Abstract
In societies of cooperative vertebrates, individual differences in contributions to offspring care are commonly substantial. Recent attempts to explain the causes of this variation have focused on correlations between contributions to care and the protein hormone prolactin, or the steroid hormone testosterone. However, such studies have seldom considered the importance of other hormones or controlled for non-hormonal factors that are correlative with both individual hormone levels and contributions to care. Using multivariate statistics, we show that hormone levels explain significant variation in contributions to pup-feeding by male meerkats, even after controlling for non-hormonal effects. However, long-term contributions to pup provisioning were significantly and positively correlated with plasma levels of cortisol rather than prolactin, while plasma levels of testosterone were not related to individual patterns of pup-feeding. Furthermore, a playback experiment that used pup begging calls to increase the feeding rates of male helpers gave rise to parallel increases in plasma cortisol levels, whilst prolactin and testosterone levels remained unchanged. Our findings confirm that hormones can explain significant amounts of variation in contributions to offspring feeding, and that cortisol, not prolactin, is the hormone most strongly associated with pup-feeding in cooperative male meerkats.
Keywords: helper, cooperative, provisioning, prolactin, cortisol, testosterone
1. Introduction
In cooperatively breeding vertebrates, individuals typically live in extended family groups in which a dominant male and female are the principal breeders, and group members delay dispersal and breeding while providing care for the dominant pair's offspring. A remaining challenge in the study of cooperative breeding is to understand the underlying causation of the large differences in individual contributions to helping observed in societies of cooperative vertebrates (Cockburn 1998; Clutton-Brock et al. 2003). Previous investigations have shown that differences may be associated with sex, age, condition and relatedness to offspring (Cockburn 1998), as well as with previous levels of investment (Russell et al. 2003a). However, such variables have limited explanatory power (Heinsohn & Legge 1999).
Recent studies in cooperative birds suggest that a significant fraction of the unaccounted variation in individual levels of offspring provisioning may be explained by hormonal differences (Schoech 2004). Two hormones have attracted particular attention: the protein hormone prolactin and the steroid hormone testosterone. For example, positive correlations between offspring provisioning rates and levels of prolactin have been documented in Harris' hawks (Parabuteo unicinctus; Vleck et al. 1991) and Florida scrub jays (Aphelocoma c. coerulescens; Schoech et al. 1996). Additionally, experimentally induced increases in levels of testosterone in male superb fairy wrens, Malurus cyaneus, have been shown to lead to significant reductions in levels of offspring provisioning (Peters et al. 2002), although effects may vary within and between species (de la Cruz et al. 2003).
Correlations between prolactin or testosterone and offspring care should be viewed with caution, however. Rather than explaining individual variation in helping behaviour, hormonal levels may be correlated with non-hormonal factors known to affect contributions to offspring care. In meerkats (Suricata suricatta), for example, oestradiol levels are positively correlated with body weight (corrected for age) in females, while levels of testosterone are positively correlated with age in males (Carlson et al. 2004), and both body weight in females and age in males are known to be associated with contributions to cooperation (Clutton-Brock et al. 2002).
The aim of this study is to simultaneously examine the demographic, phenotypic and hormonal correlates of helper contributions to pup-feeding. First, we use multivariate statistics to investigate whether variation in the pup-feeding rates of adult male meerkats is related to levels of prolactin, testosterone, or cortisol, after controlling for differences in group size, age, body weight, rate of daily weight gain, relatedness to pups and contributions to other cooperative activities. Second, we use a playback experiment to investigate whether pup-begging calls are associated with changes in hormone levels.
Meerkats are small, desert-dwelling carnivores that live in family groups of up to 50 individuals (mean=19) in southern Africa (Clutton-Brock et al. 2002; Russell et al. 2002). Reproduction is typically limited to the dominant pair. The 3–6 pups born in each litter remain at the breeding burrow for the first four weeks of life (Russell et al. 2003b), protected by one or more ‘babysitters’ as the rest of the group forages (Clutton-Brock et al. 2002). At four weeks of age, pups begin to forage with the group and are dependent on prey items donated by all group members until independence at 12 weeks of age (Brotherton et al. 2001). Individuals within the same group vary by an order of magnitude or more in their contributions to cooperation (Clutton-Brock et al. 2002, 2003). Although some of this variation is related to helper sex, age, weight, foraging efficiency and investment in the previous litter, a significant fraction remains unexplained.
