may counteract the disadvantage of FF males during the process of sperm competition. One such mechanism is male–female genotype-specific interaction as has been observed in D. melanogaster where the success of a particular male's sperm is dependent on the genotype of the female with which he mates (Clark et al. 1999). The results of our experiments where females of alternative Pgdh genotype were mated with either FF or SS males failed to reveal a significant interaction between male and female genotypes. However, our sample size was small and the power of the test low, precluding a strong conclusion.
Another possibility is a pleiotropic effect associated with the Pgdh genotype. Kozielska et al. (2004) reported a positive correlation between bulb mite males' fertilization efficiency and fecundity of their daughters. If this effect was mediated by Pgdh or a linked gene, SS females should have a higher fecundity than FF females. However, our data do not support the hypothesis of a direct effect of Pgdh on female fecundity. The fecundities of FF females did not differ significantly from the fecundities of SS females. Importantly, however, the fecundity of females was influenced by the genotype of their mate, with females mated to SS males having a lower fecundity. This demonstrates the occurrence of a sexual conflict (Parker 1979; Holland & Rice 1998; Gavrilets et al. 2001) with the SS genotype conferring a positive effect on fertilization efficiency of males but a negative effect on females that mated with males having such a genotype. These findings may provide an explanation for the observation that females with experimentally reduced access to males have higher fecundity than females constantly exposed to males (Kołodziejczyk & Radwan 2003).
Finally, a third possibility is that females prefer to mate with males with the less harmful genotypes as has been observed in the cockroach Nauphoeta cinerea (Moore et al. 2003). If females consistently discriminate against SS males, this may be a potent selective force maintaining the F allele in the wild. This possibility remains to be investigated. However, the loss of the F allele we observed in our stock culture hints against this mechanism.
We found that the number of eggs laid by females in the experiment testing the effect of female genotype on their fecundity was considerably higher (109–129 eggs) than in the experiment testing male harmfulness (60–82 eggs). This difference most likely reflected a difference in rearing conditions of females between the two experiments rather than a difference of the longer exposure of females to males in the later experiment (10 days) compared to the former (3 days). Females used for the test of male harmfulness came from stock culture were mites were kept at higher density than was the case for females used in the experiment testing the effect of female genotype on their fecundity. Since, female fecundity in R. robini is very sensitive to even small environmental changes (e.g. Konior et al. 2001), it is likely that the fecundity differences stemmed from the difference in rearing conditions.
Our study is consistent with the prediction of models of antagonistic coevolution that males achieving highest reproductive success (due to their ability to manipulate female preferences, behaviour or physiology) should impose the most detrimental effects on females (Holland & Rice 1998). Similarly, experimental manipulation of the intensity of sexual selection in three fly species resulted in an increase of the harmful effect of males on females in lines selected for higher fertilization efficiency (Holland & Rice 1999; Hosken et al. 2001; Martin & Hosken 2004). In unselected populations, evidence that males achieving highest reproductive success impose more detrimental effects on females has been supported in a few other species. In the bluehead wrasse (Thalassoma bifasciatum), females mating with the males having the highest mating success have a lower proportion of their eggs fertilized because successful males transfer fewer sperm (Warner et al. 1995). In the cockroach N. cinerea males able to pheromonally manipulate females to decrease their remating rate, also negatively affect lifespan of their mating partners (Moore et al. 2003). In D. melanogaster larger male size is under positive sexual selection but is associated with an accelerated rate of ageing and reduced female lifespan (Pitnick & Garcia-Gonzalez 2002; Friberg & Arnqvist 2003). Finally, in D. grimshawi, males exhibiting higher courtship vigour have a higher probability of mating, but they also have a negative effect on female fecundity (Droney 2003). Since mating in D. melanogaster incurs costs to females (Chapman et al. 1995) and because larger males mate more often, lower fitness of females paired to larger males might stem from increased mating frequency (Friberg & Arnqvist 2003). Whether bulb mite males homozygous for the S Pgdh allele mate more often, or if they influence female fecundity by other means remains to be investigated.
