Abstract
Age-dependent breeding performance is arguably one of the best-documented phenomena in ornithology. The existence of age-related trends has major implications for life-history theory, but the proximate reasons for these patterns remain poorly understood. It has been proposed that poor breeding performance of young individuals might reflect lack of foraging skills. We investigated this possibility in a medium-sized, powerful raptor—the northern goshawk Accipiter gentilis. Male goshawks are responsible for providing their females and their offspring with food. We hypothesized that young males may generally show poor breeding performance or even delay breeding, because they lack the experience to hunt efficiently—especially, their principal avian prey, the feral pigeon Columba livia. Our study exploited a rare ‘natural experiment’, the expansion phase of an urban population, where intraspecific interference was negligible and many young males bred successfully. This enabled us to examine the improvement of foraging skills in a larger sample of young individuals, and in more controlled conditions than usually possible. Using data from individually identified male breeders, we show that, consistent with our hypothesis, the proportion of pigeons in the diet increased significantly with male age, for at least the first three years of life. Other studies have shown a parallel increase in productivity, and a positive effect of a pigeon-rich diet on brood size and nestling condition, stressing the potential fitness relevance of this prey species for goshawks. Our results suggest a causal link between patterns of age-dependence in foraging ecology and reproductive performance. Furthermore, our study is, to our knowledge, the first demonstration that prey choice of breeders, which might reflect individual hunting skills, is age-dependent in a raptor.
Keywords: Accipiter gentilis, age-dependent foraging proficiency, delayed maturation and breeding, fitness, life-history theory, northern goshawk
1. Introduction
Compared to older individuals, young birds generally start egg laying later in the season, produce smaller clutches and have lower nest success. Age-dependent breeding performance is arguably one of the best-documented phenomena in ornithology (reviews: Curio 1983; Newton 1989; Sæther 1990; Forslund & Pärt 1995; Martin 1995), but it has proved surprisingly difficult to identify the proximate reasons for the observed patterns (Forslund & Pärt 1995). Hypotheses that attempt to explain age-dependent reproduction fall into three main, not mutually exclusive, categories (for theory and empirical examples, see Forslund & Pärt 1995): (i) differential recruitment or survival (‘selection hypothesis’); (ii) optimization of reproductive effort (‘effort hypothesis’); and (iii) age-related improvement of competence (‘experience hypothesis’).
The experience hypothesis states that, in the course of their lives, individuals gain competence in various behaviours that directly or indirectly improve reproductive performance (Forslund & Pärt 1995). Interference from dominant, older birds can affect breeding attempts of young individuals in many ways, for example by forcing them to nest and forage in comparatively resource-poor habitats; age-related improvement of dominance status would then be expected to translate into enhanced breeding performance (e.g. Newton 1991; Stillman et al. 2000). On the other hand, young birds may simply lack the skills for more successful breeding, and they may need time to acquire proficiency in relevant behaviours (see Lack 1968).
Apart from previous breeding experience (see Newton 1989; Black 1996), foraging skills seem to have a major influence on reproductive success. Females need to achieve a sufficient body condition for egg laying and incubation, either through independent foraging or courtship feeding by the male (Newton 1979). Later in the season, parents have to provide their offspring with food, in addition to foraging for themselves. Foraging involves a hierarchical process of decision-making (for theory and empirical examples, see Wunderle 1991), including choosing an appropriate habitat/patch; searching for and recognizing suitable prey items; and capturing prey. At each of these stages, young individuals may suffer from deficiencies which ultimately produce the observed age-specific effects on breeding performance.
A large body of literature, covering a wide range of species, shows that foraging skills indeed improve with age (reviews: Burger 1988; Marchetti & Price 1989; Wunderle 1991). However, most studies suffer from one or several of the following limitations (Wunderle 1991): (i) study subjects are unmarked (impossible to investigate within-individual changes); (ii) birds are grouped into just two age classes (‘juvenile’ versus ‘adult’; impossible to determine accurate rates of development); and (iii) data are collected during autumn or winter (impossible to explore whether differences in foraging skills exist in differently aged breeders).
A possible causal link between patterns of age-dependence in foraging proficiency and reproductive performance is often assumed, but has rarely been investigated explicitly (e.g. Desrochers 1992a,b; Pärt 1995, 2001; Komdeur 1996; Catry & Furness 1999; Daunt et al. 1999; Galbraith et al. 1999). In some species, such as raptors, this might be due to the inherent difficulty in sampling a large number of successful breeding attempts of young individuals, which normally form only a small proportion of sparse breeding populations (see Newton 1979). Lack of foraging skills may actually prevent breeding, or incur costs which are sufficiently high to outweigh the benefits of early breeding, and first-time breeders consequently delay reproduction (Roff 1992; Stearns 1992). Furthermore, even if young breeders are present in a study population, under most conditions it is difficult to rule out interference effects from older birds, which may mask intrinsic foraging abilities. Untangling the relative contributions of adult dominance and the juveniles' natural lack of foraging skills has been identified as one of the major challenges for future studies (Wunderle 1991).
In this paper, we investigate age-dependent diet choice in the goshawk Accipiter gentilis, a raptor species showing strong age-dependence in reproduction (e.g. Kenward et al. 1999; Nielsen & Drachmann 2003). Male hawks are responsible for providing their females and, later in the season, their young with food (reviews: Glutz von Blotzheim et al. 1971; Cramp & Simmons 1980). Goshawks hunt agile avian prey (review: Rutz et al. in press), and we hypothesized that young males may lack skills for efficient foraging, which would explain their poor breeding performance (see Krüger 2005). The feral pigeon Columba livia is the principal avian prey of most European goshawk populations (Rutz et al. in press), and, according to recent independent studies, the use of pigeons may significantly enhance fitness in this raptor (Krüger & Stefener 1996; C. Rutz, unpublished data). We were able to investigate breeding attempts of an unusually large sample of young males during the expansion phase of an urban population (see Würfels 1994, 1999). This ‘natural experiment’ enabled us to examine the improvement of foraging skills in a larger sample of individuals, and in more controlled conditions than usually possible.