2. Material and methods
We studied the association between meerkat helping behaviour and individual levels of plasma prolactin, cortisol and testosterone near VanZyl's Rus in the South African Kalahari Desert (26°59′ S, 21°50′ E) between 1998 and 2002. Details of the study site, population and climate have been described elsewhere (Russell et al. 2002). Individuals in nine different groups were identified by dye marks on their pelage, with life-history pedigrees within the population having been documented since 1993. All study animals were adult males (more than 1 year of age) habituated to observation within a metre, and individuals could be weighed repeatedly each day by using crumbs of hard-boiled egg to entice them onto an electronic balance (±1 g) (Clutton-Brock et al. 2001). During the rearing of each litter we determined individual contributions to babysitting, pup-feeding, social digging and raised guarding (time spent watching for predators from an elevated position; Clutton-Brock et al. 2004). Contributions to babysitting were determined during morning visits to each group, while contributions to each of the other three activities were determined during visits to each group every 1–3 days for 3–4 h during the peak pup provisioning period (when pups are aged 35–75 days; for further details see Clutton-Brock et al. 2001, 2004).
To obtain a blood sample for hormonal analysis, the target animal was approached slowly on foot as it foraged, gently picked up by the base of the tail and placed inside a capture bag before being immobilized immediately with an intra-muscular injection of Ketamine and Dormitor administered as a weight-dependent dose (7.0 mg kg−1 ketamine hydrochloride, 0.05 mg kg−1 medetomidine hydrochloride; O'Riain et al. 2000). As soon as the animal was completely anaesthetized (2–3 min), a 0.3–1.5 ml blood sample was taken from the jugular vein using a 24.5 G needle and 1 ml syringe and placed in a heparinized vacutainer tube. Animals were re-mobilized using Antisedan (0.25 mg kg−1 atipamezole hydrochloride) and introduced back into the group once fully ambulatory. Meerkats suffered no ill effects and remained fully habituated to observers following release. Blood samples were immediately placed on ice until they were centrifuged at 20 °C at 500g later on the same day. Plasma was pipetted into a vial and frozen at −20 °C pending shipment to the UK on dry ice (O'Riain et al. 2000).
Blood sampling was standardized for the time of day, with all samples collected between 17.00 and 20.30 during the observations and between 08.30 and 10.30 during the experiment. All blood samples were collected 4–7 min after the moment of capture. These capture-to-bleed times are among the shortest obtained for a cooperative vertebrate (Reyer et al. 1986; Mays et al. 1991; Schoech et al. 1996). We interpret the hormone levels as baseline, but recognize that despite anaesthetizing our animals within a minute of capture and obtaining blood samples within 7 min, slight stress-induced elevations of plasma hormone are possible. However, capture-to-bleed times did not vary with contributions to pup-feeding (generalized linear model (GLMM), =0.04, p=0.87), so slight elevations of hormone levels from baseline would not be systematically biased towards those with high pup-feeding rates.
(a) Hormonal correlates of individual contributions to pup-feeding
We investigated whether plasma concentrations of hormones were correlated with the total number of food items adult subordinate male helpers provided to pups during the peak pup-feeding period of each litter. Data were collected from litters born between September 1998 and April 2002. Overall, we obtained 47 hormone samples from 36 individuals contributing to 19 litters in nine groups. All hormone samples were obtained during babysitting periods, before pups began to beg for solid food from group members (Brotherton et al. 2001). Using this sampling method and by controlling for a range of confounding variables in the analysis (see below), we attempt to avert two potential problems: first, that an association between hormone levels and pup-feeding is a consequence rather than a cause of pup-feeding rates; second, that an association between hormone levels and pup-feeding rates arises through an independent association with helper phenotype or contributions to another cooperative activity. Contributions to pup-feeding were fitted to a Poisson error structure in a GLMM in GenStat v. 5 (Lawes Agricultural Trust, Rothamsted Experimental Station, Harpendon, UK). GLMMs are similar to GLMs except that they allow one to control for repeated measures across error terms (individual, litter and group; Schall 1991). The primary terms of interest were plasma concentrations of prolactin, cortisol and testosterone. However, by fitting additional co-variates, we also controlled for number of observation hours, number of helpers present in the group (animals more than six months of age, mean=22, range=6–40), as well as helper age (mean=764 days, range=361–1831 days), weight, average rate of daily weight gain, relatedness to pups and contributions to other cooperative activities (babysitting, social digging, raised guarding).