In conclusion, our study reveals that a single genetic element, Pgdh itself, or one or more genes in very strong gametic disequilibrium with the locus Pgdh, influence(s) both the fertilization efficiency of males and the fecundity of females mated with males of alternative genotypes. Importantly, while the Pgdh SS genotype confers a greater fertilization efficiency to males it also reduces the fecundity of females that mated with such males. Thus, the positive selection acting on SS males in the process of sperm competition is partly opposed by the selection arising through the decreased fecundity of their mates. The balance between the magnitude of these two selection forces will depend on the level of promiscuity. Because the level of promiscuity and sperm competition is probably higher under our laboratory conditions than in the wild (Radwan & Siva-Jothy 1996) this may lead to a relative increase of the selective effect through sperm competition and a disruption of the conditions allowing polymorphism to be maintained at Pgdh. Consistent with this prediction we observed a significant decline in the proportion of FF genotypes under laboratory conditions. However, it is also possible that other, unidentified selective forces acting on Pgdh in the field were absent or less important under laboratory conditions, hence accounting for the S allele having spread to fixation in the laboratory. More generally, our study adds to the increasing number of instances showing that protein and enzymatic variants can influence important components of fitness in the wild (e.g. Watt et al. 1985; Watt 1992; Merçot et al. 1994; Lenormand et al. 1999; Ward 2000; Mauricio et al. 2003; Hoekstra et al. 2004) or even social behaviour (Keller & Ross 1998; Krieger & Ross 2002; Coates & de Bono 2002).
Acknowledgments
We thank Dave Hosken and two anonymous reviewers for their useful comments on the manuscript. This work was supported by the State Committee for Scientific Research KBN 0408/P04/2001 and the Swiss NSF.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
References
- Bernasconi G, Keller L. Female polyandry affects their sons' reproductive success in the red flour beetle Tribolium castaneum. J. Evol. Biol. 2001;14:186–193. doi: 10.1046/j.1420-9101.2001.00247.x. doi:10.1046/j.1420-9101.2001.00247.x [DOI] [PubMed] [Google Scholar]
- Birkhead T.R, Møller A.P. Academic Press; London: 1998. Sperm competition and sexual selection. [Google Scholar]
- Cavener D.R, Clegg M.T. Evidence for biochemical and physiological differences between enzyme genotypes in Drosophila melanogaster. Proc. Natl Acad Sci. USA. 1981;78:4444–4447. doi: 10.1073/pnas.78.7.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Liddie L.F, Kalb J.M, Wolfner M.F, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. doi:10.1038/373241a0 [DOI] [PubMed] [Google Scholar]
- Clark A.G, Aguade M, Prout T, Harshman L.G, Langley C.H. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics. 1995;139:189–201. doi: 10.1093/genetics/139.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A.G, Begun D.J, Prout T. Female × male interactions in Drosophila sperm competition. Science. 1999;283:217–220. doi: 10.1126/science.283.5399.217. [DOI] [PubMed] [Google Scholar]
- Coates J.C, de Bono M. Antagonistic pathways in neurons exposed to body fluid regulate social feeding in Caenorhabditis elegans. Nature. 2002;419:925–929. doi: 10.1038/nature01170. doi:10.1038/nature01170 [DOI] [PubMed] [Google Scholar]
- Conte R, Nodari R.O, Vencovsky R, dos Reis M.S. Genetic diversity and recruitment of the tropical palm, Euterpe edulis Mart., in a natural population from the Brazilian Atlantic Forest. Heredity. 2003;91:401–406. doi: 10.1038/sj.hdy.6800347. doi:10.1038/sj.hdy.6800347 [DOI] [PubMed] [Google Scholar]
- Cordero A, Miller P.L. Sperm transfer, displacement and precedence in Ischnura graellsii (Odonata, Coenagrionidae) Behav. Ecol. Sociobiol. 1992;30:261–267. doi:10.1007/BF00166711 [Google Scholar]
- Drnevich J.M, Reedy M.M, Ruedi E.A, Rodriguez-Zas S, Hughes K.A. Quantitative evolutionary genomics: differential gene expression and male reproductive success in Drosophila melanogaster. Proc. R. Soc. B. 2004;271:2267–2273. doi: 10.1098/rspb.2004.2880. doi:10.1098/rspb.2004.2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droney D.C. Females lay fewer eggs for males with greater courtship success in a lekking Drosophila. Anim. Behav. 2003;65:371–378. doi:10.1006/anbe.2003.2056 [Google Scholar]
