Abstract
Retaliation against cheaters can prevent the breakdown of cooperation. Here we ask whether the ant–plant Cordia nodosa is able to apply retaliatory sanctions against its ant symbiont Allomerus octoarticulatus, which patrols new shoots to prevent herbivory. We test the hypothesis that the modular design of C. nodosa physiologically ties the growth of housing (stem swellings known as domatia) to the successful development of the attached leaves. We experimentally simulated herbivory by cutting leaves from patrolled shoots and found that the domatia on such ‘cheated’ shoots suffered higher mortality and lower growth than did controls, evidence for a host sanction. On the other hand, patrolling is costly to the ant, and experiment shows that non-patrollers run a low risk of being sanctioned because most leaves (and the attached domatia) escape heavy herbivory even when patrollers are absent. This suggests that cheaters might enjoy a higher fitness than do mutualists, despite sanctions, but we find that patrolling provides a net fecundity benefit when the colony and plant exceed a minimum size, which requires sustained ant investment in patrolling. These results map directly onto the principal–agent (P–A) game from economics, which we suggest can be used as a framework for studying stability in mutualisms, where high sampling costs and cheating do not allow market effects to select for mutual benefits.
Keywords: biological markets, cooperation, host sanctions, market effects, mutualism, myrmecophyte
1. Introduction
Nature is rife with examples of cooperation between species, known as mutualisms (Janzen 1985). Nonetheless, it is widely acknowledged that the study of mutualisms has lacked a general theoretical framework to explain how: (i) mutualisms evolve from antagonistic interactions, and then (ii) persist in the face of (a) invasion by specialized parasites that purloin the benefits one mutualist provides another, and (b) selection for cheating behaviour in the mutualists themselves (Doebeli & Knowlton 1998; Herre et al. 1999; Hoeksema & Bruna 2000; Bronstein 2001; Wilkinson & Sherratt 2001; Yu 2001; Bergstrom & Lachmann 2003; Frank 2003; Sachs et al. 2004; Edwards et al. in preparation).
The standard explanations for intra-specific cooperation are often inadequate for mutualisms. Mutualists do not share genealogies, which precludes kin selection, specialized parasites of mutualisms do not, by definition, render by-product benefits, and policing and reciprocity require a level of recognition or localized dispersal that is rarely achieved (Yu 2001; Bergstrom & Lachmann 2003; Frank 2003; Gardner & West 2004, but see Bshary & Noë 2003). The more general model of Frank (1994, see also Bull et al. 1991) argues that if the fitnesses of mutualists are spatially correlated, then cooperation will persist. Vertical transmission (Yamamura 1993) achieves this, but for horizontally transmitted mutualisms in which partners disperse separately (Yu & Davidson 1997), allowing partner diversity to increase (West et al. 2002b), other mechanisms are needed to bring about the necessary correlation of behaviours.
One approach that is proving promising is the concept of host coercion (Murray 1985; Herre 1989; West & Herre 1994; Yu 2001; Ferdy et al. 2002; West et al. 2002a,b; Hoeksema & Kummel 2003; Yu & Ridley 2003; Yu et al. 2004a; Edwards et al. in preparation), which argues that the partner controlling the physical resources (the host) ultimately controls the relationship, as opposed to the partner providing the services (the visitor).
A straightforward manifestation of coercion is the host sanction, in which cheating visitors are punished by their hosts. For example, in the yucca–moth mutualism, yucca flowers heavily damaged by the oviposition of some species of pollinating yucca moth tend to be aborted (Pellmyr & Huth 1994), which selects for lower levels of oviposition (and thus, higher seed production). Similarly, in the legume–rhizobia mutualism, Kiers et al. (2003) have demonstrated experimentally that soybean plants selectively reduce oxygen permeability in root nodules containing non-nitrogen fixing rhizobial bacteria, reducing their fitness (West et al. 2002b, see also Hoeksema & Kummel 2003 on mycorrhizae).
Here, we test the hypothesis that host sanctions maintain costly cooperative behaviour in another classic system, protective ant–plant mutualisms (Janzen 1966; Davidson & McKey 1993; Heil & McKey 2003), in which host-plants provide housing (in the form of specialized plant cavities called ‘domatia’, figure 1), and sometimes food, to resident ant colonies, in return for protection from herbivores. We have previously rejected an alternative hypothesis that a sensory trap mimicking ant brood elicits costly patrolling in resident ant colonies (Edwards et al. in preparation), and we now test the idea that host-plants can selectively reduce the fitness of non-patrolling ant colonies.
Figure 1.

New shoot with domatium and its four attached leaves. The leaves removed in the two new leaves experiment are indicated with arrows.
We do this by experimentally simulating leaf herbivory (i.e. ‘making the ant cheat’) and measuring the effects on the growth and survivorship of the associated domatia. If host sanctions are acting, leaf herbivory should reduce survivorship and/or growth of the domatia. We also quantify the risk of herbivory to unprotected leaves and the worker biomass investment made by ant colonies to patrolling, and we combine these results in a simple model to calculate whether the cost of sanctions likely outweighs the benefit of cheating, which is reduced investment in patrolling workers. Finally, we show how the ant–plant mutualism can be usefully viewed as a principal–agent (P–A) game from economics.
(a) Study system
Our system is the ant-plant Cordia nodosa Lam. (Boraginaceae) and its most common obligate ant-symbiont Allomerus cf. octoarticulatus var. demerarae Wheeler (Myrmicinae, named A. demerarae in previous papers (Yu & Pierce 1998; Yu et al. 2001, 2004b)). C. nodosa is an understorey treelet found across Amazonia (Wheeler 1942), and is principally inhabited by Allomerus and four species of Azteca Forel (Dolichoderinae), with one colony of any of the species per plant (Yu et al. 2001, 2004b). The plant provides its resident ant colony with food bodies (Solano et al. 2005) and housing (domatia) in the form of hollow swellings at branch internodes, and Allomerus and Azteca workers actively patrol and protect new shoots (Yu & Pierce 1998, Edwards et al. in preparation; figure 1). We focus on the more abundant Allomerus. The allocation of colony biomass and energy to patrolling ant workers represents a proximate diversion of colony resources from reproduction to host-plant defence.