(b) Playback experiment, helper behaviour and hormone levels
To test which of the three hormones was most responsive to pup-begging calls in male helpers, we conducted playback experiments between December 2000 and November 2002. An experimental and a control session playback were performed 8–10 days apart on nine different males from six groups. Any group that was used for more than one experiment was sampled during different breeding attempts, and each male was used in one set of experiments only. Playback experiments were conducted in groups that met two criteria: (i) group members were currently babysitting a litter of pups; and (ii) the previous litter of pups was begging for food less than 10% of the time when foraging with the group. These criteria ensured that our results were not influenced by the presence of dependent pups begging for food.
The experimental tape comprised pup-begging calls and the control tape comprised adult contact calls; the order of the experimental and control tape presentation was switched each time an experiment was conducted. During the playback experiments, an observer walked 2–4 m behind the subject while holding the loudspeaker 5–15 cm above the ground. The volume was set to simulate the normal amplitude of a calling pup following a potential feeder (Manser & Avey 2000). When an individual approached the loudspeaker with a food item, the volume was temporarily turned down, leading the feeder to leave the speaker and attempt to feed the food item to one of the older pups in the group or to eat the prey itself before resuming foraging.
Playbacks were broadcast with a Sony digital audio recorder (DAT) Pro II TCDD10 connected to a Sony Walkman loudspeaker SRSA60. As group members commonly approached the loudspeaker upon first hearing the pup-begging calls, we spent 10–45 min habituating all animals to the tape without recording behavioural data. In instances in which the control tape was used first, we played the contact calls for a 30 min habituation period.
Pup-begging calls for the playback experiment had been recorded from pups in other groups at the peak of their begging (Manser & Avey 2000), using a Sony DAT recorder and a Sennheiser directional microphone MKH816. To make each experimental tape, we copied 5 min of original recordings into a computer. All such recordings had been edited with Canary v.1.2.1 to eliminate non-pup begging calls and reduce background noise. The 5 min edited periods were then copied repeatedly onto a DAT tape to create a 70 min experimental tape. Contact calls that had been recorded in groups without pups were similarly edited to create a 70 min control tape. To avoid pseudo-replication during the experiments, we prepared four tapes of pup-begging and four tapes of contact calls from different groups.
During each 1 h playback, we recorded the behaviour of the focal male, including the number and size of prey items that he captured, the volume of sand removed to obtain the prey item (Brotherton et al. 2001) and the number and size of prey items that he attempted to feed to the loudspeaker. Immediately after the completion of each playback, the focal male was captured, anaesthetized and blood sampled.
(c) Hormonal assays
All hormonal assays were carried out at the Medical Research Centre laboratories at the University of Edinburgh in Scotland, where samples were assayed in duplicate. We assayed our samples for prolactin using a highly specific heterologous radioimmunoassay of rabbit antiserum to human prolactin (final dilution 1 : 105 000) and canine [125I]iodo-prolactin, validated for meerkat plasma (see Carlson et al. 2003 for assay details). Assay sensitivity was 0.05 ng ml−1. Intra- and inter-assay coefficients of variation for a meerkat plasma pool were 8.3 and 12.6%, respectively, for the multivariate analyses and the measurement of the acute stress response (n=6 assays), and 9.5 and 13.1%, respectively, for the three experimental studies (n=3 assays).
We assayed our samples for testosterone using Coat-a-Count testosterone kits (Diagnostic Products Corporation, Los Angeles, California) validated for meerkat plasma (Carlson et al. 2004). Assay sensitivity was 20 ng dl−1. Intra- and inter-assay coefficients of variation for a meerkat plasma pool were 4.4 and 7.5%, respectively, for the multivariate analyses and the measurement of the acute stress response (n=6 assays) and 5.7 and 8.1%, respectively, for the three experimental studies (n=2 assays).
We assayed our samples for cortisol using Coat-a-Count cortisol kits (Diagnostic Products Corporation, Los Angeles, California) validated for meerkat plasma (Carlson et al. 2004). Assay sensitivity was 0.25 μg dl−1. Intra- and inter-assay coefficients of variation for a meerkat plasma pool were 4.5 and 6.8%, respectively, for the multivariate analyses and the measurement of the acute stress response (n=5 assays) and 6.8 and 8.8%, respectively, for the three experimental studies (n=2 assays).
