Abstract
There is increasing evidence that the environment experienced early in life can strongly influence adult life histories. It is largely unknown, however, how past and present conditions influence suites of life-history traits regarding major life-history trade-offs. Especially in animals with indeterminate growth, we may expect that environmental conditions of juveniles and adults independently or interactively influence the life-history trade-off between growth and reproduction after maturation. Juvenile growth conditions may initiate a feedback loop determining adult allocation patterns, triggered by size-dependent mortality risk. I tested this possibility in a long-term growth experiment with mouthbrooding cichlids. Females were raised either on a high-food or low-food diet. After maturation half of them were switched to the opposite treatment, while the other half remained unchanged. Adult growth was determined by current resource availability, but key reproductive traits like reproductive rate and offspring size were only influenced by juvenile growth conditions, irrespective of the ration received as adults. Moreover, the allocation of resources to growth versus reproduction and to offspring number versus size were shaped by juvenile rather than adult ecology. These results indicate that early individual history must be considered when analysing causes of life-history variation in natural populations.
Keywords: development, phenotypic plasticity, trade-off, growth, reproduction, cichlids
1. Introduction
Animal life-history decisions depend on an individual's current phenotype, taking into account temporally changing internal states and ambient external conditions (e.g. Houston & McNamara 1999; Clark & Mangel 2000). However, presently observed phenotypic traits may have developed through different ontogenetic trajectories that were influenced by an animal's previous environment (see Schlichting & Pigliucci 1998). Observed life-history trajectories may hence critically depend on an individual's early history. Several long-term studies have revealed how ontogenetic experience may influence key life-history traits such as fecundity and survival (Lindström 1999; Metcalfe & Monaghan 2001; Lummaa & Clutton-Brock 2002). For example, poor environmental conditions early in life can result in smaller adult size, lower energy reserves or inferior competitive ability and, ultimately, in reduced life-time fitness of individuals. If conditions become more favourable animals may compensate for a bad start, for example by a period of rapid growth (reviewed in Metcalfe & Monaghan 2001; Ali et al. 2003). However, growth compensation may cause immediate (e.g. Gotthard 2000) or long-term fitness costs (e.g. Metcalfe & Monaghan 2001). The impact of early environment may be transmitted between generations by non-genetic parental effects (Mousseau & Fox 1998; Lindström 1999; Lummaa & Clutton-Brock 2002), which may even affect the third generation (Huck et al. 1987).
A trade-off between growth and reproduction exists in unicellular and multicellular organisms and can be regarded as universal characteristic of life (e.g. Cavalier-Smith 1980). Animals with indeterminate growth like most fishes, reptiles, amphibians and many invertebrates face this trade-off over their entire lives. The growth conditions an individual encounters early in life should influence the solution of this trade-off during adulthood. First, both growth rates and reproductive output are usually related to body size (e.g. Roff 1992), and early growth and development can influence the size of organisms throughout life (Arendt 2000); second, early growth may cause irreversible changes to an animal's metabolism (Desai & Hales 1997). Nevertheless, the effect of early environment on resource allocation to growth and reproduction in adults remains largely unexplored. One reason for this deficit may be a research bias towards animals with determinate growth, namely mammals, birds and insects, when investigating long-term effects on life histories and fitness (reviewed in Mousseau & Fox 1998; Lindström 1999; Metcalfe & Monaghan 2001; Lummaa & Clutton-Brock 2002). Studies of indeterminately growing animals have hitherto focussed mainly on the effects of short-term growth inhibition on growth rates directly after these manipulations (Aune et al. 1997; Metcalfe & Monaghan 2001; Ali et al. 2003). A few studies have considered the effects of early nutrition on life-history traits related to reproduction (Reznick 1990; Reznick & Yang 1993; Reznick et al. 1996; Sinervo & Doughty 1996) and survival (Sinervo & Doughty 1996), but a simultaneous look at both growth and reproduction is almost entirely missing (but see Siems & Sikes 1998).
Here I present results from a long-term experiment investigating how past and present environments determine growth, reproductive performance and major life-history trade-offs during adulthood. Females of the cichlid fish Simochromis pleurospilus were raised either on a high-food or low-food diet as juveniles, resulting in diverging growth rates between treatments. After maturation, half of the fishes in each group were switched to the opposite diet, while the other half stayed with the original treatment. Growth conditions and the resulting body sizes are important determinants of life-history trajectories in fishes, where usually mortality decreases with size (Sogard 1997) while fecundity normally increases (Wootton 1990). Life-history models predict that faster juvenile growth favours maturation at a larger size (e.g. Stearns & Koella 1986; Berrigan & Koella 1994; Day & Rowe 2002). However, whether fast growth should favour maturation at a later or at an earlier age depends critically on the assumptions made about the relationship between mortality and growth rate (Berrigan & Koella 1994). Delayed maturation at a larger size and age is predicted to occur when juvenile mortality strongly increases with decreasing growth or if both juvenile and adult mortality increase as growth rate decreases (Stearns & Koella 1986).
