Abstract
The evolution of parental care and egg size has attracted considerable attention and theoretical debate. Several different hypotheses have been proposed concerning the trajectories of parental care and egg size evolution and the order of specific evolutionary transitions. Few comparative studies have investigated the predictions of these hypotheses. Here, we investigate the evolutionary association between parental care and egg size in frogs in a phylogenetic context. Data on egg size and presence or absence of parental care in various species of frogs was gathered from the scientific literature. As a basis for our comparative analyses, we developed a phylogenetic supertree, by combining the results of multiple phylogenetic analyses in the literature using matrix representation parsimony. Using phylogenetic pairwise comparisons we demonstrated a significant association between the evolution of parental care and large egg size. We then used recently developed maximum likelihood methods to infer the evolutionary order of specific transitions. This analysis revealed that the evolution of large egg size typically precedes the evolution of parental care, rather than the reverse. We discuss the relevance of our results to previous hypotheses concerning the evolution of parental care and egg size.
Keywords: parental care, egg size, anurans, phylogenetic supertree, pairwise comparisons
1. Introduction
The relationship between parental care and number and size of offspring has been a pivotal and often contentious point in our understanding of reproductive strategy (Clutton-Brock 1991; Stearns 1992). Shine (1978) proposed an influential yet controversial hypothesis: that the evolution of parental care creates a ‘safe harbour’ that causes selection to favour increases in egg size. This hypothesis predicts that the evolution of parental care will typically precede the evolution of large egg size. Shine's (1978) hypothesis generated considerable debate.
Shine's (1978) hypothesis was based on a model that assumed a constant instantaneous mortality rate for juveniles, unaffected by egg size variation. Sargent et al. (1987) incorporated size-dependent survival and growth rates into Shine's (1978) model. Removing the assumption of constant mortality removed some anomalous predictions made under Shine's (1978) original model, such as the prediction of a bimodal distribution of egg size. Hence Sargent et al.'s (1987) analysis extended the applicability of Shine's (1978) model and improved the accuracy of its predictions (Shine 1989).
Nussbaum (1985, 1987) proposed an alternative to Shine's (1978) hypothesis. He proposed that the evolution of large egg size typically precedes the evolution of parental care, rather than the reverse. Using a comparative analysis of salamanders as a specific example, Nussbaum (1985, 1987) proposed that selection for the ability of hatchling juveniles to consume large food items in lotic environments favoured large hatchling size (and hence large egg size). Nussbaum argued that the larger eggs produced by this process of selection take longer to develop, and (given a tradeoff between offspring size and number) will be produced in smaller numbers. These two factors should increase egg mortality (both in absolute terms and in relation to clutch size), leading to selection in favour of behaviours that reduce this increased mortality, such as parental care. Shine (1989) argued that this was unlikely to be a general trend explaining an association between parental care and large egg size. In particular, he argued that a variety of disparate factors commonly drive the evolution of parental care. If these factors are unrelated to egg size, then any correlation between egg size and parental care is likely to reflect the evolution of egg size in response to the evolution of parental care, rather than the reverse.
Nussbaum & Schultz (1989) developed a model that suggested that whichever evolved first (large egg size or parental care), parental care and egg size should tend to coevolve thereafter, making it difficult to disentangle the selective forces driving any correlation between parental care and egg size. To the extent that this is the case, there should be no clear trends in the order of parental care and egg size evolution.
Finally, Shine (1989) suggested that a third factor, such as certain forms of predation, might simultaneously select for both large egg size and parental care, leading to a correlation between these two characteristics that is not due to their interaction. In this case, the increase in both egg size and parental care would be simultaneous, and hence no trends with respect to evolutionary order would be expected.
Other than Nussbaum's (1985, 1987) comparative investigations of parental care and egg size in salamanders, there have been few if any empirical investigations of the predictions of the safe harbour hypothesis (Kolm & Ahnesjo 2005). In this paper, we first investigate whether or not there is a correlation between parental care and large egg size in frogs, as this has not been investigated in a phylogenetically controlled context (see below). Next, we investigate the pattern of transitions in parental care and egg size, to see if the results are more consistent with one or another of the hypotheses described above.