- Friberg U, Arnqvist G. Fitness effects of female mate choice: preferred males are detrimental for Drosophila melanogaster females. J. Evol. Biol. 2003;16:797–811. doi: 10.1046/j.1420-9101.2003.00597.x. doi:10.1046/j.1420-9101.2003.00597.x [DOI] [PubMed] [Google Scholar]
- Gavrilets S, Arnqvist G, Friberg U. The evolution of female mate choice by sexual conflict. Proc. R. Soc. B. 2001;268:531–539. doi: 10.1098/rspb.2000.1382. doi:10.1098/rspb.2000.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra H.E, Drumm K.E, Nachman M.W. Ecological genetics of adaptive color polymorphism in pocket mice: geographic variation in selected and neutral genes. Evolution. 2004;58:1329–1341. doi: 10.1111/j.0014-3820.2004.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Holland B, Rice W.R. Chase-away sexual selection: antagonistic seduction versus resistance. Evolution. 1998;52:1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- Holland B, Rice W.R. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl Acad. Sci. USA. 1999;96:5083–5088. doi: 10.1073/pnas.96.9.5083. doi:10.1073/pnas.96.9.5083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosken D.J, Garner T.W.J, Ward P.I. Sexual conflict selects for male and female reproductive characters. Curr. Biol. 2001;11:489–493. doi: 10.1016/s0960-9822(01)00146-4. doi:10.1016/S0960-9822(01)00146-4 [DOI] [PubMed] [Google Scholar]
- Keller L, Reeve H.K. Why do females mate with multiple males? The sexually selected sperm hypothesis. Adv. Stud. Behav. 1995;24:291–315. [Google Scholar]
- Keller L, Ross K.G. Selfish genes: a green beard in the red fire ant. Nature. 1998;394:573–575. doi:10.1038/29064 [Google Scholar]
- Kirby J.D, Froman D.P. Comparative metabolism of spermatozoa from subfertile Delaware and Wyandotte roosters. J. Reprod. Fertil. 1991;91:125–130. doi: 10.1530/jrf.0.0910125. [DOI] [PubMed] [Google Scholar]
- Kołodziejczyk M, Radwan J. The effect of mating frequency on female lifetime fecundity in the bulb mite, Rhizoglyphus robini (Acari: Acaridae) Behav. Ecol. Sociobiol. 2003;53:110–115. [Google Scholar]
- Kołodziejczyk M, Heinze J, Radwan J. Enzyme polymorphisms in Rhizoglyphus robini and R. echinopus and their application in paternity analysis. Exp. Appl. Acarol. 2002;26:161–168. doi: 10.1023/a:1021193411454. doi:10.1023/A:1021193411454 [DOI] [PubMed] [Google Scholar]
- Konior M, Radwan J, Kołodziejczyk M. Polyandry increases offspring fecundity in the bulb mite. Evolution. 2001;55:1893–1896. doi: 10.1111/j.0014-3820.2001.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Konior M, Keller L, Radwan J. Effect of inbreeding and heritability of sperm competition success in the bulb mite Rhizoglyphus robini. Heredity. 2005;94:577–581. doi: 10.1038/sj.hdy.6800649. [DOI] [PubMed] [Google Scholar]
- Kozielska M, Krzemińska A, Radwan J. Good genes and the maternal effects of polyandry on offspring reproductive success in the bulb mite. Proc. R. Soc. B. 2004;271:165–170. doi: 10.1098/rspb.2003.2585. doi:10.1098/rspb.2003.2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M.J.B, Ross K.G. Identification of a major gene regulating complex social behavior. Science. 2002;293:328–332. doi: 10.1126/science.1065247. doi:10.1126/science.1065247 [DOI] [PubMed] [Google Scholar]
- Krzanowska H, Wabik-Śliz B, Rafiński J. Phenotype and fertilizing capacity of spermatoza of chimaeric mice produced from two strains that differ in sperm quality. J. Reprod. Fertil. 1991;91:667–676. doi: 10.1530/jrf.0.0910667. [DOI] [PubMed] [Google Scholar]
- Lenormand T, Bourguet D, Guillemaud T, Raymond M. Tracking the evolution of insecticide resistance in the mosquito Culex pipiens. Nature. 1999;6747:861–864. doi: 10.1038/23685. doi:10.1038/23685 [DOI] [PubMed] [Google Scholar]
- Lewis S.M, Austad S.N. Sources of intraspecific variation in sperm precedence in red flour beetles. Am. Nat. 1990;135:351–359. doi:10.1086/285050 [Google Scholar]
- Martin O.Y, Hosken D.J. Reproductive consequences of population divergence through sexual conflict. Curr. Biol. 2004;14:906–910. doi: 10.1016/j.cub.2004.04.043. doi:10.1016/j.cub.2004.04.043 [DOI] [PubMed] [Google Scholar]
