Abstract
Life-history theory predicts that an individual should reduce its reproductive efforts by laying a smaller clutch size when high risk of nest predation reduces the value of current reproduction. Evidence in favour of this ‘nest predation hypothesis’, however, is scarce and based largely on correlative analyses. Here, we manipulated perceived risk of nest predation in the Siberian jay Perisoreus infaustus using playback involving a mixture of calls by corvid nest predators in the vicinity of nest sites. In response to being exposed to this acoustic cue simulating increased risk of nest predation, the jays chose a nest site offering more protective covering and reduced clutch size. This is the first experimental demonstration of clutch size adjustment and nest site selection as a result of phenotypic plasticity in an open nesting passerine reflecting a facultative response to the perceived risk of nest predation.
Keywords: clutch size, nest site selection, predation, phenotypic plasticity, life-history
1. Introduction
Clutch size reduction under high risk of nest predation has been hypothesized to be adaptive for at least two reasons (Roff 1992). First, when nest predation increases with clutch size (Safriel 1975), smaller broods will shorten the period when the nest is susceptible to nest predators and reduce the number of nest visits that could attract the attention of predators (Skutch 1949; Martin et al. 2000a,b). Second, if parental survival declines with clutch size, then a reduction in clutch size will improve parental survival prospects and future reproduction, thereby spreading the risk of nest predation between broods and ultimately increasing lifetime reproductive success (Slagsvold 1984; Roff 1992; Martin 1995). Despite these predictions being consistent with life-history theory, there is little experimental support for nest predation affecting clutch size variation in birds. This is surprising because many theoretical and experimental studies on other taxa provide evidence for predator-induced life-history shifts through phenotypic plasticity (Crowl & Covich 1990; Reznick et al. 1990; Ball & Baker 1996). Past work on bird clutch sizes has mainly involved intra- and interspecific comparative analyses, which provides important insights into factors associated with clutch size variation, but does not necessarily infer a causal relationship (Martin & Clobert 1996; Julliard et al. 1997; Martin et al. 2000a). Only one experimental study of hole-nesting collared flycatchers Ficedula albicollis has addressed the issue of behavioural responses in terms of clutch size modification, by simulating nest predation during the previous breeding season through removal of nestlings and short presentations of predator models (Doligez & Clobert 2003). Here, we consider nest site selection of an open-nesting species while manipulating perceived predation risk during the current reproductive attempt, thereby allowing us to examine the clutch size by nest site interaction. This is crucial because any response in terms of selecting a safer nest site might also affect the relative value of the reproductive attempt and so clutch size. Furthermore, nest sites may affect thermoregulatory costs (Marzluff 1988). Yet few studies have tested whether parent birds are able to assess variation in current nest predation risk and choose their nest site accordingly (Larson 2000; Boulton et al. 2003; Forstmeier & Weiss 2004).
The Siberian jay provides an opportunity to test predator-induced plasticity in both nest site selection and clutch size. Parents produce a single brood per season with a relatively small but highly variable clutch size (range: 1–5 eggs, mean±s.e.: 3.9±0.1 eggs, n=57). Nest predation by other corvid species, which hunt mostly using visual cues, is a main cause of reproductive failure, and this is reflected in both circumspect parental behaviours and the cryptic nature of nest sites (Ekman et al. 1994; Ekman et al. 2001; Eggers et al. 2005a,b).
To test if Siberian jays shift their nest site to one offering more nesting cover, and to test if they reduce clutch size in response to perceived high activity of visually oriented predators, we simulated the presence of their main predators (Eurasian jay Garrulus glandarius, hooded crow Corvus corone, and raven Corvus corax) by playback of a mixture of calls. Because previous experience of nest predation risk can potentially alter nest site selection and reproductive investment we explored if previously unsuccessful parents were more susceptible to shifting nest sites and/or clutch size reduction (Marzluff 1988; Doligez & Clobert 2003).
