Abstract
Symbiosis is prevalent throughout the tree of life and has had a significant impact on the ecology and evolution of many bacteria and eukaryotes. The benevolence of symbiotic interactions often varies with the environment, and such variation is expected to play an important role in shaping the prevalence and distributions of symbiosis throughout nature. In this study, we examine how the fitness of aphids is influenced by infection with one of three maternally transmitted bacteria, ‘Candidatus Serratia symbiotica’, ‘Candidatus Hamiltonella defensa’ and ‘Candidatus Regiella insecticola’, addressing how symbiont benevolence varies with temperature. We find that the effects of these ‘secondary’ symbionts on Acyrthosiphon pisum depend on when and whether aphids are exposed to a brief period of heat shock. We also demonstrate that symbionts—even closely related isolates—vary in their effects on hosts. Our results indicate similar effects of S. symbiotica and H. defensa in conferring tolerance to high temperatures and a liability of R. insecticola under these same conditions. These findings reveal a role for heritable symbionts in the adaptation of aphids to their abiotic environments and add to an expanding body of knowledge on the adaptive significance of symbiosis.
Keywords: aphid, secondary symbiont, Wolbachia, fitness, heat, maternal transmission
1. Introduction
Bacteria commonly interact with insects in intimate associations known as symbioses. Many of these microbes behave in a fashion analogous to cytoplasmic elements, moving among hosts via maternal transmission. Due to their dependence on host reproduction, natural selection is expected to favour symbionts that increase host fitness (May & Anderson 1982; Ewald 1983; Bull et al. 1991). Accordingly, several heritable bacteria of insects have been shown to confer benefits on their hosts (e.g. Buchner 1965; Wicker & Nardon 1982).
For the past several decades, aphids (Hemiptera: Aphididae) have received attention from biologists interested in dissecting the mechanisms and consequences of symbiosis. These insects are specialized on diets of plant phloem sap, which are typically lacking in essential amino acids (e.g. Sandström & Moran 1999). Nearly, all aphids harbour an essential, maternally inherited primary symbiont known as Buchnera aphidicola (Munson et al. 1991a,b) which supplements host nutrition through synthesis and provisioning of essential amino acids (Douglas 1988; Douglas & Prosser 1992; Lai et al. 1994; Bracho et al. 1995; Sandström & Moran 2001). Several aphids harbour additional maternally transmitted bacteria, known as secondary symbionts (Buchner 1965; Unterman et al. 1989; Chen et al. 1996; Darby et al. 2001; Fukatsu 2001a,b; Sandström et al. 2001; Russell et al. 2003). Most secondary symbionts are considered non-essential, and they are generally found at intermediate frequencies within host populations (e.g. Chen & Purcell 1997; Tsuchida et al. 2002; Haynes et al. 2003; Leonardo & Muiru 2003; Simon et al. 2003; but see Fukatsu 2001a and Sandström et al. 2001).
Though documented over 50 years ago, the significance of these bacteria remained elusive until recent studies on the secondary symbionts of Acyrthosiphon pisum (Hemiptera: Aphididae) revealed several effects on aphid fitness and phenotypes. Both ‘Candidatus Hamiltonella defensa’ (a.k.a. PABS, T-type) and ‘Candidatus Serratia symbiotica’ (a.k.a. PASS, R-type) were shown to defend against the parasitoid Aphidius ervi (Hymenoptera: Braconidae), though a third symbiont, ‘Candidatus Regiella insecticola’ (a.k.a. PAUS, U-type), played no role in this defence (Oliver et al. 2003). R. insecticola was, however, found to enable A. pisum to utilize white clover as a host plant (Tsuchida et al. 2004; but see Leonardo 2004). In addition, S. symbiotica has been demonstrated to confer heat tolerance in A. pisum (Chen et al. 2000; Montllor et al. 2002). The generality of the heat tolerance effect is currently unclear. It is not known whether this phenotype is conferred by multiple isolates of S. symbiotica or whether other species of secondary symbionts of A. pisum can confer the same protections.
Here, we examine the effects of three secondary bacterial symbionts on A. pisum fitness, emphasizing their roles in aphids exposed to heat shock. We compare the survival, fecundity, and generation times between clonal lines of aphids which are free of secondary symbionts or infected with isolates of S. symbiotica, H. defensa, or R. insecticola (γ-Proteobacteria, Moran et al. 2005). Our findings reveal that benevolence varies even among closely related symbionts, depending on the exposure of their hosts to high temperatures.
2. Material and methods
(a) Aphids and plants
Aphids are cyclical parthenogens, and can be reared in the laboratory as asexual lineages. This enabled us to create clonal lines that were either free of secondary symbionts (N), or infected with either S. symbiotica (SsWI, SsNY, SsAZ), H. defensa (Hd) or R. insecticola (Ri). Due to the asexual nature of aphid reproduction, fitness differences among these lines can be attributed to the effects of symbionts rather than those of aphid genotype.
Artificially infected lines were established by microinjecting juveniles of the pink A. pisum clone (‘5A’, collected from alfalfa in Madison, Wisconsin) with body fluids from A. pisum donors that harboured either S. symbiotica, H. defensa or R. insecticola (Oliver et al. 2003). Briefly, injections were performed on the stage of a dissecting microscope using a glass microcapillary tube, pulled into a fine needle point. Body fluids were extracted from donor individuals and injected into the abdomens of recipients. Donors used to establish infected lines belonged to one of five aphid clones collected from alfalfa in Cayuga County or Tompkins County, New York (for SsNY, Hd, Ri), from black medic in Madison, Wisconsin (for SsWI), or from fava bean in Tucson, Arizona (for SsAZ). Before injection, each of these donor clones was screened for S. symbiotica, H. defensa, R. insecticola and pea aphid Rickettsia (Chen et al. 1996) using diagnostic PCR (see Russell et al. 2003, for methods). Donors harboured only a single type of secondary symbiont. Members of the recipient clone (5A) were free of each of these heritable bacteria, harbouring only B. aphidicola.