2. Material and methods
Fieldwork was conducted during 1989–1997 in a study area (ca 200 km2) covering much of the city of Cologne, west of the River Rhine (for a detailed description, see Würfels 1994). Each year, an attempt was made to find all goshawk nesting territories in the study plot. During the breeding season, active nests were visited at least once every fortnight to collect moulted goshawk feathers and prey items, and to record whether broods were successful (at least one chick fledged). Data on clutch or brood sizes were not available for this population, because nest trees were not climbed, and no dedicated observation sessions were conducted (see Würfels 1994, 1999). The population expanded from three pairs in 1989 to 21 pairs in 1997 (Würfels 1999). A total of 118 successful breeding attempts were investigated during the 9 year study period.
Moulted flight feathers of goshawks can be used reliably for sexing, ageing and identifying individuals without the need to trap and mark birds (Opdam & Müskens 1976). According to differences in feather shape, colouration and patterning, three age classes can be distinguished: 1, 2 and more than or equal to 3 years. After the first year of life, the basic patterns of primaries remain fairly consistent, permitting individual identification by comparing feathers of equivalent position, which are found in subsequent years at the same nest site. Mark–recapture studies employing multiple marking techniques (feather-stamping, banding) have demonstrated the reliability of this technique in goshawks (Ziesemer 1983) and related species (Newton 1986). The method is routinely used in goshawk population studies (for further methodological details, see Kühnapfel & Brune 1995; Rust & Kechele 1996; Bijlsma 1997), including those that are concerned with age-related trends in breeding performance (e.g. Nielsen & Drachmann 2003; Risch et al. 2004; Krüger 2005).
In nesting goshawks, moulted feathers are typically much more difficult to find for males than for females, because male hawks—being responsible for provisioning their families with food—range farther than their mates, who stay at the nest to protect the offspring (Glutz von Blotzheim et al. 1971; Cramp & Simmons 1980). Therefore, only a handful of male feathers are shed at any given territory. This is another aspect where our study benefited from the special circumstances presented by an urban environment. In the city of Cologne, goshawks breed in urban parks and cemeteries, and because of little or no understorey at nest sites, collecting moulted feathers was much easier than in natural or rural settings. Thus, an unusually large number of feathers from male breeders was found. Careful feather comparisons among and between years enabled us to monitor most males through large parts of their reproductive lives, and to obtain exact ages beyond the third year of life for birds which were recruited when 1 or 2 years old.
Diet composition of pairs was assessed by systematically searching nesting territories for remains of plucked prey items (hair and fur) during the breeding season (15 March–15 July), following standard procedures (Würfels 1994; Rutz 2003). Continuous radio-tracking has been shown to yield slightly more accurate diet estimates (Ziesemer 1983; Rutz 2003), but is logistically not feasible for investigating the diets of a large number of breeding pairs (in our case, an entire local population) over several years. As with any other field method, searching for prey remains is affected by certain biases. In goshawks, small items may be missed because they are hard to detect or eaten away from the nest, and large items may be under-represented, because they are partly plucked at kill sites (Rutz 2003). In our study population, both biases were likely to apply equally to all breeding territories investigated, and had, therefore, little or no effect on analyses. Territories were very similar in terms of access to feeding habitat for hawks (see §4), and they were all particularly suited for conducting rigorous scans for prey remains (lack of understorey around nests). Nevertheless, to avoid sampling artefacts and maximize the reliability of diet estimates for particular pairs, we followed the methodological approach of earlier studies (Götmark & Post 1996; Krüger & Stefener 1996) and restricted our dataset to broods for which more than or equal to 30 prey items had been collected (63±3 items, mean±s.e.m.), resulting in a pooled sample of 5069 prey items. It is worth noting that, compared to other brood-level diet analyses in raptors, a sample size of greater than or equal to 30 prey items per breeding pair is exceptionally large—other studies used less conservative selection criteria (more than or equal to 15–20 items).
Male goshawks do most of the food-provisioning during the breeding cycle (see §1), but females may also hunt toward the late nestling period (mid-June; Rutz et al. in press). Therefore, prey samples may have contained a few kills made by female hawks. Data had been collected for other purposes (see Würfels 1994) and were originally grouped into two distinct time blocks (15 March–30 April; 1 May–15 July). Using the first period only was inappropriate for brood-level analyses because of insufficient prey sample sizes, but we were able to carry out analyses on pooled data.
After having established that diet composition changed with male age, we explored whether the pattern was indeed due to longitudinal, within-individual changes, as hypothesized above (see §1). To this end, we assessed diet trajectories for individual male goshawks, which had bred for at least 2 years. Because only a few individuals qualified for this analysis, we attempted to corroborate results by examining the alternative hypothesis of population-level phenotypic variation (i.e. ‘good hunters’ and ‘bad hunters’ differentially appeared or disappeared from the population). We therefore tested the prediction of a positive correlation between diet composition of first-time breeding males (proportion of pigeons in diet of first breeding attempt) and longevity (number of years breeding), which is exclusive for the selection hypothesis (Forslund & Pärt 1995; Mauck et al. 2004). For this analysis, we had to exclude seven males from the dataset, which had not yet died or emigrated by the end of the study in 1997.
Most statistical analyses were carried out by means of generalized linear mixed models (GLMMs) with binomial error structure and logit link function (REML method; Genstat 6). Model fit was checked by inspecting diagnostic scatter plots, and significance of fixed effects was assessed using Wald statistics, which are approximately χ2-distributed (see Payne 2000). ‘Year’ was modelled as a random term to acknowledge the hierarchical design of the dataset and to control for possible temporal effects. Additionally, we fitted ‘identity of male hawks’, as inferred from moulted feathers, as a random effect to account for the fact that datasets contained non-independent multiple records from individual birds across years (Payne 2000). In 87% of all male-years with sufficient prey samples (n=81), we were able to establish the identity of male breeders, and in all remaining cases, we conservatively assumed that the male from the previous year was still present. According to this classification, diet data were available for a total of 41 different males.