Frogs, which display an extraordinary diversity of reproductive strategies (Duellman & Trueb 1986), are an excellent taxonomic assemblage in which to investigate the relationships between parental care and egg size in an evolutionary context. Reproductive diversity in anurans is associated with a spectrum of parental behaviours, including the production and defence of foam nests or burrows, and the transport of eggs or tadpoles to phytotelmata (small pools that form in plant structures, such as bromeliad tanks), among many others. The diversity of these strategies has been the subject of numerous investigations (e.g. Crump 1974; Resetarits & Wilbur 1989), and there have been many summaries (e.g. Salthe & Duellman 1973; Duellman & Trueb 1986). A number of studies have investigated various aspects of both egg and clutch size in frogs (e.g. Salthe & Duellman 1973; Crump 1974; Kuramoto 1978). Some comparative studies have indicated that egg size increases in the presence of parental care (e.g. Crump 1995, 1996). However, none of the studies done to date have taken phylogeny into account, calling the statistical validity of any conclusions into question (Harvey & Pagel 1991).
In general, there have been few attempts to investigate the evolution of frog reproductive strategies using comparative methods that control for phylogenetic relationships in a statistical framework (e.g. Beck 1998). This stands in contrast to recent trends in comparative biology, where the use of phylogenetic trees to statistically inform comparative analyses has become the rule rather than the exception (e.g. Garland et al. 1991; Balshine et al. 2001; Liker et al. 2001; Bennett & Owens 2002; Kolm et al. in press).
The reason for the dearth of phylogenetic comparative analyses on anurans is simple: until very recently there were few well-supported phylogenies available for amphibians in general and frogs in particular. Frogs have been an especially difficult group for traditional phylogenetic analyses relying on morphology, because variation among species is usually insufficient. Recent efforts by phylogenetic systematists using molecular characters (particularly DNA sequences) have changed things dramatically over the last ten years, and there are now numerous molecular phylogenetic analyses of various clades of frogs available. In this paper, we present a phylogenetic supertree for several hundred species of frogs, constructed by combining the results of many of the recent molecular phylogenetic analyses. We then use that tree to analyse the relationship between the evolution of parental care and egg size using several comparative methods.
2. Material and methods
To investigate the correlation between parental care and egg size in a phylogenetic context, we used a method of phylogenetic pairwise comparison developed by Maddison (2000). Pairwise comparisons are useful when comparing species that differ in one categorical variable (the independent variable; in this case, presence or absence of parental care) and one continuous variable (the dependent variable; egg size). In our analysis, we used data on egg size directly. We did not control for either female body size or clutch size. Female body size was excluded from the analysis because egg size and body size were not significantly associated in our data. A regression analysis of egg size on maximum female body size revealed no significant relationship (N=469, R2=0.00016, F=0.079, p=0.78). Similar results were found using average female body size. We did not control for clutch size because doing so might obscure the hypothesized patterns (the hypotheses that we addressed focus on egg size as the target of selection—see §3). Egg and clutch size are typically correlated, but the selective forces that we are attempting to evaluate should affect clutch size indirectly through their direct effects on egg size. Hence, it would be inappropriate to control for clutch size in the analysis, as this variable is free to evolve in response to the hypothesized selection pressures, given an inherent tradeoff between egg and clutch size.
(a) Comparative data on egg size
Data on egg size (diameter of the ovum in millimetres) and presence or absence of parental care were taken from the scientific literature, from the references listed in electronic supplementary material, Appendix 1. We collected 790 records on 640 species. For species for which we obtained more than a single record, we took the average value across records to represent the value for that species. Comparative analyses were carried out only with data from those species that were incorporated into the phylogenetic supertree (see below). The final dataset used for the analysis is provided in electronic supplementary material, Appendix 2.
(b) Phylogenetic supertree
Once we collected data from the literature, we constructed a supertree representing the phylogenetic relationships of as many species from our dataset as possible. In order to develop the supertree, we used matrix representation parsimony (reviewed in Sanderson et al. 1998), as implemented by the program Radcon (Thorley & Page 2000). This method represents the nodes from the topologies of different phylogenetic trees as elements of a matrix. The final matrix, representing the topological structure of all of the trees used, is analysed via parsimony, yielding a topology that represents a consensus of the combined topologies. Trees from the literature were ‘pruned’ before analysis: species in the tree that were not in our dataset were removed.
To construct our supertree we used a hierarchical approach. In other words, we used a relatively small number of studies to address specific phylogenetic clades (e.g. the genus Bufo). We started with studies addressing basal relationships in the order Anura, and then worked our way up to more recent clades (the basal relationships established first were set as constraints in analyses of more recent clades). We preferentially used studies that employed DNA sequence data and maximum likelihood analysis of phylogenetic relationships. We preferred studies that used larger amounts of data, and we used the most recent studies preferentially when the same group of researchers published several different phylogenies on the same group of organisms using the same gene regions.