Survivors of microinjection were used to start single female lineages on fava bean (Vicia fava). Using diagnostic PCR, we screened these lineages for symbionts over several generations. We selected stably infected lines for continued maintenance and future experiments. These lines were screened on several occasions leading up to the experiments; no instances of transmission failure were detected, in agreement with previous findings of highly efficient maternal transfer (Chen & Purcell 1997; Darby & Douglas 2003). Moreover, the identity of aphid genotypes was confirmed with a PCR-based assay (Abbot 2001; Sandström et al. 2001), allowing us to rule out contamination by other aphid clones.
Artificially infected aphids were reared in the laboratory for at least 10 generations before use in experiments. All lines were reared in developmental synchrony and maintained at 18 °C 16L : 8D for at least two generations prior to experiments. Pre-flowering fava bean plants used for experiments were the same age within each experimental time block. These plants were grown in 10 cm diameter pots and enclosed, during experiments, using transparent SOLO cups with mesh tops.
(b) Experimental design
Our protocol was designed to mirror that used by Montllor et al. (2002). Several adult A. pisum of comparable age were placed onto fava bean plants. These were bottom-watered and then placed in a Percival Scientific growth chamber set at 18 °C. We removed adults on ‘day 1’, after 12–14 h of reproduction and designated cultures for one of three treatments: ‘control’, ‘day 2 heat shock’, or ‘day 6 heat shock’. Each plant, or culture, contained several juvenile aphids sired by several parents. Multiple replicate cultures of each line were treated in all experiments.
Nymphs subjected to the day 2 heat shock treatment were counted and placed into a separate growth chamber set at 18 °C on day 2. The growth chamber temperature was gradually increased to 37.5 °C (±0.5 °C) over a span of 2 h. After 4 h, the temperature was gradually decreased to 18 °C. Cultures were then placed back in the original growth chambers and reared side-by-side with their control treatment counterparts at 18 °C.
Juveniles subjected to the day 6 heat shock treatment were handled as in the day 2 treatment, except that nymph counting and heat shock were executed on day 6. For the control treatment, aphids were kept at 18 °C for the entire experiment. To allow side-by-side comparisons of survival with the two separate heat shock treatments, control aphids were counted at either day 2 or day 6. Since control aphids from these two classes did not differ in their fitness attributes, we pooled their fitness data in our analyses.
Two separate heat shock experiments were performed. The first (S. symbiotica or Ss experiment) included the N (uninfected), SsAZ, SsWI and SsNY lines of aphid clone 5A, and was conducted over several time blocks between September, 2003 and March, 2004. In this experiment, we executed each of the three temperature treatments. The day 2 heat shock treatment was performed over seven separate time blocks. At two of these time points, nearly all of the aphids died before adulthood. We disregarded these trials, leaving us with data from five blocks. For the control and day 6 heat shock treatments, we performed experiments over five and four time blocks, respectively. In these experiments, we employed a factorial design—all four lines were treated and examined at each time block.
The second experiment (H. defensa–R. insecticola experiment) also employed a factorial design and was conducted during May and June of 2004 on N, Hd and Ri lines of clone 5A. In these trials, only the control (three time blocks) and day 2 heat shock (two time blocks) treatments were performed. To prevent positional effects within growth chambers, individual cultures were rearranged on several occasions during each time block in both the S. symbiotica and H. defensa–R. insecticola experiments.
During both heat shock experiments growth chamber temperatures were monitored using Oregon Scientific probes. Temperatures were also measured inside the cages, in proximity to plants, using either Oregon probes or KoolTrak data loggers. We found that temperatures next to fava bean plants were comparable to external temperatures registered within growth chambers.
(c) Fitness measures
Generation time was measured as the number of days from birth to adulthood. Beginning on day 9 of the experiment, we checked cultures for adults daily until all had died or matured. We recorded the number of mature aphids in each culture observed on each day, removing adults to prevent confusion with juveniles. Using these data and the counts of pre-treated juvenile aphids, we measured the numbers of aphids surviving or dying prior to adulthood.
We selected adults from each culture for fecundity assays, using those that reached adulthood earliest. These aphids were placed, individually, on fava bean plants and reared at 18 °C 16L : 8D. We counted offspring of each female at days 5 and 9 of adulthood, removing those produced by day 5 to prevent confusion with later-born progeny. Typically, four individuals per treated culture were selected for fecundity measures. However, this sampling scheme was limited by the fact that several heat shocked cultures had fewer than four survivors.
(d) Statistical analyses
The distributions of development times and fecundities were non-normal, even after attempts to transform the data. Since these deviations violated assumptions of parametric statistics, we used non-parametric Wilcoxon rank-sum tests to analyse these fitness measures. We conducted pairwise comparisons between all lines within each experiment, allowing us to estimate the effects of their symbionts.
Due to our use of non-parametric statistics, we were limited to considering single explanatory factors within analyses. To address potentially confounding explanatory effects, we performed several additional analyses, examining variables such as time, rearing history, and the presence of winged and wingless adult morphs. Though these factors accounted for some of the variation in our development time and fecundity data, we found that all of our interpretations on the effects of symbionts were robust and had not been distorted by confounding variables.
To analyse survival, we used logistic regression, computing the log odds of survival for infected lines relative to their uninfected counterparts. In the text, we discuss back-transformed (inverse natural log) values for regression coefficients, which represent the magnitudes of survival differences between infected and secondary-free aphid lines.
3. Results
(a) S. symbiotica experiment
Exposure to heat shock at both the day 2 and day 6 stages had drastic effects on A. pisum performance. Compared to control aphids, heat-shocked individuals suffered from prolonged development as well as large reductions in survival and fecundity. Many aphids were sterilized by heat shock, and some gave birth to malformed offspring whose limbs were stuck to the thorax and abdomen. These aphids were visually distinguishable from healthy offspring and typically died within hours of birth. We did not include such individuals in fecundity measures.