3. Results
(a) Hormonal correlates of individual contributions to pup-feeding
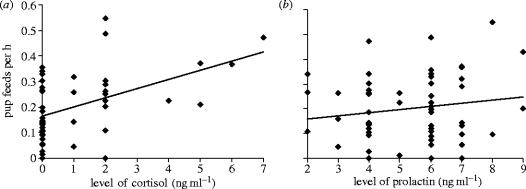
Individual contributions to pup-feeding over the peak provisioning period decreased with both increasing helper number and individual age. No significant effects of weight, daily weight gain, relatedness to offspring or contributions to any other cooperative activity were found (table 1). After controlling for these effects, we found that individual contributions to pup-feeding were significantly and positively associated with plasma levels of cortisol (figure 1a), but not with prolactin or testosterone (table 1). Two findings are of particular note. First, plasma levels of prolactin were significantly correlated with individual contributions to pup-feeding when cortisol data were removed from the analysis (=8.29, p=0.004), but not when cortisol data were included (table 1; figure 1b). Second, levels of cortisol not only explained significant variation in contributions to cooperation after controlling for non-hormonal effects (ca 13%), but they also explained more than twice the variation of any other factor (helper number ca 7%; helper age ca 3%; others less than 1%).
Table 1.
Factors affecting the total number of pup feeds by subordinate male helpers during the peak pup-feeding period (pups 35–75 days old). (Observation time refers to the number of hours of observation. Whether the male was natal or an immigrant was used as a proxy for relatedness. Babysitting, digging and raised guarding refer to individual contributions to babysitting (days per period), social digging (bouts per 3 h) and raised guarding (instances per 3 h). Effect and s.e. indicate the predicted effect of each term on contributions to pup-feeding and their standard error. Prolactin had a significant effect on contributions to pup-feeding in the absence of cortisol data only (χ21=8.29, p=0.004, effect=0.067±0.023). Degrees of freedom were 1 in each case.)
| model terms | Wald statistic (χ2) | p-value | effect | s.e. |
|---|---|---|---|---|
| observation time | 12.75 | <0.001 | 0.043 | 0.012 |
| helper number | 6.83 | 0.009 | −0.027 | 0.010 |
| age | 4.18 | 0.041 | −0.0035 | 0.0017 |
| body weight (g) | 1.40 | 0.24 | 0.0041 | 0.025 |
| daily weight gain (g h−1) | 0.03 | 0.87 | 0.054 | 0.045 |
| natal versus immigrant | 1.82 | 0.18 | 0.28 | 0.21 |
| babysitting contribution | 0.42 | 0.52 | 0.026 | 0.025 |
| digging contribution | 0.32 | 0.57 | 0.0025 | 0.012 |
| raised guarding contribution | 3.11 | 0.078 | 0.021 | 0.012 |
| cortisol level | 11.01 | 0.001 | 0.52 | 0.016 |
| prolactin level | 0.03 | 0.87 | 0.0094 | 0.059 |
| testosterone level | 1.64 | 0.20 | −0.00027 | 0.00021 |
| constant | −3.13 |
Figure 1.
Association between levels of (a) cortisol and (b) prolactin on individual contributions to pup-feeding. Graphs show results after controlling for significant terms shown in table 1. Since the sensitivity of cortisol assays was 0.25 mg dl−1, any value below this was set as 0 mg dl−1 and considered baseline.
(b) Playback experiment, helper behaviour and hormone levels
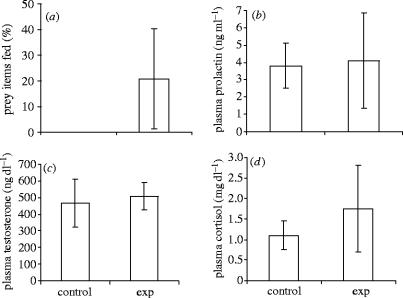
Playbacks of pup-begging calls had a significant effect on the pup-feeding behaviour of focal males. Males brought 20.8±19.4% (mean±s.d.) of the prey items they found to the loudspeaker during the experimental session, compared with 0±0% of prey items during the control session (T+=0, N=9, p=0.008, figure 2a). Despite this, focal males did not differ in the rate at which they found prey, with a median (±inter-quartile range) of 25±5.8 prey items found in the experimental session and 30±9.5 prey items found in the control session (Wilcoxon paired test: n=9, T+=21.5, p=0.90).