Our analysis utilized the following references for the following groups: order Anura: (Hoegg et al. 2004; Roelants et al. 2004); Neobatrachia: (Darst & Cannatella 2004; Hoegg et al. 2004); African–Asian Ranoids: (Richards & Moore 1996; Emerson et al. 2000a,b; Marmayou et al. 2000; Dawood et al. 2002; Wilkinson et al. 2002; Van der Meijden et al. 2004): African Ranoids (Madagascan taxa): (Richards et al. 2000; Glaw & Vences 2002; Vences et al. 2003a): Leptodactylids: (Larson & de Sa 1998); Hylids: (Da Silva 1997; Mendelson et al. 2000; Chek et al. 2001; Faivovich 2002; Salducci et al. 2002; Moriarty & Cannatella 2003; Faivovich et al. 2004); Bufonids: (Graybeal & Cannatella 1995; Graybeal 1997); Dendrobatids: (Clough & Summers 2000; Vences et al. 2003,b; Santos et al. 2003); Australian frogs: (Schauble et al. 2000; Read et al. 2001), Microhylids: (Zweifel 1986). The final supertree consisted of 383 species of frogs. In order to carry out the pairwise comparisons and the discrete character analysis, we needed a fully resolved phylogenetic hypothesis. The final supertree was close to full resolution, but there were six polytomies remaining. These were arbitrarily resolved for the analysis. This did not appear to affect the results of the analyses, as alternative resolutions of the polytomies did not qualitatively affect the results (data not shown). The phylogenetic supertree used for the analysis is provided in Nexus format in electronic supplementary material, Appendix 3.
(c) Pairwise comparisons
We employed a method of phylogenetic pairwise comparison developed by Maddison (2000) and implemented in the Pairwise module in the program Mesquite (Maddison & Maddison 2004). In most published studies using phylogenetic pairwise comparisons, traits are compared only between the focal taxon (a taxon characterized by a specific trait value, such as large egg size), and its sister clade (e.g. Gotmark 1994). Maddison (2000) developed methods that find all possible maximal sets of comparisons (sets that include the maximal number of phylogenetically independent pairs) for the case in which the characters are chosen at random (all possible pairs), chosen to contrast the categorical (independent) variable only (one pair), or chosen to contrast both the categorical (independent) and continuous (dependent) variable (two pairs). We used the ‘onepair’ method, which chooses pairs to contrast values of the independent variable among species. The large size of our phylogenetic supertree made it impossible to exhaustively evaluate all possible maximal sets of pairwise comparisons. Therefore, we limited the number of pairwise comparisons performed to 100 000 for the analysis.
We also used Mesquite to estimate the ancestral states of both parental care and egg size (coded as binary discrete characters), using both parsimony and maximum likelihood. This can be done with the program Discrete (Pagel 1994, 1999a,b; see below), but is very time consuming with a tree this large.
(d) Comparative analysis of discrete characters
To investigate the order of transitions in parental care and egg size evolution, we used maximum likelihood methods developed by Pagel (1994, 1997, 1999a,b), and implemented in his program Discrete (v.1.0). These methods allow one to compare the likelihood of a model in which the evolutionary patterns of two discrete characters are independent with one in which they are correlated. Using a likelihood ratio test (LRT), one can statistically test the hypothesis of correlated evolution. The log likelihood ratio is asymptotically χ2 distributed for the comparison between the independent and dependent models, and simulation studies (Pagel 1997) indicate that four degrees of freedom are appropriate for this type of comparison. Pagel's methods also allow one to make inferences concerning the rate (and the hence the typical order) of specific types of character transitions, making them very useful for the issues addressed in this paper. For these tests, one degree of freedom is generally appropriate for the log LRT. These methods require discrete binary characters as data. Hence, the continuous variable (egg size) was set to 0 for all species falling below the mean egg size, and to 1 for all species falling above the mean, following the coding method used in several previous studies (e.g. Kruger & Davies 2002). Since branch lengths were not available for our supertree (which was constructed from the results of multiple analyses based on different characters) we set all branch lengths equal to one (see Pagel 1994).