Though all lines performed poorly when exposed to heat shock as 2 day olds, aphids infected with S. symbiotica isolates were substantially more fit than uninfected, genetically identical aphids under this treatment. S. symbiotica infection led to a higher odds of survival, with advantages over the uninfected line ranging from 19 to 30% according to logistic regression (table 1). Similarly, S. symbiotica-infected aphids reached adulthood significantly faster than uninfected aphids, according to Wilcoxon rank-sum tests (table 2). Uninfected aphids matured in an average time of 13.3 days. Infected lines matured, on average, at 11.0 (SsAZ), 11.6 (SsNY) and 12.4 (SsWI) days—revealing a 1–2 days acceleration conferred by the symbionts. There were also differences among the infected lines, with SsAZ reaching adulthood faster than both SsNY (p<0.0001) and SsWI (p<0.0001) and SsNY maturing faster than SsWI (p=0.0002).
Table 1.
Logistic regression analyses of aphid survival.
| treatment | regression equationa | β1b | β2b | β3b |
|---|---|---|---|---|
| controlSs | Y=−2.60−0.58SsWI−0.25SsNY+0.12SsAZ | p=0.0184; 95% CI: −1.06 to −0.10 | p=0.3199; 95% CI: −0.74 to 0.24 | p=0.6793; 95% CI: −0.44 to 0.67 |
| day 2 heat shockSs | Y=−0.03+0.30SsWI+0.29SsNY+0.19SsAZ | p<0.0001; 95% CI: 0.15 to 0.45 | p<0.0001; 95% CI: 0.15 to 0.43 | p=0.0099; 95% CI: 0.04 to 0.33 |
| day 6 heat shockSs | Y=0.90−0.28SsWI− 0.46SsNY−0.44SsAZ | p=0.0005; 95% CI: −0.44 to −0.12 | p<0.0001; 95% CI: −0.61 to −0.30 | p<0.0001; 95% CI: −0.60 to −0.27 |
| controlHd/Ri | Y=−2.92−0.01Hd+0.58Ri | p=0.9652; 95% CI: −0.58 to 0.56 | p=0.1544; 95% CI: −0.22 to 1.39 | n.a. |
| day 2 heat shockHd/Ri | Y=0.77+0.29Hd−0.27Ri | p=0.0111; 95% CI: 0.07 to 0.52 | p=0.0439; 95% CI: −0.53 to −0.01 | n.a. |
Regression equation is (for S. symbiotica experiments) or (for H. defensa and R. insecticola experiments) .
Statistics indicate whether β parameter estimates (representing the relative difference in the log odds of survival between infected and uninfected aphids) differed significantly from zero.
Table 2.
Time to adulthood for uninfected and S. symbiotica-infected A. pisum.
| treatment | statistics | aphid line | |||
|---|---|---|---|---|---|
| N | SsWI | SsNY | SsAZ | ||
| control | number of aphids (number of treated cultures) | 195 (16) | 109 (12) | 186 (17) | 214 (19) |
| mean; median days to adulthood | 9.9; 10 | 9.8; 10 | 9.7; 10 | 9.6; 10 | |
| Wilcoxon rank-sum testa | n.a. | p=0.0359 | p=0.0006 | p<0.0001 | |
| day 2 heat shock | number of aphids (number of treated cultures) | 116 (20) | 166 (18) | 182 (21) | 177 (22) |
| mean; median days to adulthood | 13.3; 13 | 12.4; 12 | 11.6; 11 | 11.0; 11 | |
| Wilcoxon rank-sum testa | n.a. | p=0.0003 | p<0.0001 | p<0.0001 | |
| day 6 heat shock | number of aphids (number of treated cultures) | 201 (16) | 156 (14) | 155 (18) | 143 (20) |
| mean; median days to adulthood | 10.5; 10 | 10.3; 10 | 10.5; 10 | 10.1; 10 | |
| Wilcoxon rank-sum testa | n.a. | p=0.0375 | p=0.4278 | p<0.0001 | |
Pairwise Wilcoxon rank-sum tests were performed, comparing development times for each S. symbiotica-infected line (SsWI, SsNY and SsAZ) to those from the uninfected line (N) under each temperature treatment.
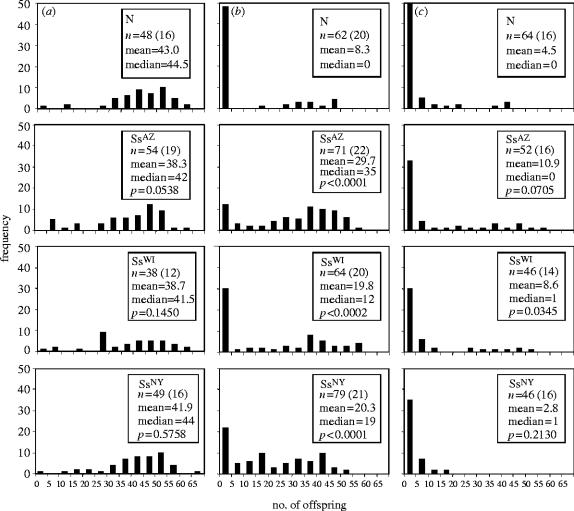
S. symbiotica-infected aphids heat-shocked as 2 day olds were far more fecund than aphids lacking secondary symbionts (figure 1b). Whereas uninfected aphids produced an average of 8.3 offspring per female, infected insects gave birth to averages of 19.8 (SsWI), 20.3 (SsNY) and 29.7 (SsAZ) offspring per female. This was due, in part, to higher sterility in uninfected aphids. Of aphids surviving 8 days of adulthood, 63% of uninfected individuals produced no offspring compared to 2–29% of those harbouring S. symbiotica.
Figure 1.