To check that our key results obtained with a mixed effects modelling approach were robust (for details, see §3), and, in particular, to rule out the possibility that changes in pigeon availability might have produced the observed effects, we also modelled the diet data using a resampling approach. We randomly picked one datum from each of the 41 individual males and fitted a GLM in Minitab (normal error structure; identity link function), with age as a covariate (1, 2 and more than or equal to 3 years) and year as a three-level factor (‘early period’=1989–91, ‘middle period’=1992–94, ‘late period’=1995–97). We repeated this process 100 times and report the mean F-statistic; the number of replicates was sufficient, as each run provided a valid test statistic. Throughout, probabilities are two-tailed.
3. Results
The proportion of feral pigeons in the diet of breeding goshawks increased highly significantly with male age (figure 1a; n=57 broods, for which the exact age of the male was known; GLMM: , p=0.002). This result was confirmed by a similarly conservative analysis, employing a resampling procedure (figure 1b,c; F1,37=7.49, p=0.009), which also controlled for the increasing use of pigeons through the study (F1,37=3.58, p=0.038).
Figure 1.
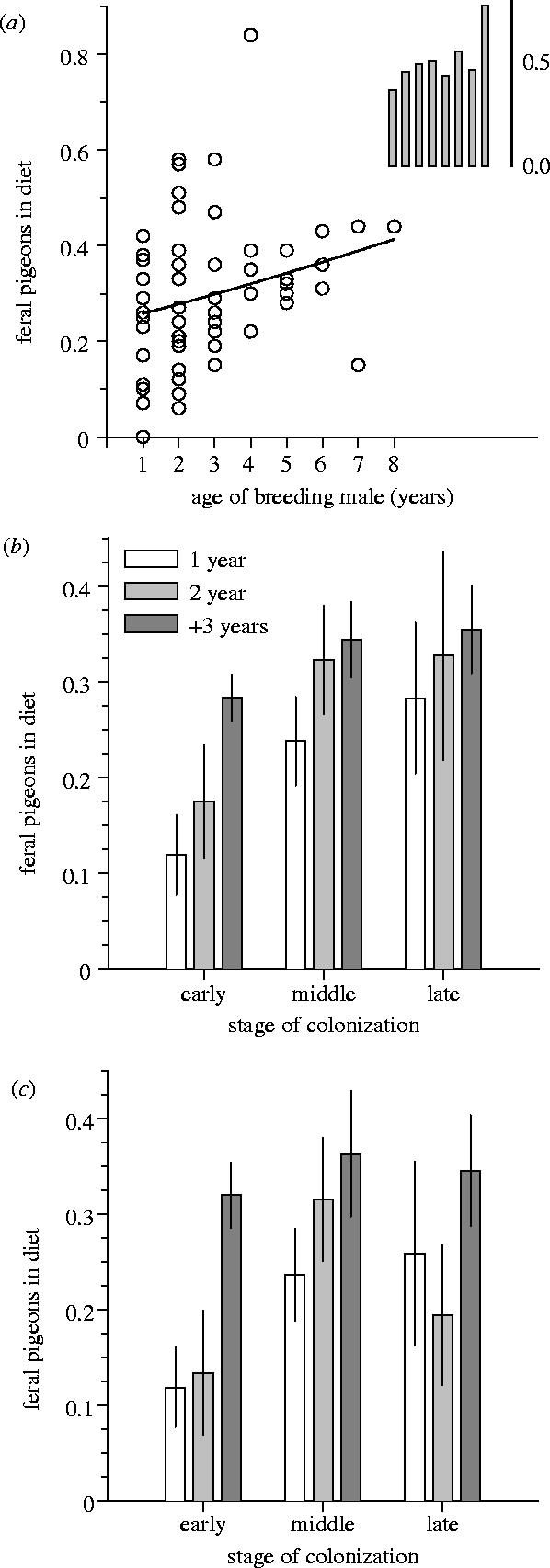
The proportion of feral pigeons in the breeding-season diet of goshawk pairs in the city of Cologne, Germany, in relation to the age of the respective male breeders. (a) Brood-level raw data are plotted together with the best-fit line from a GLMM (‘year’ and ‘male identity’ fitted as random effects). The inset shows the same relationship when the analysis was restricted to prey samples from a time period when only male hawks hunted (data pooled across broods, because of small sample sizes). (b) Raw data (±s.e.) for three male age classes are shown for three different time periods of the colonization event (‘early’=1989–91, ‘middle’=1992–94, ‘late’=1995–97); one datum was randomly picked per male and time block (i.e. a maximum of three data points per male was used to calculate the values for this illustration, but only one datum per male was used in the statistical analyses, see panel c and §3). (c) Estimates of coefficients (±s.e.) from a resampling approach (mean from 100 runs) are shown, where one datum was randomly picked from each of the 41 individual males (i.e. exactly one data point per male). Both (b) and (c) demonstrate that the observation of age-dependent diet (a) is unlikely to be an artefact of temporal changes in environmental pigeon availability. For statistics and methodological details, see text.
For 12 males of known exact age and identity, two or more breeding attempts were recorded (total sample: n=39 broods). Nine of these hawks had diet trajectories with positive slopes (figure 2a), and a matched-pairs analysis confirmed that diet composition changed significantly within subjects (n=12 males; Wilcoxon matched-pairs signed-rank test based on trajectory endpoints: T=14, p=0.052). The use of pigeons by first-time male breeders was positively associated with subsequent individual longevity (figure 2b; n=34 males; Spearman rank correlation: rs=0.33, p=0.059).
Figure 2.