Figure 2.
Effect of control (adult contact calls) versus experimental (exp; pup-begging calls) playbacks on (a) proportion of food brought to speaker, and levels of (b) prolactin, (c) testosterone and (d) cortisol. Graphs show (a) mean±1 s.d. and (b–d) medians±inter-quartile ranges. Circulating levels of prolactin and cortisol were significantly correlated with one another during the experimental session (Spearman rank correlation, Rs=0.833, p=0.005).
Plasma levels of prolactin (T+=37.5, n=9, p=0.098, figure 2b) and testosterone (T+=29, n=9, p=0.49, figure 2c) did not differ significantly between males in the control and experimental playback sessions. By contrast, cortisol levels were significantly higher during the experimental session when compared with the control session (n=9, T+=40, p=0.039, figure 2d). The volumes of sand displaced by foraging males in the experimental and control sessions of the playback experiment, however, were comparable (T+=27, N=9, p=0.65). These results suggest that differences in hormones between experimental and control sessions cannot be explained by differences in foraging success or effort (Kanaley & Hartman 2002). Additionally, correlations between the amount of food fed to the speaker and hormone levels were non-significant (cortisol: Rs=0.19, n=9, p=0.63; prolactin: Rs=−0.19, n=9, p=0.63; testosterone: Rs=0.22, n=9, p=0.57), and hormone levels were not correlated with the amount of food ingested by each animal during the playback experiment (Spearman rank correlations (experimental–controls): cortisol: Rs=−0.16, n=18, p=0.51; prolactin: Rs=0.31, n=9, p=0.21; testosterone: Rs=−0.13, n=9, p=0.61).
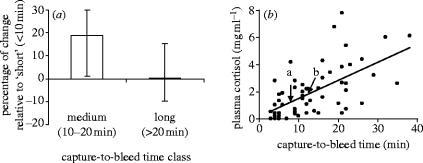
Finally, we plotted a stress profile (Freeman et al. 2000; Sapolsky et al. 2000) for meerkats by measuring hormone concentrations in 15 subordinate adults bled between two and four times each over a period of 40 min. We found that plasma levels of prolactin increased significantly in the short-term (10–20 min post-capture), but returned to baseline levels by 21–38 min post-capture (figure 3a; Carlson et al. 2003); this pattern differed from that found during the playback experiment. Concentrations of plasma cortisol rose steadily and significantly with increasing capture-to-bleed time (figure 3b; Carlson et al. 2004), but this effect was seen over a much larger range of capture-to-bleed times than those used while collecting blood samples for both studies. Comparisons of median cortisol levels during the control and experimental playbacks with the acute stress response profile revealed that helpers who were actively engaged in pup care were, at most, mildly stressed (figure 3b).
Figure 3.
Hormonal characterization of an acute stress response in subordinate adult meerkats of both sexes. (a) Prolactin levels in blood samples drawn 10–20 min post-capture were significantly higher than in blood samples collected within 10 min of capture and after 20 min of capture. Graph shows percentage change from the level measured within 10 min of capture. (b) Plasma cortisol levels increased significantly with increasing capture-to-bleed time. The ‘a’ and ‘b’ arrows mark the median level of plasma cortisol in focal males during the control and experimental playbacks, respectively.
4. Discussion
Individual differences in contributions to pup-feeding by male meerkats were negatively related to both group size and age. After controlling for these variables, we found that variation in plasma cortisol levels was positively correlated with individual differences in contributions to pup-feeding over the peak pup-feeding period, while variations in plasma prolactin and testosterone levels were not. Helpers exposed to playbacks of pup-begging calls also had significantly higher levels of plasma cortisol than when exposed to control playbacks, but there were no significant differences in plasma prolactin or testosterone. Our results provide evidence that hormone levels can account for significant amounts of variation in contributions to pup-feeding after controlling for non-hormonal effects, and that cortisol is more strongly associated with pup-feeding than prolactin.