Distributions of fecundity, for single females varying in their symbiont infection status. Offspring production of single aphid females is represented using frequency histograms. Means, medians and sample sizes are presented for each line under each of these treatments. Also, p-values are presented from Wilcoxon rank-sum tests. Each p-value represents the comparison between fecundities for the given symbiont-infected line and the uninfected line. (a) Results from control treatment for the S. symbiotica experiment. (b) Results from day 2 heat shock treatment for the S. symbiotica experiment. (c) Results from day 6 heat shock treatment for the S. symbiotica experiment. Note, sample sizes presented represent the number of aphids measured and, in parentheses, the number of treated cultures from which these aphids were derived.
Lines infected with S. symbiotica also differed in their average fecundities. Specifically, aphids from the SsAZ line produced more offspring than those from either SsWI or SsNY (SsAZ versus SsWI: p=0.0047; SsAZ versus SsNY: p=0.0012), revealing variation in heat tolerance capacity among symbionts.
In aphids exposed to heat shock as 6 day olds, S. symbiotica was less beneficial. Fecundities of all aphids were reduced compared to those of aphids heat shocked on day 2. Average numbers of offspring produced by single females were 4.5 (N), 8.6 (SsWI), 2.8 (SsNY) and 10.9 (SsAZ; figure 1c). The SsWI and the SsAZ lines were both more fertile than the secondary-free line (Wilcoxon rank-sum test, p=0.0345 for SsWI versus N and p=0.0705 for SsAZ versus N). No other differences approached significance in these analyses. Overall, sterility was more common within infected lines exposed to heat shock on day 6 than those exposed on day 2, with 31–40% of S. symbiotica-bearing females yielding no offspring over 8 days of adulthood. In comparison, 59% of secondary-free aphids failed to produce any offspring during their first 8 days of maturity, indicating that sterility remained more prevalent in aphids without secondary symbionts.
Time to adulthood was shorter when aphids were heat shocked on day 6, compared to day 2, with averages ranging from 10.1 to 10.5 days (table 2). Only SsWI and SsAZ aphids reached adulthood significantly earlier than uninfected aphids (Wilcoxon rank-sum test, p<0.0001 for SsAZ versus N and p=0.0375 for SsWI versus N). These accelerations were each less than 0.5 days in magnitude, in contrast to the 1–2 day accelerations when aphids were heat shocked as 2 day olds. Again, there were significant differences among infected lines, with SsAZ reaching adulthood faster than both SsWI (p=0.0003) and SsNY (p=0.0329).
Under the day 6 heat shock treatment the odds of survival for infected lines were reduced by 25–37% compared to uninfected aphids. Thus, symbiont infection imposed a survivorship cost when older juveniles were heat shocked (table 1). Combining these data on survival, fecundity, and development time into an age-structured Leslie matrix model revealed, that the SsAZ and SsWI lineages had slightly higher fitness (intrinsic rates of increase) than the uninfected line, though SsNY was slightly less fit (A. Ives, unpublished data).
The highest levels of fecundity and survival, and the fastest development times, were obtained for aphids reared at a constant 18 °C throughout their lifespan (figure 1a; tables 1 and 2). Under these conditions, there were no significant effects of S. symbiotica on aphid fitness with two exceptions. First, there was a slight reduction in survival of SsWI aphids compared to those without secondary symbionts. There was also a slight developmental acceleration within symbiont-bearing lines. Aphids harbouring S. symbiotica reached adulthood at 9.6–9.8 days, on average, compared to 9.9 days for uninfected aphids; these developmental accelerations were statistically significant for all infected lines according to Wilcoxon rank-sum tests (table 2). The magnitudes of these differences (2–4%) were comparable to those observed for the day 6 heat shock treatment, though substantially less than under the day 2 treatment (7–18%).
(b) H. defensa and R. insecticola experiments
To determine the generality of symbiont-mediated heat tolerance, we performed similar experiments with additional microbes, comparing the fitness of aphids infected with H. defensa or R. insecticola to that of secondary-free aphids. Again, heat shock on day 2 had drastically negative effects, causing substantial mortality, reduced fecundity and prolonged development.
The effects of the H. defensa and R. insecticola symbionts were, generally, less pronounced than those of S. symbiotica isolates. However, both had significant effects on survival under the heat shock treatment (table 1). Uninfected aphids were 24% more likely to survive to adulthood compared to R. insecticola-bearing aphids after heat shock on day 2 (table 1, logistic regression, p=0.0439). In contrast, H. defensa improved survival of heat-shocked aphids by 34%, relative to uninfected aphids (p=0.0111), on a par with the advantages conferred by S. symbiotica subjected to the same treatment. The significance (but not the trend) of this latter difference depended on the inclusion of one of the fourteen heat-treated H. defensa-infected cultures in which 20/20 individuals survived to adulthood.
Sterilization was also common among heat-treated aphids in this experiment, with 55% of all aphids that survived 8 days of adulthood failing to produce offspring. The average numbers of offspring produced per female were 1.4 (Ri, n=19), 3.7 (N, n=34) and 6.21 (Hd, n=29). These values were statistically indistinguishable, though our analyses were limited by small sample sizes imposed by high mortality. Neither H. defensa nor R. insecticola caused significant changes in the development time of heat-shocked aphids (table 3).
Table 3.
Time to adulthood for uninfected, H. defensa- and R. insecticola-infected A. pisum.
| treatment | statistics | aphid line | ||
|---|---|---|---|---|
| N | Hd | Ri | ||
| control | number of aphids (number of treated cultures) | 76 (10) | 65 (11) | 71 (10) |
| mean; median (days to adulthood) | 10.1; 10 | 10.2; 10 | 10.0; 10 | |
| Wilcoxon rank-sum testa | n.a. | p=0.1231 | p=0.7221 | |
| day 2 heat shock | number of aphids (number of treated cultures) | 55 (11) | 66 (9) | 28 (8) |
| mean; median (days to adulthood) | 13.2; 13 | 13.6; 13 | 14.17; 14 | |
| Wilcoxon rank-sum testa | n.a. | p=0.5960 | p=0.0818 | |
Pairwise tests comparing development times for each symbiont-infected line (Hd and Ri) to those for the uninfected line (N) under each temperature treatment.