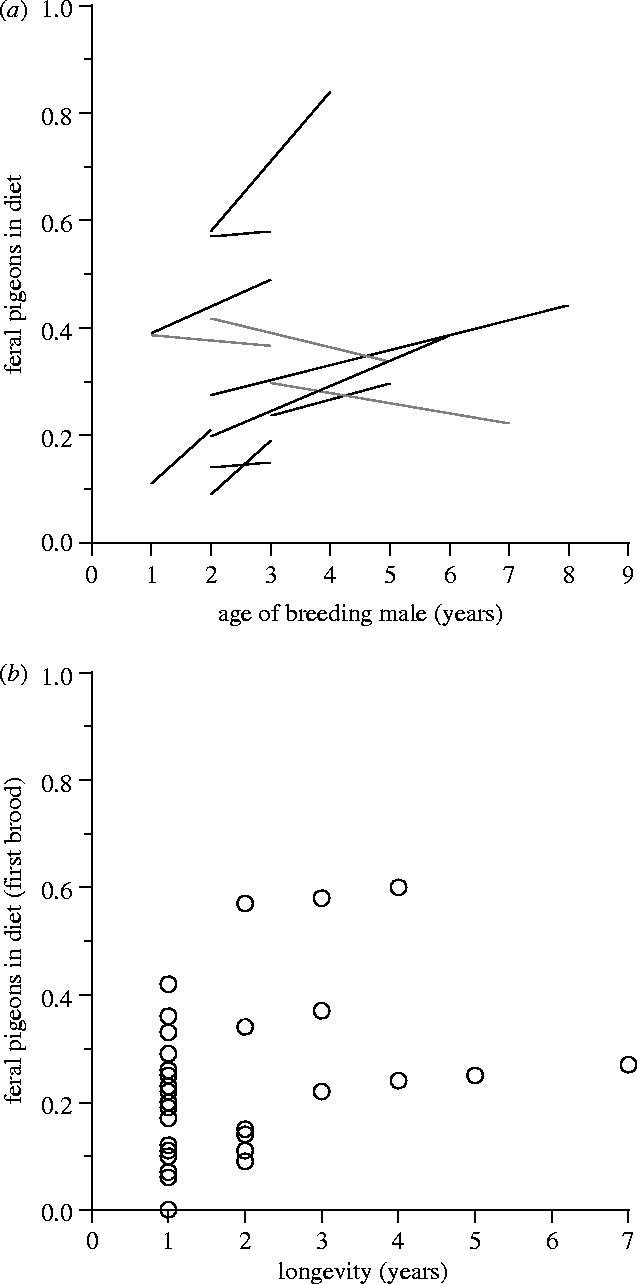
Diet trajectories for individual breeding male goshawks (a) (individuals that bred for at least 2 years; data points joined for samples of 2 years, and linear regressions fitted for samples of 3 or more years); (b) correlation between diet composition of first-time breeding males (proportion of feral pigeons in diet of first breeding attempt) and longevity (number of years recorded breeding). Panel (a) suggests improvement of individual foraging competence (increases shown in black, decreases in grey), but on the basis of (b), population-level changes in phenotype frequencies cannot be ruled out.
In the goshawk, wing and tail morphology changes from the first to the second year of life, after which no further major alterations occur (for references, see §4). However, the observed age-dependence in diet choice could not be attributed to differences in body morphology alone, because the effect remained significant after excluding all first-year birds from the analysis (n=42 broods; GLMM: , p=0.018). This conclusion was confirmed by using the alternative resampling approach (see figure 1b,c).
Prey samples used in the above analyses may have contained a few items killed by female breeders during the late nestling period (see §2). Two lines of evidence suggested, however, that this probably did not affect the conclusions. First, restricting samples to a time period when only males hunted (with subsequent pooling of samples across broods, because sample sizes were too small for a brood-level analysis; see §2), produced a similar pattern of age-dependent diet choice (inset in figure 1a; n=1099 prey items for 57 broods; Spearman rank correlation: rs=0.75, p=0.038). Second, when only broods with same-aged partners (three age categories) were considered, the proportion of pigeons in the diet still increased highly significantly with age (n=68 broods; GLMM: , p=0.002).
Could the observed pattern be an artefact of temporal or spatial changes in environmental pigeon availability? As shown above, the same pattern of age-dependence in pigeon use was observed in all three time blocks (early, middle and late), despite a general, age-independent proportional increase of pigeons in goshawk diet. Spatial artefacts could be ruled out, as controlling for the temporal order in which a territory had been established (assuming that this rank-order reflected the quality of a territory and possibly the availability of pigeons in its immediate vicinity) did not alter the conclusion that male age was the best predictor of pigeon use (n=57 broods; GLMM: territory rank: , p=0.790; male age: , p=0.002).
The proportion of the second most important prey species in the diet, the magpie Pica pica, decreased significantly with male age (n=57 broods, for which the exact age of the male was known; GLMM: , p=0.021; resampling procedure: F1,37=9.83, p=0.003). The magnitude of this effect, however, was small compared to the marked changes in the use of pigeons (percentage of magpies in the diet; coefficients from resampling procedure (three time blocks per age class); one-year-old male hawks: 7.9–10.2%; two-year-old males: 5.1–10.4%; more than or equal to three-year-old males: 3.5–4.0%; for pigeons, see figure 1b,c).
4. Discussion
In this paper, we report empirical evidence for age-dependent diet choice in breeding goshawks: the proportional use of the principal prey species, the feral pigeon, increased highly significantly with male age. In the following sections, we will (i) investigate the robustness of this relationship; (ii) explore the proximate mechanisms underlying it; and (iii) examine whether, in this species, age-specific changes in diet composition could ultimately affect fitness.
Our data were collected in a rapidly expanding population, where many breeding attempts of 1 year old hawks occurred at a time when territories were freely available and the population had not yet reached full capacity (see Würfels 1999). Throughout the study period, young hawks had, in principle at least, the same free access to nesting and food resources as older individuals. This facilitates interpretation, as we can rule out the confounding effect of intraspecific interference (see §1), which is well documented in this territorial species in populations at capacity (review: Rutz et al. in press).
Importantly, our analyses demonstrate that our results were not an artefact of temporal or spatial changes in environmental pigeon availability (no data were available on pigeon population levels in Cologne). In the course of the study, there was an overall, age-independent increase in pigeon use by breeding goshawks, possibly reflecting increasing pigeon abundance. However, when we analysed data separately for three distinct stages of the colonization process, we found a consistent pattern of age-dependence in pigeon use across time periods (resampling approach; see figure 1b,c). It is also worth noting that all our GLMMs included year as a random term to control for possible temporal effects (see §2). Spatial artefacts were unlikely, as the rank order in which a territory had been established had no significant effect on pigeon use by territory holders, suggesting that the population did not expand into either pigeon-rich or pigeon-poor areas. In fact, almost all goshawk pairs nested in a green belt of parks surrounding the city centre, having virtually identical access to nearby built-up areas with feral pigeon flocks (see fig. 1 in Würfels 1999). Goshawks in our study population showed remarkable territory fidelity (no case of breeding dispersal observed for males; and only two cases for females), so we can also exclude the possibility that males obtained better territories as they got older (cf. Newton 2001).