2. Material and methods
(a) Study area and experimental design
This study was conducted between 2001 and 2004 in a population of individually colour-banded Siberian jays northwest of Arvidsjaur, northern Sweden (65°40′ N, 19°0′ E). This population has been studied since 1989. In March each year, jay females were caught and fitted with radio transmitters (1.85 g; Holohil), and nest sites were located by tracking females during the nest-building period. In the period 1998–2004 daily monitoring of females allowed us to determine the onset of the egg laying period. We climbed trees up to nests to determine clutch size and hatching success in April and early May. In 2001, 2002 and 2004 we manipulated the perceived risk of nest predation risk by simulating a presence of corvids through playback of a unique mixture of individual calls (Eurasian jay, hooded crow and raven) to each pair. Parents were exposed to our treatment every second day between 0900 and 1800 h (1 corvid call per 6 min, TDK endless cassette, EG-12M). Nests are normally confined to a restricted area of the territory that provides high protection, with the result that nest sites in consecutive years are not far apart (mean±s.e. distance between sites: 140±23 m, n=38 territories). We, therefore, carried out the playback in the immediate vicinity of the nest site used in the previous year. Playbacks were stopped as soon as females were found sitting on the nest. We could follow the females by radio tracking and visited each territory every second day, and so at most we exposed females to playbacks only during their first day of incubation. We used two types of control. To test if there was any response to the playback equipment and artificial noise in general rather than the corvid calls specifically, control parents received playback of a mixture of individual bird songs of small European passerines (goldcrest Regulus regulus, willow tit Parus montanus, willow warbler Phylloscopus trochilus, great tit Parus major and blackbird Turdus merula). Another set of control birds was given no playback treatment at all. Control playbacks followed the same protocol as corvid calls. Each playback of song birds was unique and recordings were circulated among pairs. This procedure was, therefore, conservative because all pairs will have been exposed to any of the playback machines or tapes that produced adverse or unusual responses.
Breeding success is strongly linked to habitat structure among Siberian jays (Eggers et al. 2005a). Furthermore, acquisition of high-quality territories is linked to the timing of dispersal and the disperser's phenotype (Ekman et al. 2002). Therefore, it was imperative to stratify experimental territories to equalize the overall reproductive success between treatment and control territories. The distance between nests among territories normally exceeds 0.5 km and experimental and control nests were not grouped near one another; hence, they can be regarded as statistically independent. The population data collected since 1989 allowed us to assess the background risk of nest predation for territories under natural conditions. Hence, experimental and control territories were selected to equalize their past history of reproductive success, and the background risk of nest predation was entered into the test (NSI, nest success index; for details see Ekman et al. 2001). To determine the location and thus distances between nest sites we used the global position system (GPS; Garmin 12XL). To study effects of microclimate on reproductive efforts, we measured the temperature at nest sites. We placed temperature probes 20 cm above the nest lining, and we measured temperatures simultaneously at nest sites selected prior to and during the experimental year to control for seasonal and year effects.
(b) Statistical analysis
Clutch size was related to our experimental treatments, the previous nesting success within territories (NSI), predator protective nesting cover provided by low (<15 m) spruces (above or below five spruces per 100 m2; Eggers et al. 2005a), laying date (number of days after 1st April each year) and year (2001, 2002 and 2004) using a generalized linear mixed model with Poisson error (GLIMMIX macro of SAS 9.1, Littell et al. 1996) where the response variable is determined by both random and fixed effects. In this case the random component arose because of repeated sampling of territories across years. Territory identity was, therefore, fitted as random effect. Between-year variation in clutch size, shifts of nest sites and nesting cover among treatments were analysed using a repeated measures design were the data are collected at two time levels, namely prior to and during the experimental year. Our main interest centred on how treatment differences change over time. In other words, is there a treatment-by-time interaction and how does previous nesting success affect this interaction? All territory holders were identified and remained in the same territory between years. Intra-individual changes in clutch size were related to our two levels of time (within subjects factor), year, experimental treatment, previous nesting success (NSI) and the amount of nesting cover (above or below five spruces per 100 m2; between subject factors). Intra-individual analyses of shifts of nest sites were related to our experimental treatments only. Nesting cover changes were related to both treatments and previous nesting success to test if previously unsuccessful parents were more susceptible to shifting into denser vegetation.