Under the control treatment, we observed no effects of symbionts on either survival (table 1) or development time (table 3). Average fecundities per female were 29.0 (N, n=38), 26.9 (Hd, n=34), 28.3 (Ri, n=40) under control conditions; these values were also indistinguishable according to statistical tests (data not shown).
4. Discussion
(a) Effects of temperature on symbiosis
Our findings reveal that the effects of secondary symbionts on aphid fitness vary with temperature. We observed an overall trend toward higher fitness for aphid lines harbouring S. symbiotica or H. defensa when hosts were subjected to heat shock. These benefits extended across multiple components of fitness for S. symbiotica, though net benefits were substantially greater in aphids exposed to heat shock as younger juveniles. Benefits were limited to improved survival in the case of H. defensa, whereas lines infected with R. insecticola suffered reduced survival compared to their uninfected clone-mates. Thus, R. insecticola imposed a cost under high temperatures. In comparison, the effects of symbionts under permissive temperatures were relatively small. Neither H. defensa nor R. insecticola altered the performance of A. pisum reared constantly at 18 °C. S. symbiotica isolates had slight, though positive effects on development times of control-reared aphids. So in summary, temperature clearly plays a significant role in shaping the outcomes of these interactions.
Evidence for improved heat tolerance has been previously documented for A. pisum infected with S. symbiotica, which increased host fecundity under constant rearing at 25 °C (Chen et al. 2000) and under heat shock conditions similar to those utilized in our experiments (Montllor et al. 2002). A parallel finding was obtained from research on a Paramecium and its intracellular bacterial endosymbiont, which improved host viability after exposure to high temperatures (Hori & Fujishima 2003). In this instance, symbionts induced increased expression of a host heat shock gene, providing a potential explanation for their role in heat tolerance.
Aside from the aphids, there is scant evidence for a role of heritable symbionts in heat tolerance among insects. Yet, temperature is known to have other effects on these interactions. For instance, Wolbachia-induced effects of parthenogenesis and cytoplasmic incompatibility can both be attenuated by exposing insects to heat (Hoffmann et al. 1986, 1990; Stouthamer 1997; Feder et al. 1999; Arakaki et al. 2001). This may stem from a negative effect of high temperatures on symbiont survival, a phenomenon apparent in several insect hosts (e.g. Buchner 1965). Among the aphids, a heat shock treatment similar to our own was previously shown to reduce survival of essential Buchnera symbionts in A. pisum (Ohtaka & Ishikawa 1991). Similarly, Montllor and colleagues (2002) noted a decrease in the number of specialized Buchnera-housing cells (bacteriocytes) in A. pisum adults that survived heat treatment, suggesting reduced Buchnera survival. In this study, the authors also observed that the presence of S. symbiotica led to increased bacteriocyte persistence, thus presumably higher Buchnera survival, providing a possible mechanism for the heat tolerance phenotype. An equally compelling explanation is suggested by recent findings that S. symbiotica (a.k.a. PASS or R-type) can partially restore A. pisum fitness in the absence of the essential Buchnera (Koga et al. 2003). Thus, increased heat tolerance could stem from supplementation of Buchnera function.
(b) Variation among symbionts
In this study, we have demonstrated both variation and overlap among secondary symbionts in their effects on aphid fitness. As described above, S. symbiotica isolates conferred strong benefits on survival, fecundity and development time for aphids heat shocked as young juveniles. H. defensa improved only survival. In contrast, R. insecticola imposed a clear cost under heat shock, reducing survival of infected hosts. Previous studies on these secondary symbionts in A. pisum have also demonstrated overlap and variation in their effects on host phenotypes. For example, both H. defensa and S. symbiotica defend A. pisum against the parasitoid wasp, A. ervi (Oliver et al. 2003). In contrast, R. insecticola has no effect on defence against A. ervi (Oliver et al. 2003), yet plays a role in host plant utilization (Tsuchida et al. 2004) and, potentially, in defence against a fungal pathogen (Ferrari et al. 2004). Given these differences, as well as those observed here, it appears that different biotic and abiotic components of aphid environments have favoured the maintenance of distinct secondary symbionts. However, evidence for an overlap in symbiont effects suggests that the prevalence of H. defensa and S. symbiotica may stem from similar selective forces of parasitism and temperature.
In addition to finding variation among distantly related symbionts, we observed differences in the effects of closely related S. symbiotica isolates. The 16S rDNA sequences of the SsNY (AY136140), SsWI (AY136139) and SsAZ (AF293617) isolates differ by less than 0.4%. Moreover, we have identified only a single polymorphic site out of greater than 5000 nucleotides sequenced from protein coding genes in these three isolates (J. Russell, unpublished data). Despite these similarities, our results suggest that the fitness benefits induced by these microbes varied under high temperatures. For example, when aphids were heat-shocked as young juveniles, the line harbouring the AZ isolate of S. symbiotica had significantly higher fecundity (33% higher) and faster development (5–11% faster) compared to the NY and WI lines. In addition, the NY isolate did not appear to accelerate development or increase fecundity of aphids heat shocked as older juveniles, in contrast to its WI and AZ counterparts. Thus, the differences among these symbionts are not merely quantitative; they also extend to the presence or absence of benefits.
These findings of variation among close relatives are not unique. In fact, similar Wolbachia isolates can induce different reproductive manipulation phenotypes in their arthropod hosts (Werren et al. 1995; Jiggins et al. 2002). And recently, it has been demonstrated that isolates of H. defensa from A. pisum differ in their capacities to defend their hosts against parasitism (Oliver et al. 2005). Due to this functionally significant variation, natural selection will not only respond to heritable differences among infected and uninfected insects, but will also differentiate between hosts harbouring closely related symbionts.