Our assessment of the importance of prolactin in regulating helping behaviour would have differed had we not also investigated the importance of cortisol. Plasma levels of prolactin were significantly and positively associated with individual contributions to pup-feeding in the absence of cortisol data, but not in their presence, and prolactin and cortisol levels were significantly correlated in the experimental playback sessions. As increases in circulating cortisol can stimulate prolactin secretion (Freeman et al. 2000), it is possible that weak associations between prolactin levels and contributions to cooperation may constitute a by-product of associations between cortisol and the expression of care-giving behaviours. Previous studies have examined the relationship between helping behaviour and levels of prolactin alone (e.g. Vleck et al. 1991; Schoech et al. 1996; Brown & Vleck 1998). This study highlights the need to incorporate cortisol analyses into all future work examining links between prolactin and helping behaviour.
Owing to extensive evidence of the effects of noxious stimuli on glucocorticoid secretion (reviewed by Sapolsky et al. 2000), elevated adrenal activity is primarily associated with detrimental effects. In fact, social stimuli can modify hypothalamic–pituitary–adrenal function to positively influence the formation of social preferences and infant-care behaviours in a wide range of species (Fleming et al. 1997; DeVries 2002). For example, elevated levels of cortisol in human females are associated with increased rates of affectionate contact with infants by primiparous mothers, as well as with high levels of care-taking activities by multiparous mothers (Fleming et al. 1997). New human mothers with increased levels of cortisol also respond more sympathetically to infant cries (Stallings et al. 2001). Additionally, increased glucocorticoid levels are known to enhance foraging rates, food intake (Koch et al. 2002), attention levels and alertness (Fleming et al. 1997; Chapotot et al. 1998), indicating several potential ways in which elevated levels of cortisol could benefit caregivers.
Disentangling whether hormone levels are independent of changes in behaviour, cause changes in behaviour or result from changes in behaviour is problematic. One reason for this is that it is difficult to sample wild animals before and after a change in behaviour or experimental stimulus. The results of this study suggest that differences in cortisol levels may have, in part, caused differences in individual contributions to pup-feeding. First, given that we controlled for a large number of non-hormonal factors, it is unlikely that the significant association between levels of cortisol and pup-feeding arose independently. Second, cortisol levels were obtained prior to pup-feeding and hence, in this study, cortisol levels could not have been a consequence of pup-feeding. Additionally, the positive association between cortisol and contributions to pup-feeding was independent of contributions to babysitting, social digging and raised guarding (table 1). The possibility remains, however, that cues from pups somehow influence both pup-feeding and cortisol levels independently.
In conclusion, we found that variation in hormone levels improved our ability to explain variation in individual contributions to cooperative care. Additionally, our correlational and experimental results suggest that cortisol, not prolactin, influences contributions to pup-feeding. Finally, the positive links between glucocorticoid levels and caring behaviour in adult male meerkats suggest that small but significant increases in caregiver cortisol concentrations may facilitate an increased sensitivity to infant cues (Storey et al. 2000), although a number of studies indicate that beyond a certain upper limit threshold, further increases in circulating cortisol may disrupt offspring care (Silverin 1986; reviewed by Ziegler 2000).
Acknowledgments