3. Results
(a) Playback experiment
We exposed 24 pairs to corvid playback, with 35 control pairs nesting at the same time which were exposed either to playbacks of non-predatory song birds (n=10) or no playback treatment (n=25). There was a highly significant relationship between experimental treatment and the clutch sizes laid by females when controlling for the effects of year, lay date, amount of nesting cover and nesting success in previous years in the territory (table 1). Females exposed to corvid calls laid significantly smaller clutches (mean±s.e.: 3.3±0.2 eggs) compared to controls exposed to playbacks of song from non-predatory birds (4.0±0.3 eggs) or receiving no playbacks at all (4.2±0.2 eggs). Reaction norms depended on the natural background predation risk, as reflected in previous nesting success of individuals in the same territory (table 1). Females in territories with a high natural background risk of nest predation responded more strongly and laid fewer eggs in response to the corvid playbacks (2.6±0.2 eggs), as compared with females on territories with a low natural background risk of nest predation (3.9±0.2 eggs). The clutch size of control females did not change regardless of natural background levels of predation risk, and exposure to playbacks of songbirds had no effect compared with the no playback treatment (table 1).
Table 1.
General linear mixed model of clutch size. (Fixed effects of treatments (1, corvid playback; 2, song bird playback; 3, no playback), previous nesting success (territories with reproductive success higher or lower than average), spruce density (below or above five trees per 100 m2), laying date (partial regression coefficient: −0.03±0.02), year (2001, 2002 and 2004) and interaction terms. Territory was included as random effect. d.d.f., denominator degrees of freedom estimated by the Satterthwaite method.)
| clutch size | ||||
|---|---|---|---|---|
| fixed effects | n.d.f. | d.d.f. | F-value | p-value |
| year | 2 | 45 | 5.5 | <0.01 |
| treatment | 2 | 45 | 14.5 | <0.0001 |
| previous nesting success (NSI) | 1 | 45 | 3.7 | 0.06 |
| density of low spruces (<15 m) | 1 | 45 | 0.48 | 0.49 |
| laying date | 1 | 45 | 5.1 | <0.05 |
| treatment×NSI | 2 | 45 | 7.7 | 0.001 |
| treatment×low spruces | 2 | 45 | 1.1 | 0.33 |
| differences of LS means and contrasts | |||||
|---|---|---|---|---|---|
| label | estimate | s.e. | d.d.f. | F-value | p-value |
| corvid versus song bird | −0.76 | 0.30 | 45 | 6.5 | 0.015 |
| corvid versus no playback | −0.96 | 0.18 | 45 | 27.9 | <0.0001 |
| treat×NSI in treat 1 | 1.2 | 0.28 | 45 | 20.2 | <0.0001 |
| treat×NSI in treat 2 | −0.25 | 0.39 | 45 | 0.45 | 0.51 |
| treat×NSI in treat 3 | 0.01 | 0.25 | 45 | 0.01 | 0.99 |
(b) Intra-individual analysis of clutch size
The response to the playback of corvid calls could be confirmed in an intra-individual analysis of clutch sizes between years for 36 females with known clutch size and breeding in the same territory prior the experimental year (d.d.f.=83; year: n.d.f.=4, F=3.0, p<0.05, time: n.d.f.=1, F=8.0, p<0.01; nesting cover: n.d.f.=1, F=0.1, p=0.89, previous nesting success (NSI): n.d.f.=1, F=1.2, p=0.28, time×treatment: n.d.f.=2, F=22.8, p<0.0001; time×treatment×NSI: n.d.f.=5, F=1.0, p=0.64). Only females exposed to corvid playbacks significantly reduced clutch size (Estimated mean difference in clutch size (±s.e.) between years: −1.3±0.2 eggs, d.f.=83, t=6.3, p<0.0001), whereas control females did not (bird song playback: mean±s.e.: −0.1±0.3 eggs, d.f.=83, t=−0.32, p=0.74; no playback: −0.01±0.1 eggs, d.f.=83, t=−0.14, p=0.89). Our results provide no evidence that previous nesting success affected the degree to which females reduced clutch size in response to corvid playbacks when their preceding clutch size is accounted for. These results provide evidence for predator-induced plasticity in clutch size in response to changes in current risk of nest predation irrespective previous experience.