An alternative allocation is exhibited by a number of rarer, parasitic ants that also associate with C. nodosa, including: Brachymyrmex sp. (Formicinae); Pachycondyla crenata and Pachycondyla unidentata (Ponerinae); and Pheidole spp., Crematogaster sp., and Solenopsis sp. (Myrmicinae). These species also colonize and inhabit the domatia of saplings, but they do not patrol leaves. Thus, colony investment is directed entirely to reproduction, and many of these colonies reproduce at very small sizes (D. Yu, personal observation). Most likely, none of these ant species is an obligate symbiont, and so have not coevolved with C. nodosa.
Thus, both protecting and non-protecting ant species are found on the C. nodosa system, but protecting species are by far the dominant type, inhabiting ≈90% of plants (Yu et al. 2001, 2004b), suggesting some mechanism that favours ant protection over non-protection.
2. Material and methods
The majority of the study was conducted in the trail system of the Libertador Tambopata Lodge, bordering the Tambopata National Reserve, Madre de Dios, Peru (69°W, 12°S; 200–500 m.a.s.l.), between August 2002 and October 2003. The Fecundity cost of patrolling study was conducted at two sites in Madre de Dios: Pantiacolla Lodge (71°W, 12°S; 377 m) and Manu Wildlife Center (70°W, 12°S; 261 m), between April and May 2003. The habitat is moist to seasonal tropical primary forest (annual rainfall 2100 mm) with large-scale river meandering.
(a) Patrolling intensity by leaf age
To contrast worker investment in patrolling across leaf age, we located 43 naturally established C. nodosa plants inhabited by an Allomerus colony (min. six domatia) and producing at least one new (non-lignified and still expanding) shoot, where a shoot is defined as a branch section, a domatium, and six associated leaves, four of which grow directly from the domatium and to which we restrict our attention (figure 1). The smallest of the new shoots was selected on each plant. One of the four leaves was randomly selected using a four-sided die, measured for length along the mid-vein from the petiole to the leaf tip, and scored for number of workers patrolling. A mature shoot was also randomly selected on each plant, and the mature leaf in the same position as the new leaf was similarly measured and scored.
(b) Risk of natural herbivory to new leaves
To quantify the risk of herbivory to leaves unprotected by ants, C. nodosa were grown from seed in screen tents, and 27 saplings were selected that had both new and mature shoots. All new leaves on each plant were traced, as were a corresponding number of mature leaves. Saplings were then placed in their pots in primary forest at least 50 m apart. After seven weeks, after all new leaves had fully lignified, the original leaves were re-traced in order to calculate the area lost to herbivory. To control for the effects of leaf growth, the area of leaf eaten before and after the experiment was standardized as a percentage of the ‘uneaten’ leaf, where the size of the ‘uneaten’ leaf was estimated by filling in the areas consumed. Finally, because there was some herbivory of leaves in the screen tents (mean±s.e.: new =0.8%±0.2; mature =2.7%±0.7), the percentages of leaf consumed before the experiment were subtracted.
(c) Simulated herbivory and host sanctions
To examine the effect of leaf herbivory on the survival and growth of associated domatia, we performed three simulated herbivory experiments on naturally established C. nodosa plants (>10 domatia) inhabited by Allomerus. Two experiments used new shoots (non-lignified, <20 mm in length), and one used mature shoots. In all three experiments, we simulated herbivory by removing some number of distal leaves at the petiole with a pair of scissors (figure 1), leaving controls intact. Individual shoots were assigned to treatment or control by coin flip, and in most cases, we could use plants producing two new shoots simultaneously, which allowed in-plant controls. Plants producing only one new shoot were assigned to treatment sequentially down the transect by coin flip. Ants continued patrolling domatia and any remaining leaves.
The initial maximum diameter and length of all domatia were measured using callipers to the nearest 1 mm. Domatia were scored for mortality after four weeks and six months (and in the four new leaves experiment, also after 1 year). Surviving domatia were re-measured at four weeks, at which point they had lignified and stopped growing. Any additional shoots that grew from the focal domatia were also recorded.
To convert linear dimensions to volume, we grew 31 C. nodosa from seed and chose one domatium randomly per plant. Volume was measured directly by using a syringe to fill the domatium with water. We also estimated the volume using the equation for a cone , where L is the domatium length and r the radius. Calculated and observed volumes were highly correlated (Pearson, R2=0.91, p<0.001). Initial volumes of control and treatment domatia did not differ for any experiment (all p>0.5).
Four new leaves. We used 23 plants producing two new shoots and 12 plants producing one new shoot (six assigned to treatment). The treatment was removal of all four leaves (figure 1).
Two new leaves. We used 18 plants producing two new shoots and five plants producing one new shoot (three assigned to treatment). The treatment was removal of two leaves (figure 1).
Four mature leaves. We used 22 plants and for each, randomly selected two mature (lignified) domatia with mature leaves from the same general area of the plant, assigned one by coin flip to the treatment, and removed all four leaves.
(d) Biomass cost of patrolling
We estimate a colony's investment in patrolling as the biomass of patrolling workers. We collected all new shoots (avg. of three per plant) from 41 Allomerus-inhabited plants of known size. Because patrollers are a distinct caste (Edwards et al. in preparation), we omitted any shoots with domatia containing brood (and thus, also the brood-tending caste). The collected workers were dried at 80 °C for 48 h and weighed to the nearest tenth of a milligram.
