Abstract
Primates give alarm calls in response to the presence of predators. In some species, such as the Thomas langur (Presbytis thomasi), males only emit alarm calls if there is an audience. An unanswered question is whether the audience's behaviour influences how long the male will continue his alarm calling. We tested three hypotheses that might explain the alarm calling duration of male Thomas langurs: the fatigue, group size and group member behaviour hypotheses. Fatigue and group size did not influence male alarm calling duration. We found that males only ceased calling shortly after all individuals in his group had given at least one alarm call. This shows that males keep track of and thus remember which group members have called.
Keywords: alarm communication, Presbytis thomasi, anti-predator behaviour, memorizing
1. IntroducTion
Research on non-human primate alarm calls is an important means for investigating their cognitive abilities. The main conclusions from these investigations are that primates have the capacity to produce referential vocalizations (Marler et al. 1992; Zuberbühler et al. 1997; Zuberbühler 2000, 2001; Seyfarth et al. 1980); distinguish vocalizations based on the information they provide (Cheney & Seyfarth 1990a; Zuberbühler et al. 1999a); distinguish individual alarm calls (Cheney & Seyfarth 1990a); adjust their calling rates to the presence or composition of their audience (Cheney & Seyfarth 1990a; Wich & Sterck 2003); and, more speculatively, combine vocalizations in a syntactical manner (Zuberbühler 2002; Crockford & Boesch 2003).
These studies have yielded a good understanding of why primates give alarm calls in the presence of predators, and how the production of such calls depends on the caller's audience. An important, unanswered question is why animals stop their alarm calls and whether this depends on non-social (e.g. the caller's bodily condition) or social (e.g. behaviour of other group members or the predator) factors.
There could be several non-social and social explanations for why primates confronted with a predator stop alarm calling. First, fatigue (Cheney & Seyfarth 1981). The fatigue hypothesis states that the main factor which determines how long an individual continues calling is its stamina. It predicts that individuals in a better physical condition and, therefore, experiencing less fatigue, e.g. high-ranking ones, will continue after others have become tired.
Second, it might be more difficult for the male to convey the alarm message to all group members in a larger as opposed to a smaller group. If so, the duration of alarm calling should depend on group size. We call this the group size hypothesis.
Third, the cessation of alarm calling might depend on the behaviour of group members. In primates, where males only produce alarm calls if group members are in the vicinity (Cheney & Seyfarth 1990a; Wich & Sterck 2003), male calling requires the presence of an audience. When males do have an audience and as a result begin to give alarm calls, calling might cease when all members of the audience have noticed, directly or indirectly, the presence of the predator and/or fled to safety. Therefore, it becomes important to consider whether a male needs feedback from his audience to continue or to stop calling. In other words, by noting some behaviour (e.g. alarm calling) of its group members the male can become assured that his audience is aware of the danger and thus that he can stop his alarm calling. We term this the group member behaviour hypothesis.
We examined these three hypotheses in an experimental study of alarm calls in male Thomas langurs (Presbytis thomasi). This arboreal primate is endemic to the rainforest of northern Sumatra, Indonesia (Sterck 1997). It lives in one-male, multi-female groups (mixed-sex groups), but solitary males and all-male bands are also encountered. Mixed-sex group size ranges from 2 to 17 individuals (Sterck 1997). Adult males, adult females and juveniles of both sexes give alarm calls (alarm hiccups). Males produce alarm calls in response to a tiger model, but only when resident in a group and not when solitary (Wich & Sterck 2003). This indicates that the presence of an audience, i.e. females and their offspring, determines whether a male gives alarm calls and hence that these calls are meant for their audience and not for the predator (Wich & Sterck 2003). The previous study did not examine, however, which factors influence the duration of the period during which the male continues his alarm calling. The aim of the current paper was to examine whether the cessation of male alarm calling was consistent with either the fatigue, group size or group member behaviour hypothesis.
2. Material and methods
We conducted experiments in two study areas in the Leuser Ecosystem, North Sumatra. The langurs in one of the study areas (Ketambe 3°41′ N, 97°39′ E) have been studied from 1988 to 2001. This study area consists of primary tropical rain forest (Rijksen 1978). The langurs in the other study area (Bukit Lawang, 3 30′ N, 98 6′) were studied in the early eighties (Gurmaya 1986) and again from 1998 to 2001 (Wich & Sterck 2003). This study area consists of a mosaic of primary and secondary forest with rubber plantations on its fringes (Gurmaya 1986). In both areas, individuals were recognized individually and well habituated to the presence of observers. In each area, six mixed-sex groups were exposed to experiments, so in all statistical tests n=12. All tests are two-tailed with the critical significance level set at 0.05. The 12 males tested during the experiments were all of similar age and had similar competitive abilities since they were all resident males in their stable middle-tenure phase (Steenbeek 1999; Wich et al. 2003). This should control for large differences in physical condition in order to test the fatigue hypothesis.