It is tempting to speculate that the geographic origins of the S. symbiotica isolates can explain the variation in their effects on aphids under high temperatures. Specifically, one would expect natural selection to favour isolates that confer stronger heat tolerance in aphids colonizing warmer areas. Considering this, it is not surprising that the isolate collected from the hottest locale (SsAZ from Tucson, Arizona) appeared to confer the strongest benefits under high temperatures. This would imply that a mechanism for adapting to hot conditions is acquisition of a particular symbiont, in addition to genetic change in the insect genome itself.
(c) Ecological and evolutionary implications
Temperature is an important abiotic factor from an aphid's perspective. These insects are highly heat-sensitive, and often fail to develop or reproduce when reared at constant temperatures close to 30 °C (e.g. Turak et al. 1998; Wang & Tsai 2000, 2001; Asin & Pons 2001) or when exposed to brief periods of temperatures between 30 and 40 °C (e.g. Ma et al. 2004). In fact, A. pisum suffers drastic fitness reductions under constant temperatures as low as 25 °C (Chen et al. 2000) or when exposed to heat shock at 37 °C for periods as brief as 3 h (Ohtaka & Ishikawa 1991). Findings that secondary symbionts confer heat tolerance on A. pisum under constant rearing at 25 °C (Chen et al. 2000) and under brief exposure to heat shock (Montllor et al. 2002) suggest that maternally transmitted microbes play a role in adaptation to the abiotic environment. Therefore, we can expect these symbionts to shape the geographic and seasonal distributions of hosts.
To date, only a few published studies have attempted to determine the prevalence of secondary symbionts in relation to temperature in field populations of A. pisum. Montllor and colleagues noted that S. symbiotica (called ‘PASS’ in that study) increased in frequency in 2/3 of California localities with increasing seasonal temperature. Yet S. symbiotica frequencies were not correlated with temperature when compared across regions of California (Montllor et al. 2002) or Japan (Tsuchida et al. 2002), suggesting that heat is not the sole determinant of symbiont frequencies in the field. Furthermore, no correlations between H. defensa frequencies and temperature were found in a study that screened for this symbiont in aphids from multiple host plants (Darby et al. 2003). In contrast, R. insecticola prevalence was highest in cooler regions of Japan (Tsuchida et al. 2002), consistent with our finding that this symbiont imposed a cost at high temperatures.
Laboratory studies on the secondary symbionts of aphids have revealed that temperature is not the only factor likely to shape symbiont abundance. Due to the additional importance of parasitism (Oliver et al. 2003; Ferrari et al. 2004) and host plants (Tsuchida et al. 2004), it has become apparent that frequencies will be governed by the selective input of several biotic and abiotic forces. Future attempts to determine the natural relevance of any one of these variables will, thus, be most successful when accounting for each of the other likely determinants of symbiont prevalence.
NOTE ADDED IN PROOF
Recent studies in the Moran laboratory have revealed a mutation that may affect heat shock response in the Buchnera of some A. pisum stocks. Conceivably, this mutation could affect aphid heat tolerance. We have reviewed our results in light of these recent findings, after scoring for this mutation in our experimental lines. Our estimates of effects for particular symbiont isolates may have been affected by mutations in Buchnera genomes. However, none of our qualitative conclusions are affected. These conclusions include the findings that S. symbiotica and H. defensa isolates confer tolerance to heat stress and that the R. insecticola isolate imposes a liability under high temperatures. Also unaffected is the conclusion that the effects of symbiont species, even closely related isolates, differ under high temperatures.
Acknowledgements
We would like to thank Kim Hammond, Phat Tran, Helen Dunbar and Michelle Hoffman for technical assistance. We also thank Tony Ives for insights on the manuscript and for his work on modelling aphid fitness. In addition, we thank Molly Hunter, Yves Carrière, Kerry Oliver, Mike Worobey, Carlos Machado, Jeff Good and Steve Perlman for helpful comments on the manuscript. We also thank two anonymous reviewers for their feedback. This research was supported by NSF grant no. 0313737. J. Russell was supported by the NSF IGERT program in evolutionary genomics, the Center for Insect Science and the NSF/USDA/DOE Plant-Insect Interaction training program, all at the University of Arizona.