We assume that patroller biomass could have been invested instead in additional alates (winged reproductives). Alate investment by colony size was calculated using a previous dataset (Yu et al. 2004b) of male and female alate counts from 59 Allomerus-inhabited plants of known size. To convert to biomass, we collected three male and three female alates from each of six Allomerus colonies and dried and weighed them as above.
3. Results
(a) Patrolling intensity by leaf age
Patrolling is almost entirely concentrated on new, unlignified leaves, with the smallest (thus, newest) new leaves attracting the highest patroller density (figure 2). Once the leaf is mature (lignified), patrolling is restricted to the occasional solitary worker.
Figure 2.
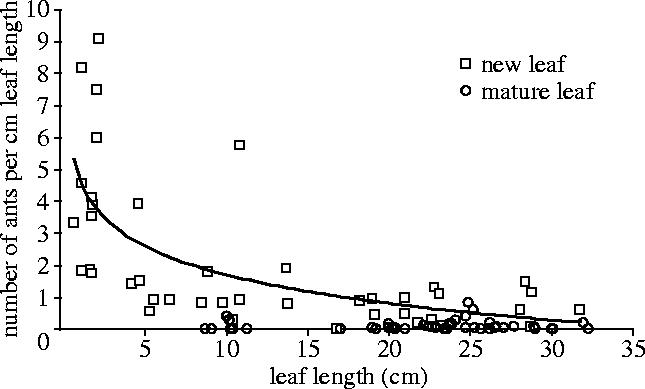
Patrolling intensity by leaf age. The relationship of leaf length (cm) to the number of patrolling workers per centimetre length (patroller density). Patroller density is higher on smaller new leaves, and is restricted to solitary workers on mature leaves (fitted line not shown), regardless of mature leaf size (ANCOVA, F1,82=17.3, R2=0.69, p<0.001). Restricting the analysis to leaf lengths ≥8.7 cm (the smallest mature leaf) still reveals a significantly higher patrolling density on new leaves (Paired t-test: n=23, t=3.6, p=0.002).
(b) Risk of natural herbivory
New leaves lost an average of three times more area to herbivory than did mature leaves (figure 3). New leaf loss was bimodally distributed: 13 of 27 shoots suffered less than 20% leaf herbivory, and 6 of 27 shoots suffered a mean loss of 96% (2.3% s.e., figure 3). The two simulated herbivory experiments, therefore, reflect the mean herbivory level (≈40%, two new leaves) and the upper mode (four new leaves).
Figure 3.
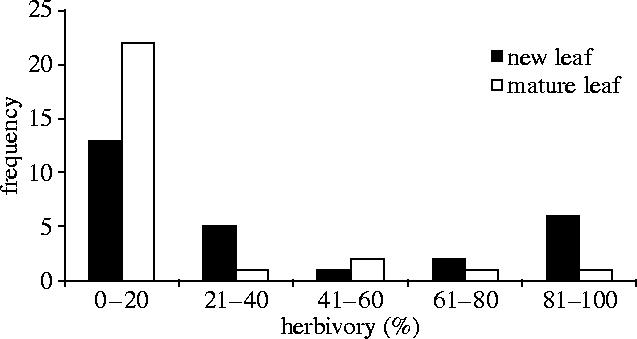
Risk of natural herbivory. The frequency distribution of new leaf and mature leaf herbivory on uncolonized C. nodosa saplings. Herbivory of new leaves (mean±s.e.: 37.5%±7.1) is bimodally distributed and significantly greater than that of mature leaves (12.3%±4.8, paired t-test: n=27, t=−4.7, p<0.001).
(c) Simulated herbivory and host sanctions
Simulated herbivory, mortality effects. Removal of four new leaves led to significantly higher mortality of domatia in all time periods, with nearly three-quarters of domatia dying in six months (figure 4). Dead treatment domatia had significantly smaller starting sizes (mean±s.e.: 0.08 cm3±0.03) than did the surviving domatia (0.25 cm3±0.04; ANOVA, F1,27=11.1, p=0.003). There were no significant mortality effects in the two-leaf and mature-leaf removal experiments (all p>0.10).
Figure 4.
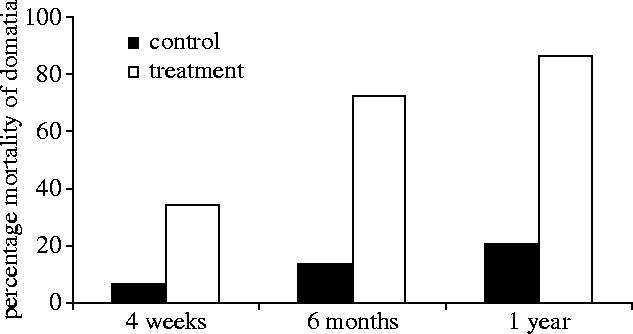
Herbivory and host sanctions—mortality effects. The percentage mortality of domatia after the removal of four new leaves (G-test with Yates correction for continuity: four weeks, ntotaldead=12, G=4.4, p<0.05; six months, ntotaldead=25, G=11.1, p<0.001; and 1 year, ntotaldead=31, G=11.1, p<0.001).
Simulated herbivory, growth effects. For the surviving domatia, removal of four new leaves prevented almost all subsequent volume growth over the next four weeks (figure 5), at which time domatia lignify and stop expanding. The removal of two new leaves also reduced volume growth, but not significantly so (figure 5). There was no growth effect, as expected, upon removal of leaves from mature domatia (figure 5).
Figure 5.