(c) Predator-induced nest site selection
When exposed to corvid calls parents shifted their nest sites more than twice the distance they had moved in the previous year (table 2). These pairs not only shifted nest sites, but they chose nest sites characterized by a denser forest structure offering more protection against visually oriented nest predators than nest sites chosen in the same territory in the previous year (table 3). We found no such shifts in nest site or nesting cover for control parents, but the reaction norms differed significantly among treatments (tables 2 and 3). Pairs exposed to corvid calls did not breed in denser vegetation for lack of sites with a more open forest structure. The spruce density at nest sites chosen for nesting (8.0±0.7 low spruces per 100 m2) was significantly higher compared to non-use sites randomly selected within the breeding territory in the same year (mean±s.e.: 6.6±0.6 low spruces per 100 m2, n=11, Wilcoxon matched-pair signed-rank test). We found no evidence that previously unsuccessful birds exposed to higher risk of predation were more susceptible to shifting nest sites into denser vegetation (time×treatment×NSI effect, ns, table 3).
Table 2.
Analysis of repeated measures data of nest site distances between years. (*Two years (yeari−1−yeari) served as control of how far the nest would be shifted in the absence of the manipulation with playback treatments. In year three (yeari+1) parents were exposed to playbacks and we determined how far the nest had been shifted from the position in the previous yeari.)
| distance (between subject effects) | d.f. | type III SS | F-value | p-value | |
|---|---|---|---|---|---|
| treatment | 2 | 56 863.4 | 0.51 | 0.603 | |
| error | 35 | 1 939 448.5 |
| distance (within subject effects) | Wilks λ | n.d.f. | d.d.f. | F-value | p-value |
|---|---|---|---|---|---|
| time | 0.80 | 1 | 35 | 8.69 | 0.006 |
| time×treatment | 0.82 | 2 | 35 | 3.74 | 0.033 |
| contrasts | |||||
|---|---|---|---|---|---|
| mean distance ±s.e.* | |||||
| treatments | yeari−1-yeari | yeari-yeari+1 | d.f. | F-value | p-value |
| corvids | 102±33 | 259±51 | 1 | 19.9 | <.0001 |
| song birds | 201±47 | 261±72 | 1 | 1.4 | 0.24 |
| no playback | 153±42 | 159±65 | 1 | 0.02 | 0.89 |
Table 3.
Analysis of repeated measures data of nesting cover between years. (*Nesting cover was described from a sample of four 2×50 m plots each starting at the nest tree and heading north, east, south and west. All low spruces (<15 m) within 1 m on each side of a 50 m trajectory were counted to characterize nesting cover. Yeari prior the experimental year served as control of how parents select nesting cover in the absence of experimental manipulations. In year two (yeari+1) parents were exposed to playbacks.)
| nesting cover (between subject effects) | d.f. | type III SS | F-value | p-value | |
|---|---|---|---|---|---|
| treatment | 2 | 3.6 | 0.1 | 0.91 | |
| previous nesting success (NSI) | 1 | 0.1 | 0.0 | 0.96 | |
| treatment×NSI | 2 | 11.1 | 0.62 | 0.54 | |
| error | 43 | 772.8 |
| nesting cover (within subject effects) | Wilks λ | n.d.f. | d.d.f. | F-value | p-value |
|---|---|---|---|---|---|
| time | 0.84 | 1 | 43 | 7.7 | 0.01 |
| time×treatment | 0.78 | 2 | 43 | 5.8 | 0.01 |
| time×NSI | 0.99 | 1 | 43 | 0.05 | 0.81 |
| time×treatment×NSI | 0.95 | 2 | 43 | 1.14 | 0.32 |
| estimates and contrasts | |||||
|---|---|---|---|---|---|
| mean spruce density ±s.e.* | |||||
| treatments | yeari−1 | yeari | d.f. | F-value | p-value |
| corvids | 5.5±0.6 | 8.0±0.7 | 1 | 23.0 | <.0001 |
| song birds | 7.3±0.9 | 7.0±1.1 | 1 | 1.1 | 0.30 |
| no playback | 6.3±0.8 | 6.7±0.9 | 1 | 0.28 | 0.60 |
(d) Trade-off between predator protection and energetic requirements?