We are grateful to members of the Mammal Research Institute at the University of Pretoria, without whom this study would not have been possible, including Professor J. Du Toit and M. Haupt. We thank Mr and Mrs H. Kotze for generously allowing us to work on their land, and Northern Cape Conservation for granting us permission to conduct research in South Africa. R. Carlson and J. Fourie collected blood samples and A. Detoeuf-Boulade, H. Banyard-Smith, H. Webster and L. Taylor provided assistance with the playback experiments. We are grateful to V. Lance and T. Ziegler for suggestions and discussion. This research was funded by grants from the Natural Environmental Research Council, the Biotechnology and Biological Sciences Research Council (T.C.-B.), the Swiss National Science Foundations (M.B.M.), the Royal Society (A.F.R.) and the Zoological Society of San Diego (A.A.C.). All research protocols were approved by the Research Ethics Committee at the University of Pretoria and complied with regulations stipulated in the Guidelines for the Use of Animals in Research.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
References
- Brotherton P.N.M, Clutton-Brock T.H, O'Riain M.J, Gaynor D, Sharpe L.L, Kansky R, McIlrath G.M. Offspring food allocation by parents and helpers in a cooperative mammal. Behav. Ecol. 2001;12:590–599. doi:10.1093/beheco/12.5.590 [Google Scholar]
- Brown J.L, Vleck C.M. Prolactin and helping in birds: has natural selection strengthened helping behaviour? Behav. Ecol. 1998;9:541–545. doi:10.1093/beheco/9.6.541 [Google Scholar]
- Carlson A.A, Nicol L, Young A.J, Parlow A.F, McNeilly A.S. Radioimmunoassay of prolactin for the meerkat (Suricata suricatta), a cooperatively breeding carnivore. Gen. Comp. Endocrinol. 2003;130:148–156. doi: 10.1016/s0016-6480(02)00610-x. doi:10.1016/S0016-6480(02)00610-X [DOI] [PubMed] [Google Scholar]
- Carlson A.A, Young A.J, Russell A.F, Bennett N.C, McNeilly A.S, Clutton-Brock T.H. Hormonal correlates of dominance in meerkats (Suricata suricatta) Horm. Behav. 2004;46:141–150. doi: 10.1016/j.yhbeh.2004.01.009. doi:10.1016/j.yhbeh.2004.01.009 [DOI] [PubMed] [Google Scholar]
- Chapotot F, Gronfier C, Jouny C, Muzet A, Brandenberger G. Cortisol secretion is related to electrocephalographic alertness in human subjects during daytime wakefulness. J. Clin. Endocrinol. Metab. 1998;83:4263–4268. doi: 10.1210/jcem.83.12.5326. doi:10.1210/jc.83.12.4263 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Brotherton P.N.M, O'Riain M.J, Griffin A.S, Kansky R, Sharpe L, McIlrath G.M. Contributions to cooperative rearing in meerkats. Anim. Behav. 2001;61:705–710. doi:10.1006/anbe.2000.1631 [Google Scholar]
- Clutton-Brock T.H, Russell A.F, Sharpe L.L, Young A.J, Balmforth Z, McIlrath G.M. Evolution and development of sex differences in cooperative behaviour in meerkats. Science. 2002;297:253–256. doi: 10.1126/science.1071412. doi:10.1126/science.1071412 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Russell A.F, Sharpe L.L. Meerkat helpers do not specialize in particular activities. Anim. Behav. 2003;66:531–540. doi:10.1006/anbe.2003.2209 [Google Scholar]
- Clutton-Brock T.H, Russell A.F, Sharpe L.L, Jordan N.R. Behavioural tactics of breeders in cooperative meerkats. Anim. Behav. 2004;68:1029–1040. doi:10.1016/j.anbehav.2003.10.024 [Google Scholar]
- Cockburn A. Evolution of helping behaviour in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 1998;29:141–177. doi:10.1146/annurev.ecolsys.29.1.141 [Google Scholar]
- de la Cruz C, Solis E, Valencia J, Chastel O, Sorci G. Testosterone and helping behaviour in the azure-winged magpie, Cyanopica cyanus: natural covariation and an experimental test. Behav. Ecol. Sociobiol. 2003;55:101–111. doi:10.1007/s00265-003-0674-4 [Google Scholar]
- DeVries A.C. Interactions among social environment, the hypothalamic–pituitary–adrenal axis, and behaviour. Horm. Behav. 2002;41:405–413. doi: 10.1006/hbeh.2002.1780. doi:10.1006/hbeh.2002.1780 [DOI] [PubMed] [Google Scholar]
- Fleming A.S, Steiner M, Corter C. Cortisol, hedonics, and maternal responsiveness in human mothers. Horm. Behav. 1997;32:85–98. doi: 10.1006/hbeh.1997.1407. doi:10.1006/hbeh.1997.1407 [DOI] [PubMed] [Google Scholar]
- Freeman M.E, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol. Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Heinsohn R, Legge S. The cost of helping. Trends Ecol. Evol. 1999;14:53–57. doi: 10.1016/s0169-5347(98)01545-6. doi:10.1016/S0169-5347(98)01545-6 [DOI] [PubMed] [Google Scholar]