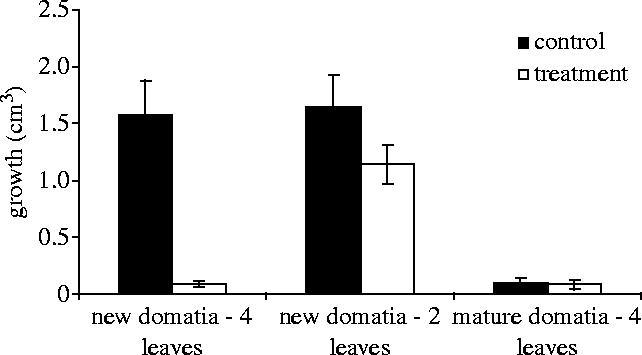
Herbivory and host sanctions—growth effects. Domatia volume growth after four weeks (four new leaves, Mann–Whitney U-test: n=46; U=10.0, p<0.001; two new leaves, ANOVA: F1,35=2.2, p=0.15; four mature leaves: n=43, U=210.5, p=0.61).
Finally, treatment domatia (pooled over both new-leaf removal experiments) produced slightly fewer new shoots after six months than did the control domatia, but not significantly so (mean±s.e.: treatment, 0.65±0.17; control, 0.98±0.26. Mann–Whitney U-test, n=70, U=543.5, p=0.68).
(d) Biomass cost of patrolling
Colony-level biomass investment in patrolling workers is a positive, linear function of the number of new shoots only (F1,39=37.0, R2=0.50, p<0.001); mature domatia and (new domatia)2 are not significant predictors (both p>0.25). Thus, the per-shoot biomass investment in patrolling workers, Pc, is taken as the slope of the regression, 9.02 mg±0.93 s.e., and independent of how many new shoots are being produced at the same time (over the observed range=1–7) or how big the colony is.
4. Discussion
Allomerus workers concentrate their patrolling activity on new leaves (figure 2), as is usual for plant-ants (Yu & Pierce 1998, reviewed in Heil & McKey 2003). After lignification, leaves appear to rely mainly on toughness for defence (Coley 1983). Thus, for each domatium, patrolling is a one-time ‘sunk cost’ for the ants, which is paid off by the fecundity benefit of more domatia volume to house alates (figures 4 and 5). However, because the two new leaf removal experiment produced no excess mortality nor a significant growth reduction (figure 5), we conclude that: (i) host sanctions exist but (ii) are triggered only when leaves are heavily damaged.
However, the risk of heavy damage is low. Almost half of unprotected new shoots escaped with almost no herbivory, and a further quarter escaped with less herbivory than necessary to trigger sanctions (figure 3). We hypothesize that the low risk derives from a small target area, a short maturation time (≈4 weeks), and spatially variable herbivore densities. As a result, even non-patrolling cheater ants should still be able to derive substantial benefits from their host-plants, which raises the possibility that the observed sanction is not sufficient to select for patrolling behaviour.
(a) Calculating the net benefit of patrolling
We, therefore, make some simple calculations of the net benefit of patrolling, allowing cheaters to save the sunk cost of patrolling but paying the cost of reduced housing. The net marginal benefit of patrolling, B(D), is
| 4.1 |
where AP(D) is marginal benefit of a domatium when patrolled, and AN(D) when not patrolled. The marginal benefit is the total alate biomass housed over the life of the next domatium to be patrolled. The terms are functions of the number of domatia, D, but we drop the (D) notation for simplicity. If B>0, then given enough food, it pays to patrol the next new shoot.
The marginal benefits of patrolled and unpatrolled domatia are
| 4.2a |
| 4.2b |
where dM/dD is the marginal gain in alate biomass M with plant size D, G is the number of alate generations produced over the lifetime of a domatium, S is the risk-weighted reduction in marginal alate biomass caused by a host sanction (0≤S≤1), and Pc is the per-shoot ant biomass ‘sunk cost’ of patrolling (9.0 mg), which we conservatively assume is entirely available to the cheater species for investment in alates. We can omit terms for the ‘background mortality’ of domatia not caused by differences in ant patrolling, as these are equal in both strategies and thus cancel.
In the electronic supplementary material, we estimate the other values, and find that the net marginal benefit of patrolling B is positive for plants bigger than 13 or 26 domatia (figure 6), depending on the value of G. Also, the net cumulative benefit of patrolling (that is, after paying back the costs accumulated while patrolling the plant when small) is positive for plants >26 or 53 domatia (figure 6), again depending on G.
Figure 6.
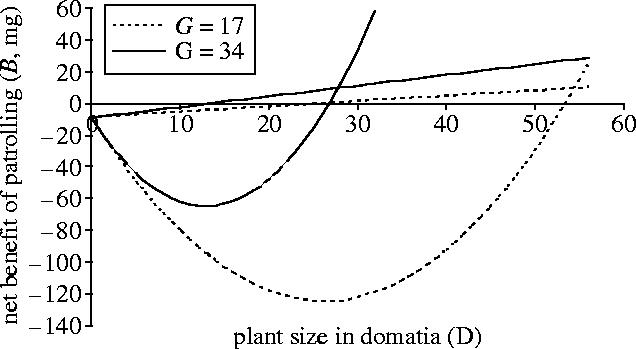
The effect of plant size on the net benefit of patrolling. Net marginal (straight lines) and cumulative (curved lines) benefits of patrolling, in terms of alate biomass production over domatium lifetime, B, as a function of plant size. The cumulative benefit of patrolling becomes positive for plant sizes >26 or 53 domatia, depending on the value of G, which is the estimated number of alate generations over a domatium's lifetime. A higher G value is produced by a shorter estimate of alate development time (see electronic supplementary material).
We emphasize that our parameter values are only rough estimates. Also, this is not an evolutionary model, since we are assuming that the relationship of alate biomass production M to plant size D does not evolve, perhaps due to a minimum worker requirement for colony defence (Davidson 1988; Yu & Pierce 1998) and/or foraging (Dejean et al. 2005).