Thus, our study is, to our knowledge, the first to demonstrate age-dependent diet choice among breeders of a raptor species. Interestingly, in an unpublished study of urban goshawks in Hamburg, the proportion of white pigeons in the diet of breeding pairs was found to be significantly related to male age (p=0.019; C. Rutz, unpublished data). Age-dependent changes in diet have been described for other birds, but almost all available studies were concerned with non-breeders (see Wunderle 1991). For raptors, a general improvement in hunting skills during early development is well documented (e.g. Newton 1979; Johnson 1986; Edwards 1989a,b), but no study known to us has investigated, under controlled conditions, the diets of individually identified breeders of known ages (for studies outside the breeding season, see Bourne 1985; Village 1990).
Hypotheses for age-dependent effects are not mutually exclusive, and, as exemplified by our study, several factors may act in concert to produce the observed pattern (for age-specific reproduction, see Newton 1989; Forslund & Pärt 1995). Our analyses indicate that diet choice changed within subjects (see figure 2a), and below we will briefly discuss this finding. On the other hand, we also obtained support for the hypothesis of differential selection of goshawk phenotypes: those birds best able to catch pigeons when young tended to live longest (see figure 2b). Empirical data support the finding that differential recruitment and/or mortality patterns play a role in goshawks; recruitment age is known to vary in this species (review: Rutz et al. in press), and mortality rates are comparatively high and decrease steeply with age (Bijlsma 1993; Kenward et al. 1999; Krüger 2005). We were unable to test the effort hypothesis (Forslund & Pärt 1995), because we lacked direct measures of individual hunting skills (e.g. attack success rate, or prey searching time; see §5).
It has been noted that wing and tail characteristics change in goshawks from the first to the second year of life and remain fairly constant thereafter, although few quantitative studies exist to support this claim (Whaley & White 1994; Tornberg et al. 1999; see also Marcström & Kenward 1981). Anyway, such possible morphological changes might affect flight performance and manoeuvrability (see Hamilton 1961), but were an insufficient explanation for the observed age differences in diet, because the effect remained after excluding all 1 year old males from the analysis. The same result was obtained with both modelling approaches (GLMM and resampling procedure).
We have presented evidence that pigeon use increased within individual hawks (see above and figure 2a). There are at least three potential proximate explanations for such longitudinal changes. First, hunting abilities may improve with age, and hawks may become more able to target manoeuvrable prey (see Wunderle 1991). Under this scenario, the ratio of prey types attacked as goshawks age remains constant. Radio-tracking studies indeed illustrate that the hunting of agile avian prey is demanding for goshawks (e.g. Kenward 1982), and almost certainly hawks have to learn how to hunt pigeons efficiently (see Palleroni et al. 2005; C. Rutz, unpublished data). Second, young hawks may be less aware than older ones of the profitability of particular prey relative to other species, and they may have to learn to target pigeons as they get older. In this case, the ratio of different prey types on which goshawks initiate attacks changes through time. Our observations show that even yearling hawks had a substantial proportion of pigeons in their diet. We checked whether male hawks exhibited a consistent dietary switch during maturation by investigating their use of the second most important prey species, the magpie. The proportional use of this species decreased significantly with age, but the magnitude of this effect was insufficient to explain on its own the marked age-dependent increase in the use of pigeons. Finally, hawks may make more effort or take more risks with increasing age, as their residual reproductive value declines and/or their competence increases. At present, our data do not permit a rigorous test of these ideas. Nevertheless, our results show age-dependence in goshawk diet, and our arguments developed below do not assume any particular mechanism.
Two pieces of evidence from other long-term studies on goshawks strongly suggest that the ability to catch pigeons—irrespective of whether this is a learned skill—may ultimately affect reproductive output in this raptor. First, brood size has been shown to increase significantly with the proportion of pigeons in the diet in a rural study plot close to the city of Cologne, where our study was carried out (figure 3a; Krüger & Stefener 1996). Second, in another urban population (Hamburg), residual body mass of goshawk nestlings was found to increase significantly with the proportion of feral pigeons in the diet (n=67 nestlings from 23 broods; p=0.011, C. Rutz, unpublished data). These results provide a crucial link between our findings and the well established pattern of age-dependent reproduction in goshawks. At least three studies have reported an increase in breeding performance with male age (figure 3b(i); Bijlsma 1993; Kenward et al. 1999; Nielsen & Drachmann 2003), and five studies have found a similar pattern for breeding females (figure 3b(ii); Bijlsma 1993; Kenward et al. 1999; Nielsen & Drachmann 2003; Risch et al. 2004; Krüger 2005). In fact, we are not aware of any relevant studies that have failed to find an age-related increase in reproductive success in nesting goshawks. A recent thorough analysis of life-history variation in goshawks concluded that age-dependence of (foraging) skills was the most likely explanation for improvement of reproductive performance with increasing age (Krüger 2005), although other factors, such as breeding experience, mate and territory quality may also play a role (see Newton 1989; Forslund & Pärt 1995; Black 1996).
Figure 3.