In the experiments, we used a fake tiger skin as a stimulus. The fake skin was presented to the monkeys by a human observer carrying the skin over his shoulders and the rest of his body while walking on all fours. As soon as, one of the monkeys was observed staring at the stimulus, the observer carrying the stimulus moved slowly out of sight. Simultaneously, two other observers collected the behavioural data.
Although it might seem difficult to ensure that all alarm calls were assigned correctly to the right individuals, in practice this was not difficult for several reasons. First, male alarm calls differ from those of females and independent offspring (hereafter offspring), which made scoring of male calls during the experiment simple. Second, alarm calls of females differ in pitch from that of offspring, which made alarms calls from these two classes easily distinguishable. Third, dependent offspring did not alarm call, which made the maximum number of individuals that needed to be distinguished only 10. Fourth, independent offspring consistently approached their mother as soon as alarm calling started, which facilitated keeping track of those individuals and their calls. Fifth, langurs are very easy to recognize individually because of characteristic tails, facial marks, shape of crest, scars and overall physical appearance. Sixth, except for the male, individuals do not give alarm calls at a high rate, and therefore it is straightforward to track which individual makes the alarm calls. Seventh, group spread was usually not large during the experiments (less than 25 m), which in addition to the fact that the group did not move up higher than 20–30 m in the trees facilitated our observations. Finally, although individuals avoided the location of the tiger skin, they did so slowly which allowed us to keep track of individuals. For all the above reasons, we are very confident that we assigned alarm calls correctly to the individuals that gave the calls.
We conducted trials from 1998 to 2001. Trials were cancelled if there were alarm calls from individuals in the group before the moment we aimed to conduct the experiment. In such cases, we tried to conduct the experiment on the following day, until the day when there were no alarm calls before the time of the experiment. During each trial, we noted the number of adult females, dependent and independent offspring in the group and we collected data on the time when the stimulus was first observed, the time of each alarm call (in minute blocks) and which individual made the alarm call.
3. Results
The average total number of male alarm calls given after the stimulus was noticed was 250.1 (s.d.=176.3) and the average alarm calling duration was 18.0 min (s.d.=12.8). Males always started to call within the first minute after they noticed the tiger skin. Even if, the male was not the first individual to notice the tiger skin, the male's frantic scanning of the area after an alarm call from another group member ensured that a male noticed the skin during each experiment. The male then stared at the skin and kept on staring at it when it moved away, until it was out of sight. The average female and offspring alarm calling duration was 3.2 min (s.d.=0.5) and was significantly shorter than that of males (Wilcoxon matched-pairs test, p=0.002). In addition, the calling duration of the female or offspring that called for the longest duration in a group (mean=6.9 min, s.d.=1.6) was still significantly shorter than that of the male (Wilcoxon matched-pairs test, p=0.003). The average duration from the time the stimulus was noticed to the females' last alarm call was 6.3 (s.d.=2.3) minutes. This was significantly shorter than that of the males (Wilcoxon matched-pairs test, p=0.002).
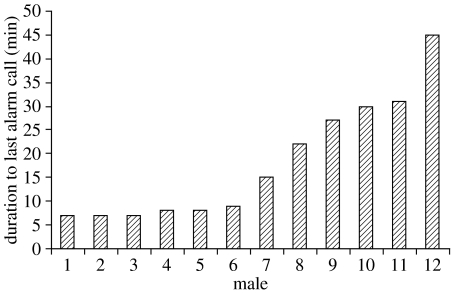
Because all males had a similar physical condition, the fatigue hypothesis predicted that the range of male alarm calling durations would be small and normally distributed. However, the range of alarm calling durations was large and not normally distributed (figure 1; Kolmogorov-Smirnov test with Lilliefors significance correction, p=0.025; Shapiro-Wilk test, p=0.029).
Figure 1.
The length of time that male Thomas langurs give alarm calls after noticing a fake tiger. Data for 12 males are presented. Numbers 1, 3, 4, 7, 10 and 11 are from Bukit Lawang, the other numbers from Ketambe.