Nonetheless, the shape of the net benefit curve, which requires only that proportional reproductive allocation rise with colony size, has some interesting implications. First, as the mean size of Allomerus-inhabited plants in Madre de Dios is around 28 domatia (Yu & Pierce 1998; Edwards et al. in preparation), the observed level of host sanctions appears sufficient to select for patrolling. This is because the sample mean includes juvenile plants, so most colonies can expect to exceed the threshold required to achieve a net cumulative benefit (figure 6). If anything, we have erred on the conservative side because the estimated biomass cost of patrolling, Pc, is probably too high, as patrollers could be allocated to multiple shoots over their lifetimes.
Second, if the cumulative benefit of patrolling really is negative for small plant sizes (figure 6), the calculations suggest that C. nodosa has evolved to minimize domatia size, which both reduces host costs and forces the ant to invest in patrolling over a long time before it can enjoy a net benefit. We speculate that when the expected maximum plant size varies across the environment, evolutionary diversification or ecological niche partitioning might take place, with some ant associates evolving not to patrol (specializing in environments with smaller plants), and other species evolving to be patrollers. Obligate, specialist non-protecting ant parasites do co-occur with protecting species in some ant–plant systems (Janzen 1975; Gaume & McKey 1999; Raine et al. 2004), and plant size can vary spatially (e.g. caused by differential disturbance levels), suggesting that such diversification has occurred.
(b) Adaptation, preadaptation and modularity
Is the host sanction an adaptation or a preadaptation? After all, in plants, leaf loss generally leads to reduced proximal branch development and death (Heichel & Turner 1984; Parsons et al. 2003, but see Avila-Sakar et al. 2003). Expanding leaves are a sink for plant resources, so removal naturally shuts down resource flux through the branch, precluding uptake by the growing domatium.
We, therefore, consider the key adaptation enabling host sanctions not to be the death of domatia, but the dispersed spatial distribution of domatia: i.e. modularity (Yu 2001). In all protective ant–plant mutualisms that we are aware of (with one exception), each domatium's growth is physically tied to the growth of a specific leaf or leaf set (‘hostage trading’, Yu 2001). The interesting exception is Barteria nigritana (Passifloraceae), the branches of which produce domatia only along the basal half of their length. None of this plant's ant symbionts is thought to be a specialist, and the most frequent symbiont often fails to remove herbivores (Djiéto-Lordon et al. 2004). Similarly, we do not expect ant associates of ant-fed plants (Janzen 1974; Huxley 1978), which house ant colonies in non-modular tubers, to have evolved leaf-protection behaviours other than as an incidental by-product of foraging (Yu 2001).
Analogous preadaptation arguments can be made regarding host sanctions in yucca–moth and legume–rhizobia symbioses (Introduction, Bronstein 2003). Many genera in the Agavaceae, of which Yucca is a member, abscise flowers after damage by insect feeding or oviposition (Sutherland 1987; Becerra & Lloyd 1992; Pellmyr 1997), despite not being associated with pollinating seed predators (see also Marr & Pellmyr 2003). Similarly, non-leguminous plants, which do not associate with rhizobia, respond to nitrogen soil availability via differential root investment, which would be a preadaptation for legume host sanctions if the trait occurs in the non-rhizobia-associated ancestors of legumes (Hodge et al. 1998, 1999; Farley & Fitter 1999; Wijesinghe et al. 2001). Thus, it is modularity that provides stronger evidence of adaptation. As in ant-plants, excess floral production in yuccas and multiple root nodules in legumes modularize the interaction between host and visitors.
(c) Principal plants and agent ants
Mutualisms can be modelled as markets when cheating cannot occur and alternative partners can be sampled and chosen at low cost (Noë & Hammerstein 1994). However, neither assumption holds in many mutualisms (Yu 2001), and instead, the principal–agent game from economics appears to be a more appropriate theoretical construct (Gintis 2000; Bowles & Hammerstein 2003). In P–A games, principals are defined by their ‘power’ to propose incentive contracts to agents, but agents are allowed to keep private some information about themselves. A straightforward example is the employer–employee relationship, in which employers can offer wages but cannot monitor employee effort directly. Employers are thus forced to rely on correlated signals, such as company profits, to assess effort, and those signals are used to design an incentive contract: different wages for different profit levels. To persuade an agent to expend effort, the agent's expected wage upon doing so must be greater than that for no effort (Gintis 2000).
The parallel with ant-plants is straightforward. Host-plants are principals that offer contracts to ants. Plants cannot monitor ant-patrolling effort directly but can monitor a correlated signal, leaf growth, that triggers incentives (domatia provision or loss) for the ant. To select for patrolling, the expected fecundity from doing so must be greater than that from not patrolling, as we have shown (figure 6).
Clearly, the key parameter of cooperation is the strength of the correlation between agent action and the signal monitored by the host. The weaker the correlation, the more likely that incentives will be applied incorrectly, rewarding cheaters and/or punishing cooperators, and thus, making it less likely that the participation constraint will be achieved. In this light, modules increase the correlation between action and signal (see also Frank 1994; Yu 2001; West et al. 2002a,b), so that each instance of an action is more likely to be paid correctly. Thus, a module can be seen as a substitute for partner choice and as a ‘natural contract’ between a principal plant and its agent ants.
We speculate that sanctions based on pre-adapted responses should be advantageous to the principal, since that part of the contract is more credibly enforced (following Bergstrom & Lachmann 2003). Also, the P–A game clarifies the mechanisms that increase or decrease the power that principals have over their agents. Power increases when: (i) principals have more resources (are more capable of offering the wage necessary to elicit effort) and (ii) can detect cheating reliably (signal correlation is strong). Because plants tend to control more physical resources and are inherently modular in construction, this might explain why plants generally appear to be in control over their symbionts (e.g. Herre 1989; Yu et al. 2004a). The key role of correlation is echoed in other models of cooperation (Grafen 1985; Connor 1986; Nowak & May 1992; Frank 1994; Doebeli & Knowlton 1998; Gardner & West 2004; Doebeli & Hauert 2005).