References
- Abbot, P. 2001 Individual and population variation in invertebrates revealed by Inter-simple Sequence Repeats (ISSRs). J. Insect Sci 1.8. See http://insectscience.org/1.8 [PMC free article] [PubMed]
- Arakaki N, Miyoshi T, Noda H. Wolbachia-mediated parthenogenesis in the predatory thrips Franklinothrips vespiformis (Thysanoptera: Insecta) Proc. R. Soc. B. 2001;268:1011–1016. doi: 10.1098/rspb.2001.1628. doi:10.1098/rspb.2001.1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asin L, Pons X. Effect of high temperature on the growth and reproduction of corn aphids (Homoptera: Aphididae) and implications for their population dynamics on the northeastern Iberian peninsula. Environ. Entomol. 2001;30:1127–1134. [Google Scholar]
- Bracho A.M, Martinez-Torres D, Moya A, Latorre A. Discovery and molecular-characterization of a plasmid localized in Buchnera sp. bacterial endosymbiont of the aphid Rhopalosiphum padi. J. Mol. Evol. 1995;41:67–73. doi: 10.1007/BF00174042. doi:10.1007/BF00174042 [DOI] [PubMed] [Google Scholar]
- Buchner P. Interscience; New York: 1965. Endosymbiosis of animals with plant microorganisms. [Google Scholar]
- Bull J.J, Molineux I.J, Rice W.R. Selection of benevolence in a host–parasite system. Evolution. 1991;45:875–882. doi: 10.1111/j.1558-5646.1991.tb04356.x. [DOI] [PubMed] [Google Scholar]
- Chen D.-Q, Purcell A.H. Occurrence and transmission of facultative endosymbionts in aphids. Curr. Microbiol. 1997;34:220–225. doi: 10.1007/s002849900172. doi:10.1007/s002849900172 [DOI] [PubMed] [Google Scholar]
- Chen D.-Q, Campbell B.C, Purcell A.H. A new Rickettsia from a herbivorous insect, the pea aphid Acyrthosiphon pisum (Harris) Curr. Microbiol. 1996;33:123–128. doi: 10.1007/s002849900086. doi:10.1007/s002849900086 [DOI] [PubMed] [Google Scholar]
- Chen D.-Q, Montllor C.B, Purcell A.H. Fitness effects of two facultative endosymbiotic bacteria on the pea aphid Acyrthosiphon pisum, and the blue alfalfa aphid, A. kondoi. Entomol. Exp. Appl. 2000;95:315–323. doi:10.1023/A:1004083324807 [Google Scholar]
- Darby A.C, Douglas A.E. Elucidation of the transmission patterns of an insect-borne bacterium. Appl. Environ. Microbiol. 2003;69:4403–4407. doi: 10.1128/AEM.69.8.4403-4407.2003. doi:10.1128/AEM.69.8.4403-4407.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby A.C, Birkle L.M, Turner S.L, Douglas A.E. An aphid-borne bacterium allied to the secondary symbionts of whitefly. FEMS Microbiol. Ecol. 2001;36:43–50. doi: 10.1111/j.1574-6941.2001.tb00824.x. doi:10.1016/S0168-6496(01)00117-9 [DOI] [PubMed] [Google Scholar]
- Darby A.C, Tosh C.R, Walters K.F.A, Douglas A.E. The significance of a facultative bacterium to natural populations of the pea aphid Acyrthosiphon pisum. Ecol. Entomol. 2003;28:145–150. doi:10.1046/j.1365-2311.2003.00492.x [Google Scholar]
- Douglas A.E. Sulfate utilization in an aphid symbiosis. Insect Biochem. 1988;18:599–605. doi:10.1016/0020-1790(88)90012-1 [Google Scholar]
- Douglas A.E, Prosser W.A. Synthesis of the essential amino acid tryptophan in the pea aphid (Acyrthosiphon pisum) symbiosis. J. Insect Physiol. 1992;38:565–568. doi:10.1016/0022-1910(92)90107-O [Google Scholar]
- Ewald P. Transmission modes and the evolution of the parasitism-mutualism continuum. Ann. NY. Acad. Sci. 1983;503:295–306. doi: 10.1111/j.1749-6632.1987.tb40616.x. [DOI] [PubMed] [Google Scholar]
- Feder M.E, Karr T.L, Yang W, Hoekstra J.M, James A.C. Interaction of Drosophila and its endosymbiont Wolbachia: natural heat shock and the overcoming of sexual incompatibility. Am. Zool. 1999;39:363–370. [Google Scholar]
- Ferrari J, Darby A.C, Daniell T.J, Godfray H.C.J, Douglas A.E. Linking the bacterial community in pea aphids with host-plant use and natural enemy resistance. Ecol. Entomol. 2004;29:60–65. doi:10.1111/j.1365-2311.2004.00574.x [Google Scholar]
- Fukatsu T. Secondary intracellular symbiotic bacteria in aphids of the genus Yamatocallis (Homoptera: Aphididae: Drepanosiphinae) Appl. Environ. Microbiol. 2001a;67:5315–5320. doi: 10.1128/AEM.67.11.5315-5320.2001. doi:10.1128/AEM.67.11.5315-5320.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukatsu T. Spiroplasma symbiont of the pea aphid, Acyrthosiphon pisum (Insecta: Homoptera) Appl. Environ. Microbiol. 2001b;67:1284–1291. doi: 10.1128/AEM.67.3.1284-1291.2001. doi:10.1128/AEM.67.3.1284-1291.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S, Darby A.C, Daniell T.J, Webster G, van Veen F.J.F, Godfray H.C.J, Prosser J.I, Douglas A.E. Diversity of bacteria associated with natural aphid populations. Appl. Environ. Microbiol. 2003;69:7216–7223. doi: 10.1128/AEM.69.12.7216-7223.2003. doi:10.1128/AEM.69.12.7216-7223.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A.A, Turelli M, Simmons G.M. Unidirectional incompatibility between populations of Drosophila simulans. Evolution. 1986;40:692–701. doi: 10.1111/j.1558-5646.1986.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann A.A, Turelli M, Harshman L.G. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics. 1990;126:933–948. doi: 10.1093/genetics/126.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori M, Fujishima M. The endosymbiotic bacterium Holospora obtusa enhances heat-shock gene expression of the host Paramecium caudatum. J. Eukaryot. Microbiol. 2003;50:293–298. doi: 10.1111/j.1550-7408.2003.tb00137.x. doi:10.1111/j.1550-7408.2003.tb00137.x [DOI] [PubMed] [Google Scholar]
- Jiggins F.M, Bentley J.K, Majerus M.E.N, Hurst G.D.D. Recent changes in phenotype and patterns of host specialization in Wolbachia bacteria. Mol. Ecol. 2002;11:1275–1283. doi: 10.1046/j.1365-294x.2002.01532.x. doi:10.1046/j.1365-294X.2002.01532.x [DOI] [PubMed] [Google Scholar]