3. Results and discussion
On the basis of 100 000 optimal sets of pairwise comparisons, we determined that egg size is significantly associated with parental care in frogs (for 100 000 sets of pairs, the range of p=1.235×10−4 to 0.0012). Hence, in all cases the results were highly significant. A representative maximal pairing set showed 47 pairs, of which 34 showed a positive relationship, 11 showed a negative relationship, and two were neutral (p=4.120×10−4). Hence parental care is strongly associated with increased egg size, as a number of researchers have argued on the basis of non-phylogenetically controlled analyses.
The results from the maximum likelihood analysis using Discrete also supported the inference of correlated evolution between the evolution of parental care and egg size in a phylogenetically controlled framework. The final log-likelihood under the maximum likelihood model of independent evolution was −361.19, whereas that under the model of dependent evolution was −333.23. This yields a log-likelihood ratio of 27.97, which is a highly significant difference under a χ2 distribution with four degrees of freedom (p<0.0001).
The reconstruction of the ancestral state of parental care across our phylogenetic tree indicated that no parental care is the most likely state. Under maximum likelihood reconstruction, the probability that absence of parental care is ancestral was 0.92 (log-likelihood=−166.25). Reconstruction using parsimony also returns no parental care as the ancestral state. The reconstruction of the ancestral state of egg size (large versus small) was equivocal. Under maximum likelihood, the probability of small egg size was 0.33, and of large egg size was 0.67 (log-likelihood=213.15). Under parsimony, the most likely ancestral state was equivocal. Hence the ancestral state of egg size was uncertain. For our purposes, the assumption that small egg size is primitive is the most favourable for Shine's (1978) original hypothesis, which proposed that the evolution of parental care in species with small eggs led to the evolution of larger eggs. Hence we will use that assumption as a starting point in our discussion of the order of the evolution of parental care and egg size.
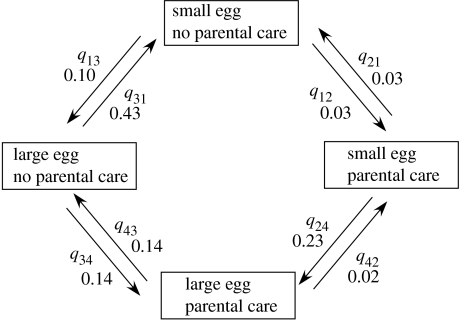
Figure 1 shows the pattern of evolutionary transitions, with rates for each type of transition. We first tested to see if all the transitions toward the state with both parental care and large egg size from no parental care and small egg size were significantly different than 0. This is accomplished by setting the particular rate parameter (e.g. q12, for the transition rate from no parental care and small eggs to parental care and small eggs) to 0, then comparing the log-likelihood of the appropriate maximum likelihood model to the log-likelihood of the unrestricted model (again using a LRT). Each of these rates was significantly different than 0 under the LRT. The log-likelihoods and LRT results for each specific rate parameter (rates in parentheses) were as follows: q12 (0.03): −333.53 (p<0.05), q24 (0.23): −338.96 (p<0.05), q13 (0.1): −352.05 (p<0.01), q34 (0.14): −339.17 (p<0.05).
Figure 1.
Path diagram illustrating transitions between different states of parental care and egg size calculated with Discrete. Simultaneous transitions between both parental care and egg size are not calculated. Parameters q12, etc. give transition rates between different state combinations.
For the purpose of distinguishing between the order of transitions proposed as common by Shine (1978) versus Nussbaum (1985, 1987), we can compare the transition rates from no parental care, small eggs to parental care, small eggs, and from there to parental care, large eggs (the pathway proposed as prevalent by Shine 1978) to the alternative of no parental care, small eggs to no parental care, large eggs, and from there to parental care and large eggs (the pathway proposed as prevalent by Nussbaum 1985 and 1987).
The rate of the transition from no parental care, small eggs to parental care, small eggs (q12) is very low. Although this rate is significantly different than zero (see above), it is also significantly lower than the rate from no parental care, small eggs to no parental care, large eggs (log-likelihood=−335.63, LRT=4.8, p<0.05), and significantly lower than the rate from no parental care, large eggs to parental care, large eggs (log-likelihood=−335.61, LRT=4.76, d.f. =1, p<0.05). The rate from parental care, small eggs to parental care, large eggs is quite high (0.23), but is not significantly different from the rate from no parental care, small eggs to no parental care, large eggs (log-likelihood=−334.69, LRT=2.92, d.f.=1, p>0.05).