Finally, there is always the possibility that evolution can allow agents to re-shape the principal–agent game. For example, we recall that Allomerus is a castration parasite, in that Allomerus workers destroy flowers of C. nodosa, which prevents fruiting and increases host-plant growth rate and domatium size (Yu & Pierce 1998; Edwards et al. in preparation), and thereby moves the colony more quickly to the right on the benefit graph (figure 6). Castration can be interpreted as the agent seizing power and re-writing the incentive contract. Castration, therefore, seems to be a qualitatively different form of cheating from a failure to patrol, and so perhaps it is not surprising that the ultimate persistence of this system rests on ecological and evolutionary mechanisms that allow the truly mutualistic Azteca spp. symbionts to coexist stably in competition with Allomerus (Yu et al. 2001, 2004b).
Acknowledgments
Special thanks to our field assistants Arturo Balareso, Pedro Cauper-Vallez, Charlotte Forder, and Simon Mahood. We also thank Jo Ridley, Niclas Kölm, Tony Davy, Doyle McKey, Stuart West and an anonymous reviewer for intellectual support, Thomas Hendrickson and the Libertador Tambopata Lodge, Laurel Hanna and the Picaflor Research Centre, Marianne van Vlaardingen and the Pantiacolla Lodge, and Barry Walker and the Manu Wildlife Center for logistical assistance and permission to conduct the study on their land, and Peru's Instituto de Recursos Naturales (INRENA) for research permits. D.P.E. is supported by a Natural Environment Research Council (NERC) studentship.
Supplementary Material
References
- Avila-Sakar G, Leist L.L, Stephenson A.G. Effects of the spatial pattern of leaf damage on growth and reproduction: nodes and branches. J. Ecol. 2003;91:867–879. doi:10.1046/j.1365-2745.2003.00819.x [Google Scholar]
- Becerra J.X, Lloyd D.G. Competition-dependent abscission of self-pollinated flowers of Phormium tenax (Agavaceae)—a 2nd action of self-incompatibility at the whole flower level. Evolution. 1992;46:458–469. doi: 10.1111/j.1558-5646.1992.tb02051.x. [DOI] [PubMed] [Google Scholar]
- Bergstrom C.T, Lachmann M. The Red King effect: when the slowest runner wins the coevolutionary race. Proc. Natl Acad. Sci. USA. 2003;100:593–598. doi: 10.1073/pnas.0134966100. doi:10.1073/pnas.0134966100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles S, Hammerstein P. Does market theory apply to biology? In: Hammerstein P, editor. Genetic and cultural evolution of cooperation. MIT Press; Cambridge, MA: 2003. pp. 153–165. [Google Scholar]
- Bronstein J.L. The exploitation of mutualisms. Ecol. Lett. 2001;4:277–287. doi:10.1046/j.1461-0248.2001.00218.x [Google Scholar]
- Bronstein J.L. The scope for exploitation within mutualistic interactions. In: Hammerstein P, editor. Genetic and cultural evolution of cooperation. MIT Press; Cambridge, MA: 2003. pp. 186–202. [Google Scholar]
- Bshary R, Noë R. Biological markets: the ubiquitous influence of partner choice on the dynamics of cleaner fish–client reef fish interactions. In: Hammerstein P, editor. Genetic and cultural evolution of cooperation. MIT Press; Cambridge, MA: 2003. pp. 167–184. [Google Scholar]
- Bull J.J, Molineux I.J, Rice W.R. Selection of benevolence in a host–parasite system. Evolution. 1991;45:875–882. doi: 10.1111/j.1558-5646.1991.tb04356.x. [DOI] [PubMed] [Google Scholar]
- Coley P.D. Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecol. Monogr. 1983;53:209–233. [Google Scholar]
- Connor R.C. Pseudoreciprocity: investing in mutualism. Anim. Behav. 1986;34:1652–1654. [Google Scholar]
- Davidson D.W. Ecological studies of neotropical ant gardens. Ecology. 1988;69:1138–1152. [Google Scholar]
- Davidson D.W, McKey D. Ant plant symbioses—stalking the Chuyachaqui. Trends Ecol. Evol. 1993;8:326–332. doi: 10.1016/0169-5347(93)90240-P. doi:10.1016/0169-5347(93)90240-P [DOI] [PubMed] [Google Scholar]
- Dejean A, Solano P.J, Ayroles J, Corbara B, Orivel J. Arboreal ants build traps to capture prey. Nature. 2005;434:973. doi: 10.1038/434973a. doi:10.1038/434973a [DOI] [PubMed] [Google Scholar]
- Djiéto-Lordon C, Dejean A, Gibernau M, Hossaert-McKey M, McKey D. Symbiotic mutualism with a community of opportunistic ants: protection, competition, and ant occupancy of the myrmecophyte Barteria nigritana (Passifloraceae) Acta Oecol. 2004;26:109–116. doi:10.1016/j.actao.2004.03.007 [Google Scholar]
- Doebeli M, Hauert C. Models of cooperation based on the Prisoner's Dilemma and the Snowdrift game. Ecol. Lett. 2005;8:748–766. doi:10.1111/j.1461-0248.2005.00773.x [Google Scholar]
- Doebeli M, Knowlton N. The evolution of interspecific mutualisms. Proc. Natl Acad. Sci. USA. 1998;95:8676–8680. doi: 10.1073/pnas.95.15.8676. doi:10.1073/pnas.95.15.8676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, D. P., Arauco, R., Hassall, M., Sutherland, W. J., Chamberlain, K., Wadhams, L. J. & Yu, D. W. In preparation. Protection in an ant–plant mutualism: an adapted or a pre-adapted trait?