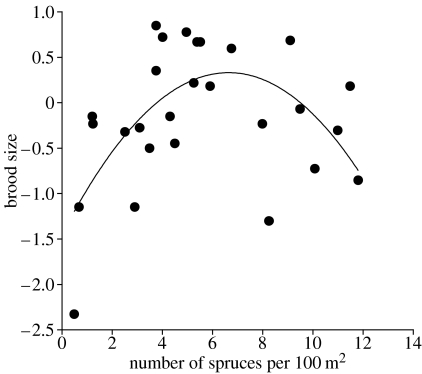
Several findings suggest that denser vegetation may involve a cost for predator protection in increased thermoregulatory costs and reduced egg hatchability. (i) Ambient mean temperatures at nest sites selected in response to corvid playbacks were on average 2.3 °C±0.3 (s.e.) colder as compared to what they were at the nest sites selected without experimental manipulations in the previous year (p<0.01; Wilcoxon matched-pair signed-rank test). (ii) Presumably to reduce the negative effects of low temperatures on breeding performance, Siberian jay parents normally build their nest in spruce or pine trees so that they are exposed to the warmth of the sunny side of tree trunks (mean direction±s.e.: 154±13°, n=14). (iii) There seems to be an optimal amount of nesting cover in that parents trade-off predator protection against the higher energetic demands of incubating and raising nestlings in denser vegetation (figure 1). Starting from more open forest, brood sizes increase as the amount of nesting cover increases. Eventually brood size peaks and decreases again as the forest get very dense. Consistent with this finding (iv) choice of denser vegetation in response to corvid playbacks was associated with a lower proportion of eggs that hatched (proportion: 0.81, n=41 eggs) when compared with control sites (0.98, n=62 eggs; p<0.001, χ2-test, d.f.=2), thereby reinforcing the effect of our experimental treatment on brood size. However, clutch size in nests on corvid playback territories with denser (mean±s.e.: 10.9±0.9 spruces per 100 m2, n=11) versus less denser vegetation (5.1±0.7 spruces per 100 m2, n=11) did not differ significantly (mean±s.e. clutch size: dense, 3.2±0.2 eggs; less dense, 3.3±0.2 eggs; proc Glimmix, d.d.f.=16, year: n.d.f.=2, F=2.7, p=0.1, date: n.d.f.=1, F=0.4, previous nesting success: n.d.f.=1, F=13.9, p<0.01 and nesting cover: n.d.f.=1, F=0.02, p=0.89). This result indicates that nest site choice had little impact on clutch size although our experiment cannot exclude it as a contributing factor.
Figure 1.
Polynomial regression (second order) of residual brood size means (controlling for year) on mean density of low (<15 m) spruces surrounding nest sites. Brood size is calculated from sequences of data in 25 territories that vary between 4 and 11 years (d.f.=2, MS=2.71, R2=0.36, F=6.74, p<0.005).
4. Discussion
Our results indicate that Siberian jays select for saver nest sites as a mechanism to reduce predator-attracting nest visits in addition to the clutch size reduction, maximization of food load sizes, prevention of allofeeding (feeding of non-breeding ‘helpers’) and modification of daily nest visitation patterns (Ekman et al. 1994; Strickland & Waite 2001; Eggers et al. 2005a,b). Our findings are highly consistent with Skutch's (1949) hypothesis that provisioning visits impose a cost in risk of predation if parental activity alerts predators to the location of the nest. This cost in risk of predation must be balanced against the risk of starvation, and it can, therefore, be expected to constrain the rate at which parents can deliver food to young and thereby constrains clutch size by limiting the number of young that parents can feed. Yet, this interaction between nest predation and food limitation should derive from an increase in nest predation with clutch size through higher parental activity but evidence for this relationship is scarce (Roff 1992; Martin et al. 2000a,b). Our findings do not exclude the possibility that the observed reduction in clutch size under increased nest predation risk reflects a trade-off between clutch size and age-dependent adult mortality (Slagsvold 1984; Martin 1995; McCleery et al. 1996). However, Siberian jays produce a single brood each season and normally do not re-lay after nest failure. This eliminates the possibility that parents simply reduced clutch size in response to high probability of nest failure because it permits rapid renesting within the breeding season. It also excludes any confounding effects of nest predation and seasonal changes in food supplies on clutch size (Slagsvold 1984; Martin 1995).