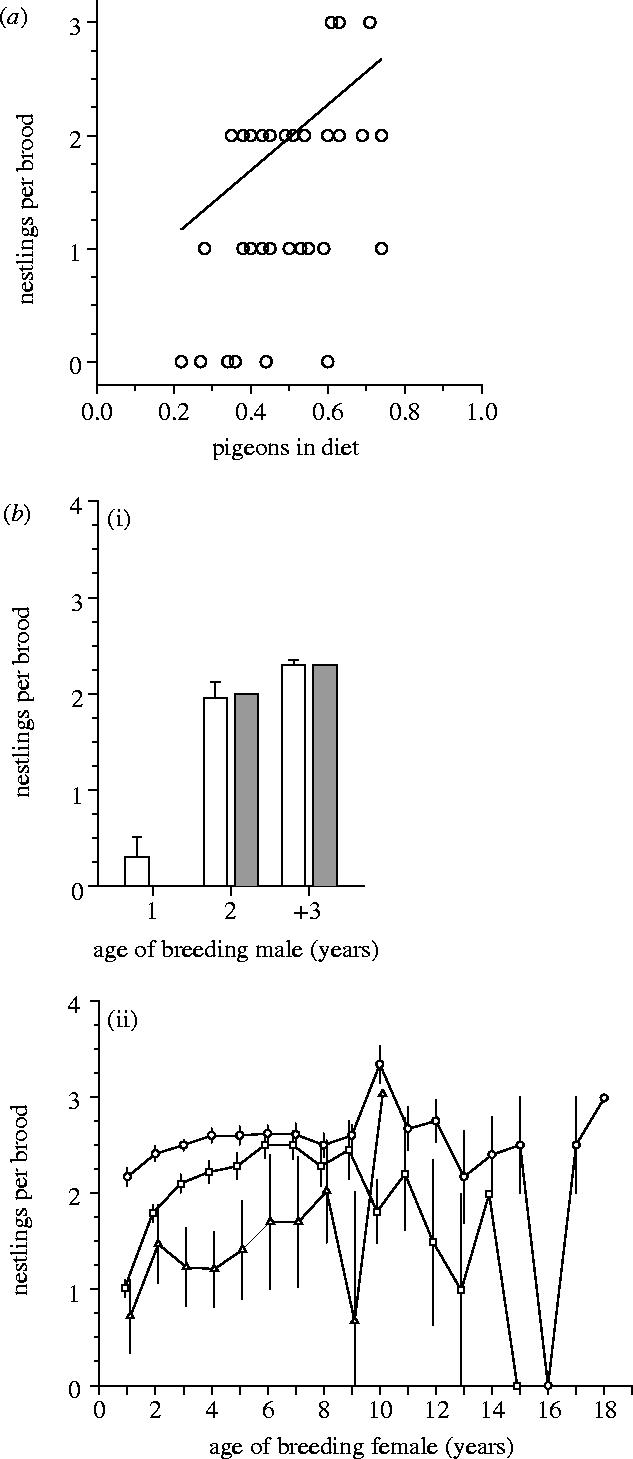
Reproduction in relation to (a) diet composition and (b) age in goshawks. (a) Brood size increases significantly with the proportion of pigeons in the diet of pairs (a) (n=37 broods; linear regression: p<0.01; redrawn from Krüger & Stefener 1996). (b) Age-dependent reproduction in goshawks mirrors age-dependent diet choice (cf. figure 1). The relationship (mean±s.e.) is shown for (b(i)) male breeders and (b(ii)) for female breeders (male age: white bars, n=553 broods, Nielsen & Drachmann 2003; hatched bars, n=28 broods, Kenward et al. 1999; female age: circles, n=929 broods, Nielsen & Drachmann 2003; squares, n=919 broods, Risch et al. 2004; triangles, n=186 broods, Krüger 2005).
First-year male goshawks rarely recruit into breeding populations, probably because they are excluded by more dominant, older individuals: age of first-breeding varies with the degree of intraspecific competition in a population (review: Rutz et al. in press). In comparatively undisturbed goshawk populations, new breeding recruits are usually two or more years of age (Rutz et al. in press). In contrast, in situations where competition is relaxed, because a large proportion of breeding hawks is killed by man, or hitherto unoccupied habitat becomes available for (re-)colonization, goshawks occasionally breed in their first year of life (Rutz et al. in press). However, failing an early breeding attempt could incur high costs, and some young males may actively delay breeding even if breeding opportunities exist (see Krüger 2005; C. Rutz, unpublished data). Delayed breeding is widespread among raptors (Newton 1979), and our study suggests that this might be due to inadequate hunting skills of young birds, at least in species that prey on agile, highly manoeuvrable prey.
5. Concluding remarks
A recent review concluded that foraging proficiency may play a key role in shaping the patterns of age-specific reproduction (Forslund & Pärt 1995). Our study lends support to this hypothesis. Exploring this possible link further could improve our understanding of life-history variation and population dynamics in birds, but so far only few relevant studies have been conducted.
In light of our results, the goshawk seems to be an ideal model species for gaining further insights into age-dependence in life-history traits, especially if future studies could link the age of breeders to both hunting skills and reproductive performance. Ideally, in addition to a robust assessment of diet composition, hunting proficiency should be estimated directly by means of radio-telemetry, or doubly labelled water techniques (see §4). Furthermore, future work should attempt to clarify further whether the observed age-specific patterns are the result of within-subject changes, population-level alterations in phenotype frequencies, or both. Suitable datasets for addressing these issues exist for the congeneric sparrowhawk Accipiter nisus (Newton 1986; Newton & Rothery 2002), and may also be obtainable for other raptor species, for which clear predictions can be formulated on the basis of known aspects of life-history variation, hunting behaviour and diet choice.
Our whole discussion above (see §4) is based on the increase in foraging and breeding success in the first half of the natural lifespan. It is conceivable, however, that changes in foraging skills also account, at least partly, for the decline in breeding success in the second half of the lifespan—an indication of senescence, which has only recently been demonstrated in goshawks (cf. figure 3b(ii); see also Newton & Rothery 1997, 2002). We were unable to test this hypothesis, because our dataset contained only few males older than 6 years, and a measurable decline in breeding performance seems to occur at about 7–10 years of age (Nielsen & Drachmann 2003; Risch et al. 2004).
Finally, the idea that delayed reproduction is due to age-dependent foraging skills (see §4) could be tested with a powerful cross-species meta-analysis. We would predict that the effect of delayed maturation becomes more pronounced with increasing demands on foraging skills. That is, in capacity populations raptor species that prey mainly on birds would be expected to delay breeding comparatively more than those that depend on prey which are easier to hunt, such as voles.