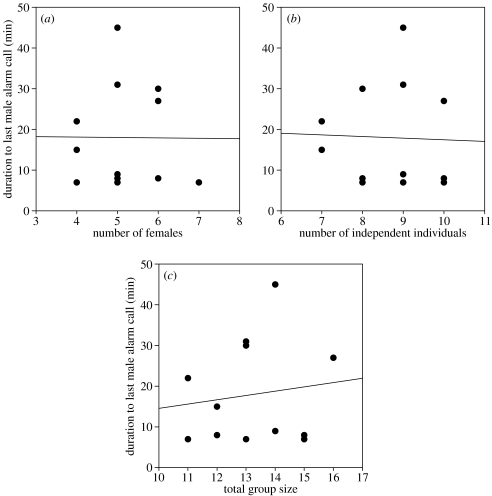
The group size hypothesis predicted that the elapsed duration of alarm calling from the first sight of the stimulus until the last male alarm call correlates positively with group size. Group size measures, however, did not correlate with the duration of male alarm calling (figure 2a–c; with number of adult females: r=−0.01, p=0.975; with number of independent individuals: r=−0.03, p=0.926; with total group size: r=0.14, p=0.664).
Figure 2.
Scattergrams of male alarm calling duration versus different measures of group size. On the y-axis the duration of the male calling period after the tiger model was noticed is presented, and on the x-axes are various measures of group size (a, number of females; b, number of independent individuals; c, total group size). None of the group size measures has a significant linear relationship with the male's calling period. Total group size was defined as the number of females and their offspring.
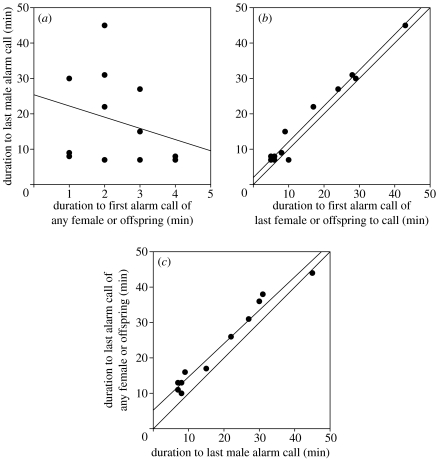
The group member behaviour hypothesis predicted that males would continue alarm calling until some behaviour by group members triggered the cessation of the male's calling. We, therefore, examined the relation between, on the one hand, the duration of a male's alarm calling after the predator was first sighted and (figure 3a; r=−0.27, p=0.396) the first alarm call by any female or offspring, (figure 3b; r=0.99, p<0.001) the first alarm call by the last female or offspring to begin calling, and (figure 3c; r=0.98, p<0.001) the last alarm call by any female or offspring. We found that correlations b and c were highly significant. Table 1 provides an overview of the duration after predator model detection to the first alarm call of females and independent offspring as well as to the last male alarm call.
Figure 3.
Scattergrams of the male alarm calling duration (in a and b on the y-axis; in c on the x-axis) versus three different durations from the initial noticing of the fake tiger, until the onset or cessation of alarm calling by females or independent offspring. (a) The male calling period does not depend on the duration until the onset of calling by females or offspring (r=−0.27, p=0.396). (b) The male stops calling shortly after the first alarm call of the last female or offspring has sounded (r=0.99, p<0.001). (c) All females and offspring have ceased calling within about five minutes after the male has stopped (r=0.98, p<0.001). In figures (b) and (c) the line y=x has been added to show that both regression lines run parallel to the line y=x.
Table 1.
Duration to first alarm call of individual females and independent offspring and to last alarm call of male. (The individual females and independent offspring in a group are numbered in the first column. For each individual female and independent offspring in each group the duration (min) to the first alarm call after detection of the predator model is presented. The duration (min) after detection of the predator model to the last male alarm call is presented in the last row.)