Overall, our results indicate that, even making the assumption (favourable to Shine's (1978) hypothesis) that small egg size is the ancestral state, the most common evolutionary trajectory is from small to large egg size in the absence of parental care, followed by the evolution of parental care, as suggested by Nussbaum (1985, 1987). The high transition rate from parental care, small eggs to parental care, large eggs supports Shine's (1978) contention that the presence of parental care will tend to select for large egg size, but the argument that this will be the most common pathway to the evolution of large egg size is not supported in this study. Our results do not negate the possibility that either of the two other hypotheses mentioned above (coevolution and a third factor affecting both egg size and parental care) affect the evolution of egg size, but they would be expected to contribute noise to the system in terms of the order of the evolution of parental care and large egg size, and hence should not bias the results in favour of either Shine or Nussbaum's hypotheses.
Finally, it should be noted that our analysis focuses on the evolution of egg size and assumes that egg size is the major target of selection (either via an interaction with parental care or with other aspects of the environment), with clutch size evolving in response to egg size as the result of an inherent tradeoff between these two traits. This is consistent with the focus of the hypotheses we have addressed in this paper (e.g. Shine 1978; Nussbaum 1985). However, it is possible that selection could act directly on clutch size (with egg size evolving in response). Although we do not address that issue in this paper, the potential importance of this possibility is highlighted in a recent comparative analysis of the evolution of egg and clutch size in cichlid fish (Kolm et al. in press).
Acknowledgments
We thank Wayne Maddison for advice and for modifying the Pairwise module in Mesquite to allow control of the number of comparisons performed in a pairwise analysis. We thank Sigal Balshine for comments and discussion and for access to papers in press. We thank two anonymous reviewers for constructive comments on previous versions of this manuscript. We thank G. Hall and W. Clark for assistance in gathering data. This study was supported by research grants from the National Science Foundation (DEB-0134191) and the National Geographic Society (7243-02) to the first author.
Supplementary Material
References
- Balshine S, Leach B.J, Neat F, Werner N.Y, Montgomerie R. Sperm size of African cichlids in relation to sperm competition. Behav. Ecol. 2001;12:726–731. 10.1093/beheco/12.6.726 [Google Scholar]
- Beck C. Mode of fertilization and parental care in anurans. Anim. Behav. 1998;55:439–449. doi: 10.1006/anbe.1997.0619. 10.1006/anbe.1997.0619 [DOI] [PubMed] [Google Scholar]
- Bennett P.M, Owens I.P.F. Evolutionary ecology of birds. Oxford University Press; Oxford, UK: 2002. [Google Scholar]
- Chek A.A, Lougheed S.C, Bogart J.P, Boag P.T. Perception and history: molecular phylogeny of a diverse group of neotropical frogs: the 30-chromosome Hyla (Anura: Hylidae) Mol. Phylogenet. Evol. 2001;18:370–385. doi: 10.1006/mpev.2000.0889. 10.1006/mpev.2000.0889 [DOI] [PubMed] [Google Scholar]
- Clough M, Summers K. Phylogenetic systematics and biogeography of the poison frogs: evidence from mitochondrial DNA sequences. Biol. J. Linn. Soc. 2000;70:515–540. 10.1006/bijl.1999.0418 [Google Scholar]
- Clutton-Brock T.H. The evolution of parental care. Princeton University Press; Princeton, NJ: 1991. [Google Scholar]
- Crump M. Reproductive strategies in a tropical anuran community. Misc. Publ. Mus. Nat. Hist. Univ. Kansas. 1974;61:1–68. [Google Scholar]