Acknowledgments
We thank M. Würfels for generously providing the original dataset for analysis; C. Perrins and A. Gosler for discussion; R. Payne and D. Garant for statistical advice; M. Shirley for help with the randomization procedures; two anonymous referees for helpful comments; and Blackwell Publishing, ‘AULA-Verlag’ and M. Risch and co-authors for permission to redraw material shown in figure 3. Work on this paper was partially funded by a Rhodes Scholarship (C.R.), an Oxford University Vice Chancellors' Fund Award (C.R.), and a BBSRC David Phillips Fellowship (M.J.W.).
References
- Bijlsma R.G. Schuyt & Co; Haarlem: 1993. Ecologische atlas van de Nederlandse roofvogels. [In Dutch, English summary.] [Google Scholar]
- Bijlsma R.G. KNNV Uitgeverij; Utrecht: 1997. Handleiding veldonderzoek roofvogels. [In Dutch.] [Google Scholar]
- Black J.M, editor. Partnerships in birds. Oxford University Press; Oxford, UK: 1996. [Google Scholar]
- Bourne G.R. The role of profitability in snail kite foraging. J. Anim. Ecol. 1985;54:697–709. [Google Scholar]
- Burger J. Effects of age on foraging in birds. In: Quellet H, editor. Proc. Nineteenth Int. Ornithol. Congr. University of Ottawa Press; Ottawa: 1988. pp. 1127–1140. [Google Scholar]
- Catry P, Furness R.W. The influence of adult age on territorial attendance by breeding great skuas Catharacta skua: an experimental study. J. Avian Biol. 1999;30:399–406. [Google Scholar]
- Cramp S, Simmons K.E.L, editors. Accipiter gentilis goshawk. Handbook of the birds of Europe, the Middle East and North Africa. The birds of the Western Palearctis. vol. II. Oxford University Press; Oxford, UK: 1980. pp. 148–157. [Google Scholar]
- Curio E. Why do young birds reproduce less well? Ibis. 1983;125:400–404. [Google Scholar]
- Daunt F, Wanless S, Harris M.P, Monaghan P. Experimental evidence that age-specific reproductive success is independent of environmental effects. Proc. R. Soc. B. 1999;266:1489–1493. doi:10.1098/rspb.1999.0805 [Google Scholar]
- Desrochers A. Age and foraging success in European blackbirds—variation between and within individuals. Anim. Behav. 1992a;43:885–894. doi:10.1016/0003-3472(92)90002-Q [Google Scholar]
- Desrochers A. Age-related differences in reproduction by European blackbirds—restraint or constraint? Ecology. 1992b;73:1128–1131. [Google Scholar]
- Edwards T.C. The ontogeny of diet selection in fledgling ospreys. Ecology. 1989a;70:881–896. [Google Scholar]
- Edwards T.C. Similarity in the development of foraging mechanics among sibling ospreys. Condor. 1989b;91:30–36. [Google Scholar]
- Forslund P, Pärt T. Age and reproduction in birds—hypotheses and tests. Trends Ecol. Evol. 1995;10:374–378. doi: 10.1016/s0169-5347(00)89141-7. doi:10.1016/S0169-5347(00)89141-7 [DOI] [PubMed] [Google Scholar]
- Galbraith H, Hatch J.J, Nisbet I.C.T, Kunz T.H. Age-related changes in efficiency among breeding common terns Sterna hirundo: measurement of energy expenditure using doubly-labelled water. J. Avian Biol. 1999;30:85–96. [Google Scholar]
- Glutz von Blotzheim U.N, Bauer K.M, Bezzel E, editors. Handbuch der Vögel Mitteleuropas, Bd. 4. Akademische Verlagsgesellschaft; Frankfurt am Main: 1971. pp. 444–478. [In German.] [Google Scholar]
- Götmark F, Post P. Prey selection by sparrowhawks Accipiter nisus—relative predation risk for breeding passerine birds in relation to their size, ecology and behaviour. Phil. Trans. R. Soc. B. 1996;351:1559–1577. [Google Scholar]
- Hamilton T.H. The adaptive significance of intraspecific trends of variation on wing length and body size among bird species. Evolution. 1961;15:180–195. [Google Scholar]
- Johnson S.J. Development of hunting and self-sufficiency in juvenile red-tailed hawks (Buteo jamaicensis) Raptor Res. 1986;20:29–34. [Google Scholar]
- Kenward R.E. Goshawk hunting behaviour and range size as a function of food and habitat availability. J. Anim. Ecol. 1982;51:69–80. [Google Scholar]
- Kenward R.E, Marcström V, Karlbom M. Demographic estimates from radio-tagging: models of age-specific survival and breeding in the goshawk. J. Anim. Ecol. 1999;68:1020–1033. doi:10.1046/j.1365-2656.1999.00347.x [Google Scholar]
- Komdeur J. Influence of age on reproductive performance in the Seychelles warbler. Behav. Ecol. 1996;7:417–425. [Google Scholar]
- Krüger O. Age at first breeding in goshawk Accipiter gentilis. J. Anim. Ecol. 2005;74:266–273. doi:10.1111/j.1365-2656.2005.00920.x [Google Scholar]
- Krüger O, Stefener U. Nahrungsökologie und Populationsdynamik des Habichts Accipiter gentilis im östlichen Westfalen. Vogelwelt. 1996;117:1–8. [In German, English summary.] [Google Scholar]
- Kühnapfel O, Brune J. Die Mauserfeder als Hilfsmittel zur Altersbestimmung und Individualerkennung von Habichten (Accipiter gentilis) Charadrius. 1995;31:120–125. [In German, English summary.] [Google Scholar]
- Lack D. Metheun; London: 1968. Ecological adaptations for breeding in birds. [Google Scholar]
- Marchetti K, Price T. Differences in the foraging of juvenile and adult birds—the importance of developmental constraints. Biol. Rev. 1989;64:51–70. doi:10.1086/416130 [Google Scholar]