These conditions apply when mortality decreases with size as it is often found in fishes. When developing under limited food, fishes grow more slowly, are smaller and hence would always be exposed to higher mortality risk than same-aged, well fed, large conspecifics under natural conditions. Slow growing fishes would benefit from reproducing as early as possible and at a fast rate to maximize reproductive output in the limited time they have. In contrast, fast growing individuals should delay first reproduction, start with a relatively low-reproductive investment and allocate more resources to growth after maturity, resulting in a slow reproductive rate but a larger size and higher fecundity later in life (Stearns 1992). Therefore, juvenile growth conditions may initiate a feedback loop resulting in individual life-history trajectories located somewhere between ‘slow juvenile growth-early reproduction-high reproductive rate’ and ‘fast juvenile growth-late reproduction-low reproductive rate’.
How should indeterminately growing animals respond if food availability, and therefore the growth potential, increases suddenly, e.g. because of environmental fluctuations or because of a niche-shift between life stages (e.g. Werner & Gilliam 1984; Takimoto 2003)? There are two main possibilities. Animals may follow the same allocation patterns as determined by juvenile growth conditions, or they may adjust energy allocation to the new conditions. Many organisms show compensatory growth if conditions improve (Metcalfe & Monaghan 2001; Ali et al. 2003). If after a period of accelerated growth an animal has caught up in size with conspecifics that always grew fast, it may then adopt the allocation pattern of these large individuals. The opposite case—a switch from good to poor conditions—has received little attention. Larger animals need more energy to maintain body functions, so under food shortage a formerly fast growing animal may face severe energy limitations. Again, it may maintain its allocation pattern and reduce both growth and reproductive rate, or it may give priority to reproduction or to growth to maximize either current or future reproduction.
A second major life-history trade-off regards resource allocation to number and size of offspring. This trade-off is limited by the proportion of total energy invested in reproduction. Hence early growth conditions may influence this trade-off through the allocation of energy to growth and reproduction. Here I analysed the respective roles of early and current environment for both trade-offs.
2. Material and methods
(a) Study species
S. pleurospilus is a small mouthbrooding cichlid of the subfamily Tropheini endemic to Lake Tanganyika, East Africa. It lives along the rocky littoral shores of the lake, where it feeds on epilithic algae. It reproduces all year round and mates promiscuously. Males defend small breeding territories visited by females only for spawning. Females mouthbrood the clutch and care for the young alone. During the first brood care phase of two weeks females continuously keep their clutch in the buccal cavity and do not feed. In the second phase, they release their young for short periods, during which both female and young may feed. When disturbed or attacked by predators, females take their young back into their mouth. After 1–2 weeks, females do not take up the young any longer, which are then independent.
Juveniles and adults live sympatrically, but juveniles are more gregarious than adults and they are confined to very shallow water (0–0.5 m) offering the highest productivity of algae (Taborsky 1999). Adults live between 0 and 3 m depth, where they experience a great variation in algae productivity, differing by two orders of magnitude along this depth range (Taborsky 1999).
(b) General experimental methods
One hundred-and-twenty 20 l Plexiglas tanks were set up in a climatized room at the Ethologische Station Hasli, University of Bern, Switzerland. Each tank was equipped with an internal biological filter and one half of a clay flower pot (10 cm diameter), which were both used as shelters by the fish, and a 3-cm layer of fine-grained river sand. Water temperature was kept at 27 °C and the light : dark cycle was set to 13 h : 11 h with 10 min dimmed light periods in the morning and evening to simulate natural light conditions at Lake Tanganyika.
Each tank was stocked with a single S. pleurospilus young directly after independence from maternal care. The young originated from 14 broods of 4–14 young. To reduce genetic variability among experimental fishes, young were bred from a stock of closely related fishes (siblings and half-siblings).