- Koga R, Tsuchida T, Fukatsu T. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc. R. Soc. B. 2003;270:2543–2550. doi: 10.1098/rspb.2003.2537. doi:10.1098/rspb.2003.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-Y, Baumann L, Baumann P. Amplification of trpEG; adaptation of Buchnera aphidicola to an endosymbiotic association with aphids. Proc. Natl Acad. Sci. USA. 1994;91:3819–3823. doi: 10.1073/pnas.91.9.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo T.E. Removal of a specialization-associated symbiont does not affect aphid fitness. Ecol. Lett. 2004;7:461–468. doi:10.1111/j.1461-0248.2004.00602.x [Google Scholar]
- Leonardo T.E, Muiru G.T. Facultative symbionts are associated with host plant specialization in pea aphid populations. Proc. R. Soc. B. 2003;270:S209–S212. doi: 10.1098/rsbl.2003.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.-S, Hau B, Poehling H.-M. The effects of heat stress on the survival of the rose grain aphid, Metopolophium dirhodum (Hemiptera: Aphididae) Eur. J. Entomol. 2004;101:327–331. [Google Scholar]
- May R.M, Anderson R.M. Coevolution of hosts and parasites. Parasitology. 1982;85:411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- Montllor C.B, Maxmen A, Purcell A.H. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 2002;27:189–195. doi:10.1046/j.1365-2311.2002.00393.x [Google Scholar]
- Moran N.A, Russell J.A, Koga R, Fukatsu T. Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl. Environ. Microbiol. 2005;71:3302–3310. doi: 10.1128/AEM.71.6.3302-3310.2005. doi:10.1128/AEM.71.6.3302-3310.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson M.A, Baumann P, Clark M.A, Baumann L, Moran N.A, Voegtlin D.J, Campbell B.C. Evidence for the establishment of aphid-eubacterial endosymbiosis in an ancestor of four aphid families. J. Bacteriol. 1991a;173:6321–6324. doi: 10.1128/jb.173.20.6321-6324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson M.A, Baumann P, Kinsey M.G. Buchnera gen. nov. and Buchnera aphidicola sp. nov., a taxon consisting of the mycetocyte-associated, primary endosymbionts of aphids. Int. J. Syst. Microbiol. 1991b;41:566–568. [Google Scholar]
- Ohtaka C, Ishikawa H. Effects of heat treatment on the symbiotic system of an aphid mycetocyte. Symbiosis. 1991;11:19–30. [Google Scholar]
- Oliver K.M, Russell J.A, Moran N.A, Hunter M.S. Facultative bacteria in aphids confer resistance to parasitic wasps. Proc. Natl Acad. Sci. USA. 2003;100:1803–1807. doi: 10.1073/pnas.0335320100. doi:10.1073/pnas.0335320100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver K.M, Moran N.A, Hunter M.S. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc. Natl Acad. Sci. USA. 2005;102:12 795–12 800. doi: 10.1073/pnas.0506131102. doi:10.1073/pnas.0506131102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.A, Latorre A, Sabater-Muñoz B, Moya A, Moran N.A. Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol. Ecol. 2003;12:1061–1075. doi: 10.1046/j.1365-294x.2003.01780.x. doi:10.1046/j.1365-294X.2003.01780.x [DOI] [PubMed] [Google Scholar]
- Sandström J, Moran N.A. How nutritionally imbalanced is phloem sap for aphids? Entomol. Exp. Appl. 1999;91:203–210. [Google Scholar]
- Sandström J, Moran N.A. Amino acid budgets in three aphid species using the same host plant. Physiol. Entomol. 2001;26:202–211. doi:10.1046/j.0307-6962.2001.00235.x [Google Scholar]
- Sandström J.P, Russell J.A, White J.P, Moran N.A. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 2001;10:217–228. doi: 10.1046/j.1365-294x.2001.01189.x. doi:10.1046/j.1365-294X.2001.01189.x [DOI] [PubMed] [Google Scholar]
- Simon J.C, Carré S, Boutin M, Prunier-Leterme N, Sabater-Muñoz B, Latorre A, Bournoville R. Host-based divergence in populations of the pea aphid: insights from nuclear markers and the prevalence of facultative symbionts. Proc. R. Soc. B. 2003;270:1703–1712. doi: 10.1098/rspb.2003.2430. doi:10.1098/rspb.2003.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer R. Wolbachia-induced parthenogenesis. In: O'Neill S, Hoffmann A.A, Werren J.H, editors. Influential passengers. Oxford University Press; New York: 1997. pp. 102–122. [Google Scholar]
- Tsuchida T, Koga R, Shibao H, Matsumoto T, Fukatsu T. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 2002;11:2123–2135. doi: 10.1046/j.1365-294x.2002.01606.x. doi:10.1046/j.1365-294X.2002.01606.x [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Koga R, Fukatsu T. Host plant specialization governed by facultative symbiont. Science. 2004;303:1989. doi: 10.1126/science.1094611. doi:10.1126/science.1094611 [DOI] [PubMed] [Google Scholar]
- Turak E, Talent R, Sunnucks P, Hales D.F. Different responses to temperature in three closely-related sympatric cereal aphids. Entomol. Exp. Appl. 1998;86:49–58. doi:10.1023/A:1003102927699 [Google Scholar]
- Unterman B.M, Baumann P, McLean D.L. Pea aphid symbiont relationships established by analysis of 16S ribosomal-RNA's. J. Bacteriol. 1989;171:2970–2974. doi: 10.1128/jb.171.6.2970-2974.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-J, Tsai J.H. Effect of temperature on the biology of Aphid spiraecola (Hompotera: Aphididae) Ann. Entomol. Soc. Am. 2000;93:874–883. [Google Scholar]
- Wang J.-J, Tsai J.H. Development, survival, and reproduction of black citrus aphid, Toxoptera aurantii (Hemiptera: Aphididae), as a function of temperature. Bull. Entomol. Res. 2001;91:477–487. [PubMed] [Google Scholar]
- Werren J.H, Zhang W, Guo L.R. Evolution and phylogeny of Wolbachia: reproductive parasites of the arthropods. Proc. R. Soc. B. 1995;261:55–71. doi: 10.1098/rspb.1995.0117. [DOI] [PubMed] [Google Scholar]
- Wicker C, Nardon P. Development responses of symbiotic and aposymbiotic weevils Sitophilus oryzae L. (Coleoptera, Curculionidae) to a diet supplemented with aromatic amino acids. J. Insect Physiol. 1982;28:1021–1024. doi:10.1016/0022-1910(82)90008-7 [Google Scholar]