The response to corvid calls reported here raises the question of why the jays normally refrain from using denser nesting habitat. Our data suggest that safer nest sites incur a net cost of predator protection through higher energy demands of incubating females and their nestlings. Evidence from the literature supports this idea. For instance, Marzluff (1988) showed in the pinyon jay, Gymnorhinus cyanocephalus that individuals shift their nest site lower into trees to conceal their nest against corvid nest predators but at the same time suffer greater thermoregulatory costs, and conversely they move up to more exposed sites if nest failure results from inclement weather (snow).
The mean ambient temperatures at Siberian jay nests in the more open breeding habitat that is usually chosen was 2.3±0.3 °C higher than temperatures in the denser stands chosen after experimental exposure to corvid calls, despite the fact that denser vegetation cuts down the process of radiational cooling during the night time. Evidence from the literature confirms our findings for thinned versus unthinned boreal coniferous forest stands showing that mean temperatures in more open habitats are generally 1–2 °C higher compared to denser habitats (Hindmarch & Reid 2001). The observed temperature differences can be explained in part by deeper snow cover in denser vegetation, resulting in prolonged sublimational and long wave cooling during the breeding season. In our study area snow cover in denser stands usually lasts for the entire nestling phase, and ambient temperatures down to −20 °C are not uncommon during incubation and early nestling stages. A warm nest site will then alleviate the energetic demands of incubating females and young, and Siberian jays normally build their nest in spruce or pine trees so that they are exposed to the warmth of the sunny side of tree trunks.
Reduced temperature and light levels in denser vegetation may thus have a dramatic impact on a parent's ability to maintain the desired incubation temperature within the nest and minor reductions in incubation temperatures may lead to lengthened incubation periods, while greater variation may cause occasional lethal chilling of eggs (Webb 1987). In the Siberian jay, females are the sole incubator and increased nest attentiveness may be required when local microclimate conditions are harsh and necessitate increased feeding of the female on the nest by the male. Yet males may reduce nest visitation rates when exposed to increased predator activity, which in turn could constrain female nest attentiveness because of energy limitations (Martin & Ghalambor 1999). Alternatively, egg temperatures may decline with reductions in female energetic state, even without changes in nest attentiveness, and prolonged reduction in average egg temperature by as little as 1 °C can have negative impacts on egg viability (Webb 1987). Hence, a reduction in clutch size may very well compensate for costs associated with lower temperatures at safer nest sites (Reid et al. 2000). In our experiment, such an indirect effect of nest site choice on clutch size is difficult to tease apart from a direct effect of predator activity because parents show strong shifts in nest sites and so direct and habitat-mediated effects of predation risk are highly confounded. However, clutch size in nests in territories exposed to corvid playbacks with denser versus less dense vegetation does not differ significantly indicating that females did not lay fewer eggs to compensate for increased thermoregulatory costs in denser vegetation.
Still, our correlative data on brood size in relation to nesting cover suggest that a colder microclimate has hatching success consequences (figure 1). Choice of denser vegetation in response to corvid playbacks was associated with a lower proportion of eggs that hatched when compared with control sites, thereby reinforcing the effect of our experimental treatment on brood size. During the study period 1998–2002 only two cases of post hatching brood reduction were observed suggesting that adjustments in brood size occur prior hatching through differential egg quality and/or incubation behaviour.