- Farley R.A, Fitter A.H. The responses of seven co-occurring woodland herbaceous perennials to localized nutrient-rich patches. J. Ecol. 1999;87:849–859. doi:10.1046/j.1365-2745.1999.00396.x [Google Scholar]
- Ferdy J.B, Desprès L, Godelle B. Evolution of mutualism between globeflowers and their pollinating flies. J. Theor. Biol. 2002;217:219–234. doi: 10.1006/jtbi.2002.3018. doi:10.1006/jtbi.2002.3018 [DOI] [PubMed] [Google Scholar]
- Frank S.A. Genetics of mutualism—the evolution of altruism between species. J. Theor. Biol. 1994;170:393–400. doi: 10.1006/jtbi.1994.1200. doi:10.1006/jtbi.1994.1200 [DOI] [PubMed] [Google Scholar]
- Frank S.A. Perspective: repression of competition and the evolution of cooperation. Evolution. 2003;57:693–705. doi: 10.1111/j.0014-3820.2003.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Gardner A, West S.A. Spite and the scale of competition. J. Evol. Biol. 2004;17:1195–1203. doi: 10.1111/j.1420-9101.2004.00775.x. doi:10.1111/j.1420-9101.2004.00775.x [DOI] [PubMed] [Google Scholar]
- Gaume L, McKey D. An ant–plant mutualism and its host-specific parasite: activity rhythms, young leaf patrolling, and effects on herbivores of two specialist plant–ants inhabiting the same myrmecophyte. Oikos. 1999;84:130–144. [Google Scholar]
- Gintis H. Princeton University Press; Princeton, NJ: 2000. Game theory evolving. [Google Scholar]
- Grafen A. A geometric view of relatedness. Oxford Surv. Evol. Biol. 1985;2:28–89. [Google Scholar]
- Heichel G.H, Turner N.C. Branch growth and leaf numbers of red maple (Acer rubrum L) and red oak (Quercus rubra L)—response to defoliation. Oecologia. 1984;62:1–6. doi: 10.1007/BF00377364. doi:10.1007/BF00377364 [DOI] [PubMed] [Google Scholar]
- Heil M, McKey D. Protective ant–plant interactions as model systems in ecological and evolutionary research. Annu. Rev. Ecol. Evol. Syst. 2003;34:425–453. doi:10.1146/annurev.ecolsys.34.011802.132410 [Google Scholar]
- Herre E.A. Coevolution of reproductive characteristics in 12 species of New World figs and their pollinator wasps. Experientia. 1989;45:637–647. doi:10.1007/BF01975680 [Google Scholar]
- Herre E.A, Knowlton N, Mueller U.G, Rehner S.A. The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol. Evol. 1999;14:49–53. doi: 10.1016/s0169-5347(98)01529-8. doi:10.1016/S0169-5347(98)01529-8 [DOI] [PubMed] [Google Scholar]
- Hodge A, Stewart J, Robinson D, Griffiths B.S, Fitter A.H. Root proliferation, soil fauna and plant nitrogen capture from nutrient-rich patches in soil. New Phytol. 1998;139:479–494. doi: 10.1046/j.1469-8137.2000.00602.x. doi:10.1046/j.1469-8137.1998.00216.x [DOI] [PubMed] [Google Scholar]
- Hodge A, Robinson D, Griffiths B.S, Fitter A.H. Nitrogen capture by plants grown in N-rich organic patches of contrasting size and strength. J. Exp. Bot. 1999;50:1243–1252. doi:10.1093/jexbot/50.336.1243 [Google Scholar]
- Hoeksema J.D, Bruna E.M. Pursuing the big questions about interspecific mutualism: a review of theoretical approaches. Oecologia. 2000;125:321–330. doi: 10.1007/s004420000496. doi:10.1007/s004420000496 [DOI] [PubMed] [Google Scholar]
- Hoeksema J.D, Kummel M. Ecological persistence of the plant-mycorrhizal mutualism: a hypothesis from species coexistence theory. Am. Nat. 2003;162:S40–S50. doi: 10.1086/378644. doi:10.1086/378644 [DOI] [PubMed] [Google Scholar]
- Huxley C.R. Ant-plants Myrmecodia and Hydnophytum (Rubiaceae), and relationships between their morphology, ant occupants, physiology and ecology. New Phytol. 1978;80:231–268. [Google Scholar]
- Janzen D.H. Coevolution of mutualism between ants and acacias in Central America. Evolution. 1966;20:249–275. doi: 10.1111/j.1558-5646.1966.tb03364.x. [DOI] [PubMed] [Google Scholar]
- Janzen D.H. Epiphytic myrmecophytes in Sarawak: mutualism through feeding of plants by ants. Biotropica. 1974;6:237–259. [Google Scholar]
- Janzen D.H. Pseudomyrmex nigropilosa—parasite of a mutualism. Science. 1975;188:936–937. doi: 10.1126/science.188.4191.936. [DOI] [PubMed] [Google Scholar]
- Janzen D.H. The natural history of mutualisms. In: Boucher D.H, editor. The biology of mutualism. Oxford University Press; New York: 1985. pp. 40–99. [Google Scholar]
- Kiers E.T, Rousseau R.A, West S.A, Denison R.F. Host sanctions and the legume–rhizobium mutualism. Nature. 2003;425:78–81. doi: 10.1038/nature01931. doi:10.1038/nature01931 [DOI] [PubMed] [Google Scholar]
- Marr D.L, Pellmyr O. Effect of pollinator-inflicted ovule damage on floral abscission in the yucca–yucca moth mutualism: the role of mechanical and chemical factors. Oecologia. 2003;136:236–243. doi: 10.1007/s00442-003-1279-3. doi:10.1007/s00442-003-1279-3 [DOI] [PubMed] [Google Scholar]