Fishes in the high and low-food treatments were fed an exact amount of Tetramin flake food corresponding to 12 and 4% of body weight, respectively, 6 days a week. Food amount was adjusted to increasing body weight every 14 d based on the mean weight of the oldest experimental cohort (n=14 fishes). Until 12 weeks of age, fishes received pulverized flake food. Afterwards, they received standardized agarose gel cubes containing the respective amount of flake food, plus 5% Spirulina algae to enrich the diet with vitamins. As these cubes did not dissolve in the water, I could easily check for food remains the next day. Until an age of 130 d all of the food was eaten. Afterwards, some of the high-food fishes occasionally left food remains, which were removed the next day. Fishes in the low-food treatment almost always ate all of the food. From an age of 196 days onward I kept their food ration constant. At this age, 91% of the high-food fishes left 20% or more of the daily ration untouched. As the high-food fishes obviously fed to satiation at this stage, further adjusting food levels to increasing weight would have diminished the 3 : 1 difference of food intake between treatment groups.
Lengths and weights of fishes were measured every other week until six weeks of age, and afterwards every four weeks (except for the oldest cohort, see above). Standard and total lengths were read from a measuring board with a 1 mm grid and were estimated to the nearest 0.1 mm by eye. Weight was read to the nearest 0.0001 g from an electronic balance. All measurements were taken before feeding the fishes. The fishes were measured by four different observers. The repeatability between observers was very high (TL: r=0.996, p<0.001, body mass: r=1.0, p<0.001; calculated after Lessells & Boag 1987).
The experiment targeted females only and consisted of two phases. The first phase covered the entire juvenile period until maturation (defined as the time of the first breeding attempt), during which the test fishes were exposed to either high or low food. To be able to compare reproductive schedules between females, it was important to start the second phase at the same developmental stage for all females. As females showed no visible sign of maturation before first spawning, the second phase was started after the first breeding attempt was finished. It served to expose females to their adult environment and to record the target life-history traits (table 1). Males received either high or low food continuously during the entire experiment.
Table 1.
Description of life-history traits measured in experimental females.
| trait (unit) | description |
|---|---|
| SGRL (%×d−1) | specific growth rate of length, , where TL1, TL2, age1 and age2 are initial and final sizes and ages of two successive measurements; as SGRL generally decreases with size, it was corrected for body length throughout |
| mean adult SGRL (%×d−1) | mean SGRL in all measuring intervals of adult individuals with at least four intervals during adulthood |
| maturation | when first spawning took place |
| raising of young | females incubated and produced viable young, rather than swallowing the clutch |
| reproductive lifespan (d) | interval from first to last spawning of a female observed in the experiment |
| spawning rate (d−1) | total number of spawnings of a female divided by its reproductive lifespan |
| rate of raising young (d−1) | total number of successful broods of a female divided by its reproductive lifespan |
| clutch size | number of independent young at the end of brood care |
| offspring size (g) | weight of independent young at the end of broodcare |
| first incubation phase (d) | period between spawning and first food uptake of females and/or young as detected from bite marks on the surface of food cubes |
| second incubation phase (d) | period from end of 1st incubation phase to end of brood care defined as a female not taking up the young in the mouth when disturbed by movements of the observer in front of its tank (for at least 1 day) |
| broodcare duration | total duration of first and second incubation phase |
| reproductive success during reproductive lifespan | two measures were calculated: (i) ‘total number of young’ produced; (ii) ‘total biomass produced’ (clutch size×mean offspring size (g)). The second measure may better reflect female and offspring fitness, as even smallest size differences enhance larvae mobility (Schürch & Taborsky 2005) and survival (McCormick & Hoey 2004) |
| correlation between growth and reproduction | growth: total length increment of females per day in the first 10 measuring intervals after maturation (cm×d−1) |
| reproduction: female mean clutch biomass at the end of brood care (g) | |
| correlation between number and size of young | number: clutch size of individual broods |
| size: mean offspring size of individual broods (g) |
(c) First phase of experiment
I introduced 120 young S. pleurospilus to the experimental tanks between 29 November 2001 and 17 June 2002. The day a fish was placed in its tank was defined as age0 for this individual. Neighbouring tanks were alternately assigned to high and low-food treatment. Siblings were placed in neighbouring tanks in random order. By this means, broods were equally split between treatments. At an age of about six months sexes could be distinguished. There were 55 females (27 Hjuv, 26 Ljuv) and 64 males (32 Hjuv, 32 Ljuv). One fish had died earlier.
Females received a male at a mean age of 202 d (s.e.±1.8 d). The age when females received their first male was determined beforehand as being three weeks before the earliest age I ever observed spawning to occur in S. pleurospilus during previous studies (B. Taborsky, unpublished data). On average, females spawned about two months after receiving a male (mean±s.e.: 56.6±14.6 d). Eighteen females matured earlier than expected, however, and already spawned once before receiving a mate. All sibling females received the first male simultaneously irrespective of treatment. Males were chosen randomly from the experimental fishes, with the constraint that they were at least 168 days old and were not a sibling of the assigned female.