- Crump M.L. Parental care. In: Heatwole H, Sullivan B.K, editors. Amphibian biology. Social behavior. vol. 2. Surrey Beatty and Sons; Chipping Norton, NSW, Australia: 1995. pp. 518–567. [Google Scholar]
- Crump M.L. Parental care among the amphibia. Adv. Stud. Behav. 1996;25:109–144. [Google Scholar]
- Darst C.R, Cannatella D.C. Novel relationships among hyloid frogs inferred from 12S and 16S mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2004;31:462–475. doi: 10.1016/j.ympev.2003.09.003. 10.1016/j.ympev.2003.09.003 [DOI] [PubMed] [Google Scholar]
- DaSilva R.H. Two character states new for Hylines and the taxonomy of the genus Psuedacris. J. Herpetol. 1997;31:609–613. [Google Scholar]
- Dawood A, Channing A, Bogart J.P. A molecular phylogeny of the genus Tomopterna in Southern Africa: examining species boundaries with mitochondrial 12S rRNA sequence data. Mol. Phylogenet. Evol. 2002;22:407–413. doi: 10.1006/mpev.2001.1060. 10.1006/mpev.2001.1060 [DOI] [PubMed] [Google Scholar]
- Duellman W.E, Trueb L. Biology of amphibians. McGraw-Hill; New York: 1986. [Google Scholar]
- Emerson S.B, Inger R.F, Iskandar D. Molecular systematics and biogeography of the fanged frogs of Southeast Asia. Mol. Phylogenet. Evol. 2000a;16:131–142. doi: 10.1006/mpev.2000.0778. 10.1006/mpev.2000.0778 [DOI] [PubMed] [Google Scholar]
- Emerson S.B, Richards C.M, Drewes R.C, Kjer K.M. On the relationships among ranoid frogs: a review of the evidence. Herpetologica. 2000b;56:209–230. [Google Scholar]
- Faivovich J. A cladistic analysis of Scinax (Anura: Hylidae) Cladistics. 2002;18:367–393. doi: 10.1111/j.1096-0031.2002.tb00157.x. 10.1111/j.1096-0031.2002.tb00157.x [DOI] [PubMed] [Google Scholar]
- Faivovich J, Garcia P.C.A, Ananias F, Lanari L, Basso N.G, Wheeler W.C. A molecular perspective on the phylogeny of the Hyla puchella species group (Anura: Hylidae) Mol. Phylogenet. Evol. 2004;32:938–950. doi: 10.1016/j.ympev.2004.03.008. 10.1016/j.ympev.2004.03.008 [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Huey R.B, Bennett A.F. Phylogeny and thermal physiology in lizards: a reanalysis. Evolution. 1991;45:1969–1975. doi: 10.1111/j.1558-5646.1991.tb02703.x. [DOI] [PubMed] [Google Scholar]
- Glaw F, Vences M. A new sibling species of the anuran subgenus Blommersia from Madagascar (Amphibia; Mantellidae: Mantidactylus) and its molecular phylogenetic relationships. Herpetol. J. 2002;12:11–26. [Google Scholar]
- Graybeal A. Phylogenetic relationships of bufonid frogs and tests of alternate macroevolutionary hypotheses characterizing their radiation. Zool. J. Linn. Soc. 1997;119:297–338. 10.1006/zjls.1996.0068 [Google Scholar]
- Graybeal A, Cannatella D.C. A new taxon of Bufonidae from Peru, with descriptions of two new species and a review of the phylogenetic status of supraspecific bufonid taxa. Herpetologica. 1995;51:105–131. [Google Scholar]
- Gotmark F. Are bright birds distasteful—a reanalysis of Cott, H.B. data on the edibility of birds. J. Avian Biol. 1994;25:184–197. [Google Scholar]
- Harvey P.H, Pagel M.D. The comparative method in evolutionary biology. Oxford University Press; Oxford, UK: 1991. [Google Scholar]
- Hoegg S, Vences M, Brinkmann H, Meyer A. Phylogeny and comparative substitution rates of frogs inferred from sequences of three nuclear genes. Mol. Biol. Evol. 2004;21:1188–1200. doi: 10.1093/molbev/msh081. 10.1093/molbev/msh081 [DOI] [PubMed] [Google Scholar]
- Kolm N, Ahnesjo I. Do egg size and parental care coevolve in fishes? J. Fish Biol. 2005;66:1499–1515. 10.1111/j.0022-1112.2005.00777.x [Google Scholar]
- Kolm, N., Goodwin, N. B., Balshine, S. & Reynolds, J. D. In press. Life history evolution in cichlids 1: revisiting the evolution of life histories in relation to parental care. J. Evol. Biol. [DOI] [PubMed]
- Kuramoto M. Correlations of quantitative parameters of fecundity in amphibians. Evolution. 1978;32:287–296. doi: 10.1111/j.1558-5646.1978.tb00644.x. [DOI] [PubMed] [Google Scholar]