- Murray M.G. Figs (Ficus spp.) and fig wasps (Chalcidoidea, Agaonidae): hypotheses for an ancient symbiosis. Biol. J. Linn. Soc. 1985;26:69–81. [Google Scholar]
- Nowak M.A, May R.M. Evolutionary games and spatial chaos. Nature. 1992;359:826–829. doi:10.1038/359826a0 [Google Scholar]
- Noë R, Hammerstein P. Biological markets—supply-and-demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. 1994;35:1–11. [Google Scholar]
- Parsons K, Quiring D, Piene H, Farrell J. Temporal patterns of balsam fir sawfly defoliation and growth loss in young balsam fir. Forest Ecol. Manag. 2003;184:33–46. doi:10.1016/S0378-1127(03)00145-2 [Google Scholar]
- Pellmyr O. Stability of plant–animal mutualisms: keeping the benefactors at bay. Trends Plant Sci. 1997;2:408–409. doi:10.1016/S1360-1385(97)01127-8 [Google Scholar]
- Pellmyr O, Huth C.J. Evolutionary stability of mutualism between yuccas and yucca moths. Nature. 1994;372:257–260. doi:10.1038/372257a0 [Google Scholar]
- Raine N.E, Gammans N, Macfadyen I.J, Scrivner G.K, Stone G.N. Guards and thieves: antagonistic interactions between two ant species coexisting on the same ant-plant. Ecol. Entomol. 2004;29:345–352. doi:10.1111/j.0307-6946.2004.00608.x [Google Scholar]
- Sachs J.L, Mueller U.G, Wilcox T.P, Bull J.J. The evolution of cooperation. Q. Rev. Biol. 2004;79:135–160. doi: 10.1086/383541. doi:10.1086/383541 [DOI] [PubMed] [Google Scholar]
- Solano P.J, Belin-Depoux M, Dejean A. Formation and structure of food bodies in Cordia nodosa (Boraginaceae) C. R. Biologies. 2005;328:642–647. doi: 10.1016/j.crvi.2005.05.004. doi:10.1016/j.crvi.2005.05.004 [DOI] [PubMed] [Google Scholar]
- Sutherland S. Why hermaphroditic plants produce many more flowers than fruits—experimental tests with Agave mckelveyana. Evolution. 1987;41:750–759. doi: 10.1111/j.1558-5646.1987.tb05850.x. [DOI] [PubMed] [Google Scholar]
- West S.A, Herre E.A. The ecology of the new world fig-parasitizing wasps Idarnes and implications for the evolution of the fig-pollinator mutualism. Proc. R. Soc. B. 1994;258:67–72. [Google Scholar]
- West S.A, Kiers E.T, Pen I, Denison R.F. Sanctions and mutualism stability: when should less beneficial mutualists be tolerated? J. Evol. Biol. 2002a;15:830–837. doi:10.1046/j.1420-9101.2002.00441.x [Google Scholar]
- West S.A, Kiers E.T, Simms E.L, Denison R.F. Sanctions and mutualism stability: why do rhizobia fix nitrogen? Proc. R. Soc. B. 2002b;269:685–694. doi: 10.1098/rspb.2001.1878. doi:10.1098/rspb.2001.1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler W.M. Museum of Comparative Zoology, Harvard College; Cambridge: 1942. Studies of Neotropical ant-plants and their ants. [Google Scholar]
- Wijesinghe D.K, John E.A, Beurskens S, Hutchings M.J. Root system size and precision in nutrient foraging: responses to spatial pattern of nutrient supply in six herbaceous species. J. Ecol. 2001;89:972–983. [Google Scholar]
- Wilkinson D.M, Sherratt T.N. Horizontally acquired mutualisms, an unsolved problem in ecology? Oikos. 2001;92:377–384. doi:10.1034/j.1600-0706.2001.920222.x [Google Scholar]
- Yamamura N. Vertical transmission and evolution of mutualism from parasitism. Theor. Popul. Biol. 1993;44:95–109. doi:10.1006/tpbi.1993.1020 [Google Scholar]
- Yu D.W. Parasites of mutualisms. Biol. J. Linn. Soc. 2001;72:529–546. doi:10.1006/bijl.2000.0514 [Google Scholar]
- Yu D.W, Davidson D.W. Experimental studies of species-specificity in Cecropia–ant relationships. Ecol. Monogr. 1997;67:273–294. [Google Scholar]
- Yu D.W, Pierce N.E. A castration parasite of an ant–plant mutualism. Proc. R. Soc. B. 1998;265:375–382. doi:10.1098/rspb.1998.0305 [Google Scholar]
- Yu D.W, Ridley J. Geopolitics in a buttercup. Trends Ecol. Evol. 2003;18:163–165. doi:10.1016/S0169-5347(03)00035-1 [Google Scholar]
- Yu D.W, Wilson H.B, Pierce N.E. An empirical model of species coexistence in a spatially structured environment. Ecology. 2001;82:1761–1771. [Google Scholar]
- Yu D.W, Ridley J, Jousselin E, Herre E.A, Compton S.G, Cook J.M, Moore J.C, Weiblen G.D. Oviposition strategies, host coercion and the stable exploitation of figs by wasps. Proc. R. Soc. B. 2004;271:1185–1195. doi: 10.1098/rspb.2003.2630. doi:10.1098/rspb.2003.2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D.W, Wilson H.B, Frederickson M.E, Palomino W, de la Colina R, Edwards D.P, Balareso A.A. Experimental demonstration of species coexistence enabled by dispersal limitation. J. Anim. Ecol. 2004b;73:1102–1114. doi:10.1111/j.0021-8790.2004.00877.x [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