Newly introduced males were separated from the female for 5 days by a 4 mm plastic mesh, allowing water exchange between male and female compartments. After 5 days of habituation, the mesh was removed for 6–8 h each day. For the remaining part of the day and at night males and females were separated by the mesh to allow individual feeding of fishes and to prevent males from injuring females when not under the control of the experimenters. If males persistently attacked a female during daytime, they were exchanged for a new male.
The day after spawning, males were transferred back to their home tank. Forty-six females (23 Hjuv and 23 Ljuv) spawned at least once and were included in the second phase of the experiment. Three females died before first spawning, and three Hjuv and three Ljuv females never spawned, for unknown reasons.
(d) Second phase of experiment
After the termination of the first breeding attempt, the second experimental phase started and females were assigned to their adult food treatment. This occurred at a mean age of 239 d (s.e.±24.0 d) and 293 d (s.e.±16.9 d) in Hjuv and Ljuv females, respectively. Among the Hjuv and the Ljuv females, siblings were assigned alternately to the high- and low-adult food levels to achieve equal brood splitting for the adult treatment. This procedure resulted in sample sizes of 13, 10, 11 and 12 for the four treatment groups with high–high (HH), high–low (HL), low–high (LH) and low–low (LL) food. For each brood, I recorded spawning date and incubation duration. Size and weight of females and of each young were measured as described above at the end of the ‘first’ and ‘second incubation phase’ (table 1). Some females did not raise their clutch, but swallowed the eggs after up to 5 days of incubation. Over the entire experiment, 23 of 46 females never raised a brood successfully, irrespective of juvenile (Fisher-exact test, p=0.24, nH=23, nL=23) or adult treatment (p=0.77, nH=24, nL=22). The day after spawning the male was removed. After a breeding attempt was finished, females remained solitary for another 5 days for recovery. Then a new male was introduced following the same procedure as for the first male. No female received the same male twice.
(e) Termination of experiment
Between June and November 2003, the number of spawnings declined steadily in the experimental population (11.9 spawnings±0.89 s.e. in the ten months before June; 7, 8, 6, 4, 2, 3 spawnings per month, respectively, between June and November). At the end of November 2003, I terminated the 4-weekly size measurements, but continued with the food treatment and monitoring of reproduction until end of May 2004. Only 5.0% of all spawnings (n=8) in this experiment occurred after November with the last one occurring on 18 February 2004. The dates of first and last spawning of individual females were positively correlated (r=0.39, p=0.033, n=30 females that spawned at least twice and survived until May 2004), indicating that females were reproductively active for similar lengths of time. These results suggest that the entire reproductive lifespan of females under the conditions provided was included in this experiment. The reproductive lifespan of females may be different, however, under different environmental conditions.
(f) Data analyses
To test for treatment effects on adult life-history traits, I calculated two-way analyses of variance with juvenile treatment (JUV) and adult treatment (AD) as factors and individual females as independent units of analysis (mean trait values per female used). If necessary, confounding variables were controlled for by including them as covariates.
Most females did not raise their first clutch (see §3), a phenomenon generally observed in mouthbrooding cichlids. Of those females that did raise their first clutch, a certain proportion was switched to the opposite food level after incubation, according to the experimental protocol. As the first young of these females were produced still under the previous (juvenile) food conditions, data of these clutches were analysed together with data of the respective non-switched groups.
For the analysis of trade-offs, I calculated correlation coefficients for growth versus clutch volume and number versus size of young, separately for the four treatment groups. Female size did not correlate consistently across the four treatments with any of the four variables. Therefore, female size was not included as a covariate in these analyses, despite an overall relationship of female size with clutch volume and clutch size.
As a trade-off between number and size of young exists only at the level of broods, the correlations between number and size of young were calculated for individual broods (n=54), with females contributing on average 1.9 broods (±0.18 s.e., range 1–4) to the sample.
Statistical analyses were done with SPSS 10.0, SPSS Inc., Chicago. Figures show untransformed results. Data for ANOVA models were log-transformed (see table 2), if variances were not homogeneous (Levene's test) or the model residuals were not normally distributed (Kolmogorov–Smirnov test). If the conditions for parametric testing were still not met after transformation, non-parametric tests were used.
Table 2.