Further studies are required to establish whether the antipredator behaviour of selecting these very dense sites has a cost in reduced female nest attentiveness because of energy limitations, reductions in female energetic state, or alternatively food stress in response to the perceived higher risk of nest predation during egg formation (Webb 1987; Martin & Ghalambor 1999; Conway & Martin 2000).
Past interspecific comparative analyses examining the adaptive significance of the coevolution of avian life history traits have strongly advanced our knowledge about the selection pressures driving life history evolution (Martin & Clobert 1996; Martin et al. 2000b). Our work along with a recent experimental study by Doligez & Clobert (2003) provides a complement to these studies supporting the hypothesis of predator-induced clutch size reduction in a proximate context. Doligez & Clobert's results suggest that current reproductive efforts in the hole-nesting collard flycatcher (Ficedula albicollis) are influenced by information on local nest predation risk gathered by parents during the previous breeding season. We demonstrate that natural variation towards elevated background predation risk interacts with experimentally induced higher perceived risk of nest predation, to further reduce clutch size in the open-nesting Siberian jay. In addition, previous experience of nest predation risk may very well contribute to influence reproductive efforts. These mechanisms are not mutually exclusive. The consideration of nest site effects on both shelter from visually oriented nest predators and thermoregulatory costs revealed that the amount of nesting cover may often exert antagonistic influences on the value of current reproduction (Martin & Li 1992; Martin 1995). Our results highlight the importance of considering the contrasting and interacting roles of nest predation and food limitation to better understand hereditary variations of parental care efforts subject to natural selection.
Acknowledgments
We thank Vittorio Baglione, Jacob Höglund, David Winkler, and Jonathan Wright for comments and discussion; Johan Wallén and Tobias Sahlman for help in the field and the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (formas), Stiftelsen Lars Hiertas Minne, Stiftelsen för Zoologisk Forskning and Swedish Biodiversity Centre for funding this work. S.E. dedicates this study to his beloved wife Jeanette and children Simon and Tim.
References
- Ball S.L, Baker R.L. Predator-induced life-history changes: antipredator behavior costs or facultative life-history shifts? Ecology. 1996;77:1116–1124. [Google Scholar]
- Boulton R.L, Cassey P.C, Schipper C, Clarke F. Nest site selection by yellow-faced honeyeaters Lichenostomus chrysops. J. Avian Biol. 2003;34:267–274. 10.1034/j.1600-048X.2003.03062.x [Google Scholar]
- Conway J.C, Martin T.E. Effects of ambient temperature on avian incubation behaviour. Behav. Ecol. 2000;11:178–188. 10.1093/beheco/11.2.178 [Google Scholar]
- Crowl T.A, Covich A.P. Predator-induced life-history shifts in a freshwater snail. Science. 1990;247:949–951. doi: 10.1126/science.247.4945.949. [DOI] [PubMed] [Google Scholar]
- Doligez B, Clobert J. Clutch size reduction as a response to increased nest predation rate in the Collard Flycatcher. Ecology. 2003;84:2582–2588. [Google Scholar]
- Eggers S, Griesser M, Andersson T, Ekman J. Nest predation and habitat change interact to influence Siberian jay numbers. Oikos. 2005a;111:150–158. 10.1111/j.0030-1299.2005.13802.x [Google Scholar]
- Eggers S, Griesser M, Ekman J. Predator-induced plasticity in nest visitation rates in the Siberian jay (Perisoreus infaustus) Behav. Ecol. 2005b;16:309–315. 10.1093/beheco/arh163 [Google Scholar]
- Ekman J, Sklepkovych B, Tegelström H. Offspring retention in the Siberian jay Perisoreus infaustus: the prolonged brood care hypothesis. Behav. Ecol. 1994;5:245–253. [Google Scholar]