- Marcström V, Kenward R.E. Sexual and seasonal variation in condition and survival of Swedish goshawks Accipiter gentilis. Ibis. 1981;123:311–327. [Google Scholar]
- Martin K. Patterns and mechanisms for age-dependent reproduction and survival in birds. Am. Zool. 1995;35:340–348. [Google Scholar]
- Mauck R.A, Huntington C.E, Grubb T.C. Age-specific reproductive success: evidence for the selection hypothesis. Evolution. 2004;58:880–885. doi: 10.1111/j.0014-3820.2004.tb00419.x. [DOI] [PubMed] [Google Scholar]
- Newton I. T. & A. D. Poyser; Berkhamsted: 1979. Population ecology of raptors. [Google Scholar]
- Newton I. T. & A. D. Poyser; Calton: 1986. The Sparrowhawk. [Google Scholar]
- Newton I, editor. Lifetime reproduction in birds. Academic Press; London: 1989. [Google Scholar]
- Newton I. Habitat variation and population regulation in Sparrowhawks. Ibis. 1991;133(Suppl. 1):76–88. [Google Scholar]
- Newton I. Causes and consequences of breeding dispersal in the sparrowhawk Accipiter nisus. Ardea. 2001;89:143–154. [Google Scholar]
- Newton I, Rothery P. Senescence and reproductive value in sparrowhawks. Ecology. 1997;78:1000–1008. [Google Scholar]
- Newton I, Rothery P. Age-related trends in different aspects of the breeding performance of individual female Eurasian Sparrowhawks (Accipiter nisus) Auk. 2002;119:735–748. [Google Scholar]
- Nielsen J.T, Drachmann J. Age-dependent reproductive performance in northern goshawks Accipiter gentilis. Ibis. 2003;145:1–8. doi:10.1046/j.1474-919X.2003.00127.x [Google Scholar]
- Opdam P, Müskens G. Use of shed feathers in population studies of Accipiter hawks (Aves, Accipitriformes, Accipitridae) Beaufortia. 1976;24:55–62. [Google Scholar]
- Palleroni A, Miller C.T, Hauser M, Marler P. Prey plumage adaptation against falcon attack. Nature. 2005;434:973–974. doi: 10.1038/434973b. doi:10.1038/434973b [DOI] [PubMed] [Google Scholar]
- Pärt T. Does breeding experience explain increased reproductive success with age? An experiment. Proc. R. Soc. B. 1995;360:113–117. [Google Scholar]
- Pärt T. Experimental evidence of environmental effects on age-specific reproductive success: the importance of resource quality. Proc. R. Soc. B. 2001;268:2267–2271. doi: 10.1098/rspb.2001.1803. doi:10.1098/rspb.2001.1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R.W, editor. The guide to GenStat. Part 2: statistics. Lawes Agricultural Trust; Hertfordshire: 2000. [Google Scholar]
- Risch M, Looft V, Ziesemer F. Alter und Reproduktion weiblicher Habichte (Accipiter gentilis) in Schleswig-Holstein—ist Seneszenz nachweisbar? Corax. 2004;19:323–329. [In German, English summary.] [Google Scholar]
- Roff D.A. Chapman & Hall; New York: 1992. The evolution of life histories: theory and analysis. [Google Scholar]
- Rust R, Kechele W. Altersbestimmung von Habichten Accipiter gentilis: Langfristige Vergleiche gemauserter Handschwingen. Ornithologischer Anzeiger. 1996;35:75–83. [In German, English summary.] [Google Scholar]
- Rutz C. Assessing the breeding season diet of goshawks Accipiter gentilis: biases of plucking analysis quantified by means of continuous radio-monitoring. J. Zool. Lond. 2003;259:209–217. [Google Scholar]
- Rutz, C., Bijlsma, R. G., Marquiss, M. & Kenward R. E. In press. Population limitation in the Northern Goshawk in Europe: a review with case studies. Stud. Avian Biol
- Sæther B.-E. Age-specific variation in reproductive performance of birds. Curr. Ornithol. 1990;7:251–283. [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford: 1992. The evolution of life histories. [Google Scholar]
- Stillman R.A, Caldow R.W.G, Goss-Custard J.D, Alexander M.J. Individual variation in intake rate: the relative importance of foraging efficiency and dominance. J. Anim. Ecol. 2000;69:484–493. doi:10.1046/j.1365-2656.2000.00410.x [Google Scholar]
- Tornberg R, Mönkkönen M, Pahkala M. Changes in diet and morphology of Finnish goshawks from 1960s to 1990s. Oecologia. 1999;121:369–376. doi: 10.1007/s004420050941. doi:10.1007/s004420050941 [DOI] [PubMed] [Google Scholar]
- Village A. T. & A. D. Poyser; Calton: 1990. The Kestrel. [Google Scholar]
- Whaley W.H, White C.M. Trends in geographic variation of Cooper's hawk and northern goshawk in North America: a multivariate analysis. Proc. West. Found. Vertebr. Zool. 1994;5:161–209. [Google Scholar]
- Wunderle J.M. Age-specific foraging proficiency in birds. Curr. Ornithol. 1991;8:273–324. [Google Scholar]
- Würfels M. Entwicklung einer städtischen Population des Habichts (Accipiter gentilis) und die Rolle der Elster (Pica pica) im Nahrungsspektrum des Habichts—Ergebnisse vierjähriger Beobachtungen im Stadtgebiet von Köln. Charadrius. 1994;30:82–93. [In German, English summary.] [Google Scholar]
- Würfels M. Ergebnisse weiterer Beobachtungen zur Populationsentwicklung des Habichts (Accipiter gentilis) im Stadtgebiet von Köln 1993–1998 und zur Rolle der Elster (Pica pica) im Nahrungsspektrum des Habichts. Charadrius. 1999;35:20–32. [In German, English summary.] [Google Scholar]
- Ziesemer, F. 1983 Untersuchungen zum Einfluß des Habichts (Accipiter gentilis) auf Populationen seiner Beutetiere. Beiträge zur Wildbiologie, Heft 2. Ph.D. thesis. University of Kiel, Kiel. [In German, English summary.]