- Mauricio R, Stahl E.A, Korves T, Tian D, Kreitman M, Bergelson J. Natural selection for polymorphism in the disease resistance gene Rps2 of Arabidopsis thaliana. Genetics. 2003;163:735–746. doi: 10.1093/genetics/163.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merçot H, Defaye D, Capy P, Pla E, David J.R. Alcohol tolerance, ADH activity, and ecological niche of Drosophila species. Evolution. 1994;48:746. doi: 10.1111/j.1558-5646.1994.tb01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A.J, Gowaty P.A, Moore P.J. Females avoid manipulative males and live longer. J. Evol. Biol. 2003;16:523–530. doi: 10.1046/j.1420-9101.2003.00527.x. doi:10.1046/j.1420-9101.2003.00527.x [DOI] [PubMed] [Google Scholar]
- Murray R, Mayes P.A, Rodwell V.W, Granner D.K. 26th edn. McGraw-Hill Companies; New York: 2003. Harper's biochemistry. [Google Scholar]
- Napier R.A.N. III. Estimation of spermatozoan quality by mixed insemination, and the inheritance of spermatozoan characters. J. Reprod. Fertil. 1961;2:273–291. doi: 10.1530/jrf.0.0020273. [DOI] [PubMed] [Google Scholar]
- Parker G.A. Sperm competition and sexual conflict. In: Blum M.S, Blum N.A, editors. Sexual selection and reproductive competition in insects. Academic Press; New York: 1979. pp. 123–166. [Google Scholar]
- Pitnick S, Garcia-Gonzalez F. Harm to females increases with male body size in Drosophila melanogaster. Proc. R. Soc. B. 2002;269:1821–1828. doi: 10.1098/rspb.2002.2090. doi:10.1098/rspb.2002.2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitnick S, Miller G.T, Reagan J, Holland B. Evolutionary responses by males to experimental removal of sexual selction. Proc. R. Soc. B. 2001;268:1071–1080. doi: 10.1098/rspb.2001.1621. doi:10.1098/rspb.2001.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T, Birkhead T.R. The sexually-selected sperm hypothesis: sex-biased inheritance and sexual antagonism. Biol. Rev. 2002;77:183–209. doi: 10.1017/s1464793101005863. doi:10.1017/S1464793101005863 [DOI] [PubMed] [Google Scholar]
- Radwan J. Intraspecific variation in sperm competition success in the bulb mite: a role for sperm size. Proc. R. Soc. B. 1996;263:855–859. [Google Scholar]
- Radwan J. Sperm precedence in the bulb mite, Rhizoglyphus robini: context-dependent variation. Ethol. Ecol. Evol. 1997;9:373–383. [Google Scholar]
- Radwan J. Heritability of sperm competition success in the bulb mite, Rhizoglyphus robini. J. Evol. Biol. 1998;11:321–327. doi: 10.1038/sj.hdy.6800649. doi:10.1007/s000360050091 [DOI] [PubMed] [Google Scholar]
- Radwan J, Siva-Jothy M.T. The function of postinsemination mate association in the bulb mite Rhizoglyphus robini. Anim. Behav. 1996;52:651–657. doi:10.1006/anbe.1996.0209 [Google Scholar]
- Radwan J, Michalczyk ł, Prokop Z. Age-dependence of male mating ability and sperm competition success in the bulb mite. Anim. Behav. 2005;69:1101–1105. [Google Scholar]
- Rice W.R. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381:232–234. doi: 10.1038/381232a0. doi:10.1038/381232a0 [DOI] [PubMed] [Google Scholar]
- Stockwell C.A, Mulvey M. Phosphogluconate dehydrogenase polymorphism and salinity in the White Sands pupfish. Evolution. 1998;52:1856–1860. doi: 10.1111/j.1558-5646.1998.tb02264.x. [DOI] [PubMed] [Google Scholar]
- Ward P.I. Cryptic female choice in the yellow dung fly Scathophaga stercoraria (L) Evolution. 2000;54:1680–1686. doi: 10.1111/j.0014-3820.2000.tb00712.x. [DOI] [PubMed] [Google Scholar]
- Warner R.R, Shapiro D.Y, Marcanato A, Petersen C.W. Sexual conflict—males with highest mating success convey the lowest fertilization benefits to females. Proc. R. Soc. B. 1995;262:135–139. doi: 10.1098/rspb.1995.0187. [DOI] [PubMed] [Google Scholar]
- Watt W.B. Eggs, enzymes, and evolution: natural genetic variants change insect fecundity. Proc. Natl Acad. Sci. USA. 1992;89:10 608–10 612. doi: 10.1073/pnas.89.22.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt W.B, Carter P.A, Blower S.M. Adaptation at specific loci. IV. Differential mating success among glycolitic allozyme genotypes of Colias butterflies. Genetics. 1985;109:157–194. doi: 10.1093/genetics/109.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley D.M, Beatty R.A. Inheritance of midpiece length in mouse spermatozoa. Nature. 1967;215:94–95. doi: 10.1038/215094a0. [DOI] [PubMed] [Google Scholar]