| group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| females and independent offspring | ||||||||||||
| 1 | 1 | 4 | 3 | 2 | 4 | 1 | 3 | 2 | 3 | 2 | 1 | 2 |
| 2 | 1 | 4 | 3 | 2 | 4 | 2 | 3 | 2 | 4 | 3 | 1 | 3 |
| 3 | 2 | 4 | 4 | 3 | 4 | 2 | 4 | 4 | 6 | 5 | 2 | 4 |
| 4 | 2 | 4 | 5 | 3 | 5 | 4 | 5 | 5 | 7 | 6 | 3 | 4 |
| 5 | 3 | 5 | 6 | 4 | 5 | 5 | 6 | 6 | 8 | 9 | 5 | 5 |
| 6 | 3 | 5 | 6 | 5 | 5 | 6 | 8 | 7 | 8 | 11 | 5 | 6 |
| 7 | 4 | 5 | 7 | 6 | 6 | 6 | 9 | 17 | 11 | 17 | 7 | 7 |
| 8 | 5 | 5 | 8 | 6 | 6 | 7 | — | — | 12 | 28 | 8 | 8 |
| 9 | — | 5 | 10 | — | 6 | 8 | — | — | 22 | — | 29 | 43 |
| 10 | — | 5 | — | — | 6 | — | — | — | 24 | — | — | — |
| male | 7 | 7 | 7 | 8 | 8 | 9 | 15 | 22 | 27 | 30 | 31 | 45 |
From the regression line in figure 3b (linear regression equation: y=1.97+1.01x, where x=duration to first alarm call of the last calling female or offspring and y=duration to last male alarm call), we can conclude that, in general, males stop their calling soon after the first alarm call by the last female or offspring has sounded. From the regression line in figure 3c (linear regression equation: y=5.23+0.94 x, where x=duration to last male alarm call and y=duration to last alarm call of any female or offspring), we can conclude that, in general, the last alarm call by any female or offspring occurs rather soon after the last call of the male.
4. Discussion
These results show that the male in a one-male, multi-female group of Thomas langurs continues to give his alarm calls until all independent individuals in the group have given an alarm call. He stops his alarm calling within on average 2 min after hearing the first alarm call of the last independent individual (figure 3b). This shows that a male keeps track of and thus remembers which independent individuals in its group have given alarm calls. The male might still give a few alarm calls after the last call from another group member to confirm he has heard them, because on several occasions we could observe that the male clearly gave these calls in the direction of that particular group member. However, more work is needed to substantiate this suggestion. Because alarm calls can be individually distinguished in a number of primate and non-primate species (Leger et al. 1984; Cheney & Seyfarth 1990a; Hare 1998; Blumstein et al. 2004), it is likely that the male langurs also recognize other group members by their individual alarm calls. The possibility that males simply reacted with alarm calls to alarm calls of the other independent group members could be excluded, because some other members continued giving alarm calls after the male had stopped calling in all but one of the 12 groups (figure 3c). After the male had given its last alarm call the calling of all other independent group members ceased within about 5 min (range 2–8 min). Many of these independent group members had already stopped calling before the male's last alarm call. This indicates that the total calling time of the group is mainly determined by the male's calling duration, which is in turn determined by the first call of the last calling independent individual in the group. It should be noted that not all group members stopped their alarm calling at about the same time, but that the male continues calling even when most females and independent offspring have long stopped alarm calling. Thus, a social factor, viz. the calls of the audience, determines the male's alarm calling duration. Because an audience effect also occurs in other primates and animal taxa (Sherman 1977; Marler & Mitani 1988; Randall et al. 2000), future studies could test the generality of these results.
One possible alternative explanation, namely that call duration is related to male dominance (Cheney & Seyfarth 1981), was not tested in this study. The reason for not testing this hypothesis is that not all langur males had adjacent home ranges and, therefore, it was not possible to construct a dominance hierarchy. However, in two male dyads we knew which male was dominant based on their behaviour during between-group encounters. In only one of these dyads did the dominant male calling period last longer. Thus, even if this hypothesis is applicable to one-male, multi-female groups, the data are not supportive.
Similarly to Thomas langurs, other non-human primates commonly give alarm calls for extensive periods after encountering a predator (e.g. Zuberbühler et al. 1997, 1999b). This is generally assumed to be adaptive because it can deter a predator to continue hunting as has been shown for leopards (Panthera pardus, Zuberbühler et al. 1999b). Because in Thomas langurs solitary males did not give alarm calls to a fake tiger skin (Wich & Sterck 2003), it is likely that male Thomas langur alarm calls are also meant for other group members and not only to deter the predator. Males might give alarm calls for long periods to warn other group members not go to the ground because a tiger is unable to climb into trees. Remaining in the trees is, therefore, a safe strategy after an encounter with a tiger. On occasions that other group members are feeding on the ground in small streams for snails or algae, the resident male usually does not follow but remains a few metres above the ground as a sentinel and continuously scans the surroundings. As soon as there is a crack of a branch or some other noise, the male will start giving alarm calls and others will immediately flee back into the safety of the trees again. The long period of alarm calling after the tiger skin has been spotted might, therefore, also function to warn other group members not to go to the ground. If so, it is also conceivable that a male would monitor the reaction of other group members to ensure that the message came across and to determine the moment to cease calling. Only after all group members have signalled that they have heard the male will the male stop giving alarm calls.