- Kruger O, Davies N.B. The evolution of cuckoo parasitism: a comparative analysis. Proc. R. Soc. B. 2002;269:375–381. doi: 10.1098/rspb.2001.1887. 10.1098/rspb.2001.1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson P.M, de Sa R.O. Chondrocranial morphology of Leptodactylus larvae (Leptodactylidae: Leptodactylinae): its utility in phylogenetic reconstruction. J. Morphol. 1998;238:287–305. doi: 10.1002/(SICI)1097-4687(199812)238:3<287::AID-JMOR2>3.0.CO;2-8. 10.1002/(SICI)1097-4687(199812)238:3%3C287::AID-JMOR2%3E3.0.CO;2-8 [DOI] [PubMed] [Google Scholar]
- Liker A, Reynolds J.D, Szekely T. The evolution of egg size in socially polyandrous shorebirds. Oikos. 2001;95:3–14. 10.1034/j.1600-0706.2001.950101.x [Google Scholar]
- Maddison W.P. Testing character correlation using pairwise comparisons on a phylogeny. J. Theor. Biol. 2000;202:195–204. doi: 10.1006/jtbi.1999.1050. 10.1006/jtbi.1999.1050 [DOI] [PubMed] [Google Scholar]
- Maddison, W. P. & Maddison, D. R. 2004 Mesquite: a modular system for evolutionary analysis Version 1.04. http://mesquiteproject.org
- Marmayou J, Dubois A, Ohler A, Pasquet E, Tillier A. Phylogenetic relationships in the Ranidae: independent origins of direct development in the genera Philautus and Taylorana. C.R. Acad. Sci. Paris Sciences de la Vie. 2000;323:287–297. doi: 10.1016/s0764-4469(00)00133-5. [DOI] [PubMed] [Google Scholar]
- Moriarty E.C, Cannatella D.C. Phylogenetic relationships of the North American chorus frogs (Psuedacris: Hylidae) Mol. Phylogenet. Evol. 2003;30:409–420. doi: 10.1016/s1055-7903(03)00186-6. 10.1016/S1055-7903(03)00186-6 [DOI] [PubMed] [Google Scholar]
- Mendelson J.R, III, Da Silva H.R, Maglia A.M. Phylogenetic relationships among marsupial frog genera (Anura: Hylidae: Hemiphractinae) based on evidence from morphology and natural history. Zool. J. Linn. Soc. 2000;128:125–148. 10.1006/zjls.1998.0229 [Google Scholar]
- Nussbaum R. The evolution of parental care in salamanders. Misc. Publ. Mus. Zool. Univ. Mich. 1985;169:1–50. [Google Scholar]
- Nussbaum R. The evolution of parental care in salamanders: an examination of the safe harbor hypothesis. Res. Pop. Ecol. (Kyoto) 1987;29:27–44. [Google Scholar]
- Nussbaum R, Schultz D. Coevolution of parental care and egg size. Am. Nat. 1989;133:591–603. 10.1086/284939 [Google Scholar]
- Pagel M. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. B. 1994;255:37–45. [Google Scholar]
- Pagel M. Inferring evolutionary processes from phylogenies. Zool. Scr. 1997;26:331–348. 10.1111/j.1463-6409.1997.tb00423.x [Google Scholar]
- Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999a;401:877–884. doi: 10.1038/44766. 10.1038/44766 [DOI] [PubMed] [Google Scholar]
- Pagel M. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol. 1999b;48:612–622. 10.1080/106351599260184 [Google Scholar]
- Read K, Keogh S.J, Scott I.A.W, Roberts D, Doughty P. Molecular phylogeny of the Australian frog genera Crinia, Geocrinia, and allied taxa (Anura: Myobatrachidae) Mol. Phylogenet. Evol. 2001;21:294–308. doi: 10.1006/mpev.2001.1014. 10.1006/mpev.2001.1014 [DOI] [PubMed] [Google Scholar]
- Richards C.M, Moore W.S. A phylogeny for the African treefrog family Hyperoliidae based on mitochondrial rDNA. Mol. Phylogenet. Evol. 1996;5:522–532. doi: 10.1006/mpev.1996.0047. 10.1006/mpev.1996.0047 [DOI] [PubMed] [Google Scholar]
- Richards C.M, Nussbaum R.A, Raxworthy C.J. Phylogenetic relationships within the Madagascan boophids and mantellids as elucidated by mitochondrial ribosomal genes. Afr. J. Herpetol. 2000;49:23–32. [Google Scholar]