- Ekman J, Eggers S, Griesser M, Tegelström H. Queuing for preferred territories: delayed dispersal of Siberian jays. J. Anim. Ecol. 2001;70:317–324. 10.1046/j.1365-2656.2001.00490.x [Google Scholar]
- Ekman J, Eggers S, Griesser M. Fighting to stay: the role of sibling rivalry for delayed dispersal. Anim. Behav. 2002;64:453–459. 10.1006/anbe.2002.3075 [Google Scholar]
- Forstmeier W, Weiss I. Adaptive plasticity in nest-site selection in response to changing predation risk. Oikos. 2004;104:487–499. 10.1111/j.0030-1299.1999.12698.x [Google Scholar]
- Hindmarch T.D, Reid M.L. Forest thinning affects reproduction in pine engravers (Coleoptera: Scolytidae) breeding in felled lodge pole pine trees. Environ. Entomol. 2001;30:919–924. [Google Scholar]
- Julliard R, Clobert M.R.H.C, Perrins C.M. Phenotypic adjustment of clutch size due to nest predation in the Great tit. Ecology. 1997;78:394–404. [Google Scholar]
- Larson T. Influence of rodent density on nesting associations involving the bar tailed godwit Limosa lapponica. Ibis. 2000;142:476–481. [Google Scholar]
- Littell R.C, Milliken G.A, Stroup W.W, Wolfinger R.D. SAS Institute; Carey, North Carolina: 1996. SAS system for mixed models. [Google Scholar]
- Marzluff J.M. Do pinyon jays alter nest placement based on prior experience? Anim. Behav. 1988;36:1–10. [Google Scholar]
- Martin T.E. Avian life-history evolution in relation to nest sites, nest predation and food. Ecol. Monogr. 1995;65:101–127. [Google Scholar]
- Martin T.E, Clobert J. Nest predation and avian life history evolution in Europe versus North America: a possible role of humans? Am. Nat. 1996;147:1028–1046. 10.1086/285891 [Google Scholar]
- Martin T.E, Ghalambor C.K. Male feeding females during incubation. I. Required by microclimate or constrained by nest predation? Am. Nat. 1999;153:131–139. doi: 10.1086/303153. 10.1086/303153 [DOI] [PubMed] [Google Scholar]
- Martin T.E, Li P. Life history traits of open versus cavity nesting birds. Ecology. 1992;73:579–592. [Google Scholar]
- Martin T.E, Martin P.R, Olson C.R, Heidinger B.J, Fontaine J.J. Parental care and clutch sizes in North and South American birds. Science. 2000a;287:1482–1485. doi: 10.1126/science.287.5457.1482. 10.1126/science.287.5457.1482 [DOI] [PubMed] [Google Scholar]
- Martin T.E, Scott J, Menge C. Nest predation increases with parental activity: separating nest site and parental activity effects. Proc. R. Soc. B. 2000b;267:2287–2293. doi: 10.1098/rspb.2000.1281. 10.1098/rspb.2000.1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery R.H, Clobert J, Julliard R, Perrins C.M. Nest predation and delayed cost of reproduction in the great tit. J. Anim. Ecol. 1996;65:96–104. [Google Scholar]
- Reid J.M, Monaghan P, Ruxton G.D. The consequences of clutch size for incubation conditions and hatching success in starlings. Funct. Ecol. 2000;14:560–565. [Google Scholar]
- Reznick D.A, Bryga H, Endler J.A. Experimentally induced life-history evolution in a natural population. Nature. 1990;346:357–359. 10.1038/346357a0 [Google Scholar]
- Roff D.A. The evolution of life histories: theory and analysis. Chapman & Hall; New York: 1992. [Google Scholar]
- Safriel U.N. On the significance of clutch size in nidifugous birds. Ecology. 1975;56:703–708. [Google Scholar]
- Skutch A.F. Do tropical birds rear as many young as they can nourish? Ibis. 1949;91:430–455. [Google Scholar]
- Slagsvold T. Clutch size variation in birds in relation to nest predation: on the costs of reproduction. J. Anim. Ecol. 1984;53:945–953. [Google Scholar]
- Strickland D, Waite T.A. Does initial suppression of allofeeding in small jays help to conceal their nest? Can. J. Zool. 2001;79:2128–2146. 10.1139/cjz-79-12-2128 [Google Scholar]
- Webb D.R. Thermal tolerance of avian embryos: a review. Condor. 1987;91:628–633. [Google Scholar]