- Kanaley J.A, Hartman M.L. Cortisol and growth hormone responses to exercises. Endocrinologist. 2002;12:421–432. [Google Scholar]
- Koch K.A, Wingfield J.C, Buntin J.D. Glucocorticoids and parental hyperphagia in ring doves (Streptopelia risoria) Horm. Behav. 2002;41:9–21. doi: 10.1006/hbeh.2001.1726. doi:10.1006/hbeh.2001.1726 [DOI] [PubMed] [Google Scholar]
- Manser M.B, Avey G. The effect of pup vocalisations on food allocation in a cooperative mammal, the meerkat (Surivata suricatta) Behav. Ecol. Sociobiol. 2000;48:429–437. doi:10.1007/s002650000248 [Google Scholar]
- Mays N.A, Vleck C.M, Dawson J. Plasma luteinizing-hormone, steroid-hormones, behavioural role, and nest stage in cooperatively breeding Harris' hawks, Parabuteo unicinctus. Auk. 1991;108:619–637. [Google Scholar]
- O'Riain M.J, Bennett N.C, Brotherton P.N.M, McIlrath G, Clutton-Brock T.H. Reproductive suppression and inbreeding avoidance in wild populations of co-operatively breeding meerkats (Suricata suricatta) Behav. Ecol. Sociobiol. 2000;48:471–477. doi:10.1007/s002650000249 [Google Scholar]
- Peters A, Cockburn A, Cunningham R. Testosterone treatment suppresses paternal care in superb fairy wrens Malurus cyaneus, despite their concurrent investment in courtship. Behav. Ecol. Sociobiol. 2002;51:538–547. doi:10.1007/s00265-002-0472-4 [Google Scholar]
- Reyer H.U, Dittami J.P, Hall M.R. Avian helpers at the nest—are they psychologically castrated? Ethology. 1986;71:216–228. [Google Scholar]
- Russell A.F, Clutton-Brock T.H, Brotherton P.N.M, Sharpe L.L, McIlrath G.M, Dalerum F.D, Cameron E.Z, Barnard J.A. Factors affecting pup growth and survival in cooperatively breeding meerkats Suricata suricatta. J. Anim. Ecol. 2002;71:700–709. doi:10.1046/j.1365-2656.2002.00636.x [Google Scholar]
- Russell A.F, Sharpe L.L, Brotherton P.N.M, Clutton-Brock T.H. Cost minimization by helpers in cooperative vertebrates. Proc. Natl Acad. Sci. USA. 2003a;100:3333–3338. doi: 10.1073/pnas.0636503100. doi:10.1073.pnas.063503100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A.F, Brotherton P.N.M, Sharpe L.L, McIlrath G.M, Clutton-Brock T.H. Breeding success in cooperative meerkats: effects of helper number and maternal state. Behav. Ecol. 2003b;14:486–492. doi:10.1093/beheco/arg022 [Google Scholar]
- Sapolsky R.M, Romero L.M, Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparatory actions. Endocrinol. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. doi:10.1210/er.21.1.55 [DOI] [PubMed] [Google Scholar]
- Schall R. Estimation in generalized linear models with random effects. Biometrika. 1991;78:719–727. [Google Scholar]
- Schoech S.J. Endocrinology. In: Koenig W.D, Dickinson J.D, editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004. pp. 128–141. [Google Scholar]
- Schoech S.J, Mumme R.L, Wingfield J.C. Prolactin and helping behaviour in the cooperatively breeding Florida scrub jay, Aphelocoma c. coerulescens. Anim. Behav. 1996;52:445–456. doi:10.1006/anbe.1996.0189 [Google Scholar]
- Silverin B. Corticosterone-binding proteins and behavioural effects of high plasma levels of corticosterone during the breeding period in the pied flycatcher. Gen. Comp. Endocrinol. 1986;64:67–74. doi: 10.1016/0016-6480(86)90029-8. doi:10.1016/0016-6480(86)90029-8 [DOI] [PubMed] [Google Scholar]
- Stallings J, Fleming A.S, Corter C, Worthman C, Steiner M. The effects of infant cries and odors on sympathy, cortisol, and autonomic responses in new mothers and nonpost-partum women. Parenting: Sci. Pract. 2001;1:71–100. doi:10.1207/S15327922PAR011&2_5 [Google Scholar]
- Storey A.E, Walsh C.J, Quinton R.L, Wynne-Edwards K.E. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol. Hum. Behav. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. doi:10.1016/S1090-5138(99)00042-2 [DOI] [PubMed] [Google Scholar]
- Vleck C.M, Mays N.A, Dawson J.W, Goldsmith A.R. Hormonal correlates of parental and helping behaviour in cooperatively breeding Harris' hawks (Parabuteo unicinctus) Auk. 1991;108:638–648. [Google Scholar]
- Ziegler T.E. Hormones associated with non-maternal infant care: a review of mammalian and avian studies. Folia Primatolog. 2000;71:6–21. doi: 10.1159/000021726. doi:10.1159/000021726 [DOI] [PubMed] [Google Scholar]