It is as yet unclear what cognitive mechanism underlies the male's calling behaviour. It is generally accepted that most non-human primates, in particular monkeys, are unable to recognize the mental states of others (Cheney & Seyfarth 1990a,b; Seyfarth & Cheney 2003). Usually other plausible and more parsimonious explanations are invoked to explain behaviour that seems to imply that non-human primates have knowledge of each other's psychological state. It is, therefore, unlikely that the male keeps on calling to make other group members aware of the danger. It is more parsimonious to assume that the male has formed an association between the behaviour of another group member and whether the other group member gave alarm calls or not. If group members that did not give alarm calls behaved in such a way that they are more prone to get caught by a predator it is beneficial for the male to keep on calling, until all group members have called. Behaviour that could make a Thomas langur more prone to a tiger attack would be coming to the ground or the area where the tiger might be. Thomas langurs seem to avoid coming near to the ground after encountering a tiger and avoid the area where the tiger was encountered for several days (personal observation).
An interesting point for future research might be that if the langur alarm calls function to deter a predator, it is expected that a predator that remains stationary would evoke a longer calling duration than a predator that moves away. During the experiments, the langurs did not mob the model or move towards the model. This might be because the tiger model itself moved. During a preliminary trial with a stationary tiger model (skin wrapped around a backpack), calling duration lasted more than an hour and the langurs did mob the model. Tigers are ambush predators, and therefore alarm calling is not very risky and can in fact function to deter the predator (Zuberbühler et al. 1999b). In the case of pursuit predators, however, it might be beneficial not to call for extended periods so that the predator gets no opportunity to localize its prey. Experiments with both ambush and pursuit predator models could address such issues. The only possible pursuit predator for the langurs is the clouded leopard (Neofelis nebulosa), but this predator is very elusive and, unfortunately, we do not have observations of clouded leopards encountering a langur group to support these predictions. Uncertainty about which type of predator is near might delay the langurs to call and explain the fact that some group members only called after 2–4 min after the tiger was observed by other group members.
In conclusion, Thomas langur males show the ability to remember which of the other independent group members have already given alarm calls. This suggests that the vocal reaction of group members shaped the evolution of alarm calling behaviour and that alarm calling behaviour in this group-living primate species is not simply the sum of individual reactions to predators but an interactive warning system, where male calling behaviour depends on the calling behaviour of females.
Acknowledgments
We thank the Indonesian Institute of Science (LIPI, Jakarta), the Indonesian Nature Conservation Service (PHKA), Universitas National (UNAS, Jakarta), and the Leuser Development Programme (LDP, Medan). We also thank the Netherlands Foundation for the Advancement of Tropical Research (WOTRO), the Netherlands Organization for Scientific Research (NWO), The Treub Foundation, Lucie Burgers Foundation, Dobberke Foundation, and the University of Utrecht for financial support. R. Seyfarth, E. H. M. Sterck, S. M. Reader, J. J. Bolhuis and two anonymous reviewers gave valuable suggestions to earlier drafts.
References
- Blumstein D.T, Verneyre L, Daniel J.C. Reliability and the adaptive utility of discrimination among alarm callers. Proc. R. Soc. B. 2004;271:1851–1857. doi: 10.1098/rspb.2004.2808. 10.1098/rspb.2004.2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney D.L, Seyfarth R.M. Selective forces affecting the predator alarm calls of vervet monkeys. Behaviour. 1981;76:25–61. [Google Scholar]
- Cheney D.L, Seyfarth R. University of Chicago Press; Chicago, IL: 1990a. How monkeys see the world. [Google Scholar]