- Roelants K, Jiang J, Bossuyt F. Endemic ranid (Amphibia: Anura) genera in southern mountain ranges of the Indian subcontinent represent ancient frog lineages: evidence from molecular data. Mol. Phylogenet. Evol. 2004;31:730–740. doi: 10.1016/j.ympev.2003.09.011. 10.1016/j.ympev.2003.09.011 [DOI] [PubMed] [Google Scholar]
- Resetarits W.J, Wilbur H. Choice of oviposition site by Hyla chrysoscelis: role of predators and competitors. Ecology. 1989;70:220–228. [Google Scholar]
- Salducci M.-D, Marty C, Chappaz R, Gilles A. Molecular phylogeny of French Guiana Hylinae: implications for the systematics and biodiversity of the Neotropical frogs. C.R. Biol. 2002;325:141–153. doi: 10.1016/s1631-0691(02)01423-3. [DOI] [PubMed] [Google Scholar]
- Salthe S, Duellman W. Quantitative constraints associated with reproductive mode in anurans. In: Vial J, editor. Evolutionary biology of the anurans: contemporary research on major problems. University of Missouri Press; Columbia, MO: 1973. pp. 229–249. [Google Scholar]
- Sanderson M, Purvis A, Henze C. Phylogenetic supertrees: assembling the trees of life. Trends Ecol. Evol. 1998;13:105–109. doi: 10.1016/S0169-5347(97)01242-1. 10.1016/S0169-5347(97)01242-1 [DOI] [PubMed] [Google Scholar]
- Santos J.C, Coloma L.A, Cannatella D.C. Multiple, recurring origins of aposematism and diet specialization in poison frogs. Proc. Natl Acad. Sci. USA. 2003;100:12 792–12 797. doi: 10.1073/pnas.2133521100. 10.1073/pnas.2133521100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent R.C, Taylor P.D, Gross M.R. Parental care and the evolution of egg sizes in fishes. Am. Nat. 1987;129:32–46. 10.1086/284621 [Google Scholar]
- Schauble C.S, Moritz C, Slade R.W. A molecular phylogeny for the frog genus Limnodynastes (Anura: Myobatrachidae) Mol. Phylogenet. Evol. 2000;16:379–391. doi: 10.1006/mpev.2000.0803. 10.1006/mpev.2000.0803 [DOI] [PubMed] [Google Scholar]
- Shine R. Propagule size and parental care: the “safe harbor” hypothesis. J. Theor. Biol. 1978;75:417–424. doi: 10.1016/0022-5193(78)90353-3. 10.1016/0022-5193(78)90353-3 [DOI] [PubMed] [Google Scholar]
- Shine R. Alternative models for the evolution of offspring size. Am. Nat. 1989;134:311–317. 10.1086/284982 [Google Scholar]
- Stearns S. The evolution of life histories. Oxford University Press; Oxford, UK: 1992. [Google Scholar]
- Thorley J.L, Page R.D.M. RadCon: phylogenetic tree comparison and consensus. Bioinformatics. 2000;16:486–487. doi: 10.1093/bioinformatics/16.5.486. 10.1093/bioinformatics/16.5.486 [DOI] [PubMed] [Google Scholar]
- Van der Meijden A, Vences M, Meyer A. Novel phylogenetic relationships of the enigmatic brevicipitine and scaphiophrynine toads as revealed by sequences from the nuclear Rag-1 gene. Proc. R. Soc. B. 2004;271(Suppl. 5):S378–S381. doi: 10.1098/rsbl.2004.0196. 10.1098/rsbl.2004.0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vences M, Kosuch J, Glaw F, Bohme W, Veith M. Molecular phylogeny of the hyperoliid treefrogs: biogeographic origin of Malagasy and Seychellean taxa and re-analysis of familial paraphyly. J. Zool. Syst. Evol. 2003a;41:205–215. 10.1046/j.1439-0469.2003.00205.x [Google Scholar]
- Vences M, Kosuch J, Boistel R, Haddad C, La Marca E, Lotters S, Veith M. Multiple evolution of aposematic coloration in Neotropical poison frogs: mitochondrial DNA evidence from basal dendrobatids. Org. Div. Evol. 2003b;3:215–226. 10.1078/1439-6092-00076 [Google Scholar]
- Wilkinson J.A, Drewes R.C, Tatum O.L. A molecular phylogenetic analysis of the family Rhacophoridae with an emphasis on the Asian and African genera. Mol. Phylogenet. Evol. 2002;24:265–273. doi: 10.1016/s1055-7903(02)00212-9. 10.1016/S1055-7903(02)00212-9 [DOI] [PubMed] [Google Scholar]
- Zweifel R.G. A new genus and species of microhylid frog from the Cerro de Neblina region of Venezuela and a discussion of relationships among new world microhylid genera. Am. Mus. Novit. 1986;2863:1–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.