- Cheney D.L, Seyfarth R.M. Attending to behaviour versus attending to knowledge: examining monkey's attribution of mental states. Anim. Behav. 1990b;40:742–753. [Google Scholar]
- Crockford C, Boesch C. Context-specific calls in wild chimpanzees. Pan troglodytes verus: analysis of barks. Anim. Behav. 2003;66:115–125. 10.1006/anbe.2003.2166 [Google Scholar]
- Gurmaya K. Ecology and behaviour of Presbytis thomasi in Northern Sumatra. Primates. 1986;27:151–172. [Google Scholar]
- Hare J.F. Juvenile Richardson's ground squirrels. Spermophilus richardsonii, discriminate among individual alarm callers. Anim. Behav. 1998;55:451–460. doi: 10.1006/anbe.1997.0613. 10.1006/anbe.1997.0613 [DOI] [PubMed] [Google Scholar]
- Leger D.W, Berney-Key S.D, Sherman P.W. Vocalizations of Belding's ground squirrels (Spermophilus beldingi) Anim. Behav. 1984;32:753–764. [Google Scholar]
- Marler P.M, Mitani J. Vocal communication in primates and birds: parallels and contrasts. In: Todt P, Goedeking P, Symmes D, editors. Primate vocal communication. Springer; Berlin: 1988. pp. 3–14. [Google Scholar]
- Marler P, Evans C, Hauser M.D. Animal signals: motivational, referential, or both? In: Papousek H, Jürgens U, Papousek M, editors. Nonverbal vocal communication: comparative and developmental approaches. Cambridge University Press; Cambridge, UK: 1992. pp. 66–86. [Google Scholar]
- Randall J.A, Rogovin K.A, Shier D.M. Antipredator behaviour of a social desert rodent: footdrumming and alarm calling in the great gerbil, Rhombomys opiums. Behav. Ecol. Sociobiol. 2000;48:110–118. 10.1007/s002650000199 [Google Scholar]
- Rijksen H.D. H. Veenman & Zonen BV; Wageningen: 1978. A field study on Sumatran orangutans (Pongo pygmaeus abelii lesson 1872) [Google Scholar]
- Seyfarth R.M, Cheney D.L. Signalers and receivers in animal communication. Annu. Rev. Psychol. 2003;54:145–173. doi: 10.1146/annurev.psych.54.101601.145121. 10.1146/annurev.psych.54.101601.145121 [DOI] [PubMed] [Google Scholar]
- Seyfarth R.M, Cheney D.L, Marler P. Monkey responses to three different alarm calls: evidence of predator classification and semantic communication. Science. 1980;210:801–803. doi: 10.1126/science.7433999. [DOI] [PubMed] [Google Scholar]
- Sherman P.W. Nepotism and the evolution of alarm calls. Science. 1977;197:1246–1253. doi: 10.1126/science.197.4310.1246. [DOI] [PubMed] [Google Scholar]
- Steenbeek R. Tenure related changes in wild Thomas's langurs I: between-group interactions. Behaviour. 1999;136:595–625. 10.1163/156853999501487 [Google Scholar]
- Sterck E.H.M. Determinants of female dispersal in Thomas langurs. Am. J. Primatol. 1997;42:179–198. doi: 10.1002/(SICI)1098-2345(1997)42:3<179::AID-AJP2>3.0.CO;2-U. 10.1002/(SICI)1098-2345(1997)42:3%3C179::AID-AJP2%3E3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- Wich S.A, Sterck E.H.M. Possible audience effect in Thomas langurs (Primates; Presbytis thomasi): an experimental study on male loud calls in response to a tiger model. Am. J. Primatol. 2003;60:155–159. doi: 10.1002/ajp.10102. 10.1002/ajp.10102 [DOI] [PubMed] [Google Scholar]
- Wich S.A, van de Post D.J, Heistermann M, Möhle M, van Hooff J.A.R.A.M, Sterck E.H.M. Tenure related changes in male loud call characteristics and testosterone levels in wild Thomas langurs. Int. J. Primatol. 2003;24:1251–1265. 10.1023/B:IJOP.0000005991.97232.2a [Google Scholar]
- Zuberbühler K. Referential labelling in Diana monkeys. Anim. Behav. 2000;59:917–929. doi: 10.1006/anbe.1999.1317. [DOI] [PubMed] [Google Scholar]
- Zuberbühler K. Predator-specific alarm calls in Campbell's monkeys. Cercopithecus campbelli. Behav. Ecol. Sociobiol. 2001;50:414–422. 10.1007/s002650100383 [Google Scholar]
- Zuberbühler K. A syntactic rule in forest monkey communication. Anim. Behav. 2002;63:293–299. [Google Scholar]
- Zuberbühler K, Noë R, Seyfarth R.M. Diana monkeys long-distance calls: messages for conspecifics and predators. Anim. Behav. 1997;53:589–604. 10.1006/anbe.1996.0334 [Google Scholar]
- Zuberbühler K, Cheney D.L, Seyfarth R.M. Conceptual semantics in a nonhuman primate. J. Comp. Psychol. 1999a;113:33–42. [Google Scholar]
- Zuberbühler K, Jenny D, Bshary R. The predator deterrence function of primate alarm calls. Ethology. 1999b;105:477–490. [Google Scholar]