(a) Analyses of variance testing the effect of juvenile (JUV) and adult treatment (AD) and their interaction on dependent variables associated with growth and reproduction of S. pleurospilus; (b) means±s.e. of the dependent variables for the four treatment groups of original, untransformed data.
| (a) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SGRLa in the first 28 d of AD | mean SGRL of adultsa | reproductive lifespana | spawning ratea,b | rate of raising younga | clutch sizea,c | residual clutch sizea,d | offspring sizea,c | corrected offspring sizec | 1st incubation phasea | 2nd incubation phasea | total number of younga | total biomass produceda | ||
| d.f. | 4,41 | 4,38 | 3,25 | 3,30 | 3,31 | 3,26 | 3,26 | 3,25 | 4,24 | 3,25 | 3,25 | 3,19 | 3,19 | |
| R2 | 0.59 | 0.67 | 0.06 | 0.11 | 0.23 | 0.56 | 0.18 | 0.35 | 0.48 | 0.22 | 0.07 | 0.32 | 0.29 | |
| full model | F | 14.86 | 19.65 | 0.51 | 1.20 | 3.01 | 10.88 | 1.90 | 4.41 | 5.58 | 2.34 | 0.62 | 2.95 | 2.63 |
| p | <0.001 | <0.001 | 0.679 | 0.362 | 0.045 | <0.001 | 0.154 | 0.013 | 0.003 | 0.097 | 0.610 | 0.059 | 0.080 | |
| covariate | initial TL | mean TL | brood care durationa | |||||||||||
| F | 34.07 | 24.82 | 6.30 | |||||||||||
| p | <0.001 | <0.001 | 0.019 | |||||||||||
| main effects | ||||||||||||||
| JUV | F | 1.47 | 0.19 | 0.18 | 3.54 | 5.24 | 19.36 | 3.27 | 11.96 | 15.60 | 0.28 | 0.27 | 2.89 | 1.43 |
| p | 0.232 | 0.665 | 0.673 | 0.070 | 0.029 | <0.001 | 0.082 | 0.002 | 0.001 | 0.599 | 0.608 | 0.106 | 0.246 | |
| AD | F | 24.35 | 74.67 | 0.03 | 0.02 | 2.01 | 8.38 | 1.59 | 1.12 | 1.26 | 7.0 | 1.53 | 5.28 | 6.15 |
| p | <0.001 | <0.001 | 0.874 | 0.876 | 0.166 | 0.008 | 0.219 | 0.301 | 0.270 | 0.014 | 0.228 | 0.033 | 0.023 | |
| JUV×AD | F | 0.07 | 0.04 | 1.36 | 0.01 | 1.07 | 0.68 | 0.59 | 0.41 | 0.94 | 0.96 | 0.001 | 1.04 | 0.73 |
| p | 0.793 | 0.847 | 0.255 | 0.910 | 0.309 | 0.417 | 0.448 | 0.528 | 0.343 | 0.388 | 0.982 | 0.321 | 0.404 | |
| (b) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| treatment | SGRLa in the first 28 d of AD | mean SGRL of adultsa | reproductive lifespana | spawning ratea,b | rate of raising younga | clutch sizea | offspring sizea | 1st incubation phasea | 2nd incubation phasea | total number of younga | total biomass produceda |
| HH | 0.069 (0.010; 13) | 0.030 (0.0032; 12) | 239.6 (55.5; 7) | 0.016 (0.0015; 8) | 0.0046 (0.0017; 9) | 18.9 (3.1; 5) | 0.044 (0.0015; 5) | 16.1 (0.57; 5) | 13.1 (0.93; 5) | 43.0 (7.8; 5) | 1.93 (0.39; 5) |
| HL | 0.029 (0.008; 10) | 0.015 (0.0029; 9) | 311.4 (55.3; 7) | 0.015 (0.0025; 8) | 0.0037 (0.0014; 8) | 11.2 (1.6; 4) | 0.043 (0.0022; 4) | 13.2 (0.48; 4) | 15.2 (1.54; 4) | 22.2 (4.7; 4) | 0.95 (0.23; 4) |
| LH | 0.078 (0.012; 11) | 0.036 (0.0024; 11) | 279.6 (57.4; 8) | 0.022 (0.0032; 10) | 0.013 (0.0036; 10) | 9.2 (0.8; 8) | 0.063 (0.0062; 8) | 15.7 (0.61; 8) | 12.3 (1.25; 8) | 26.0 (5.9; 8) | 1.33 (0.26; 8) |
| LL | 0.041 (0.011; 12) | 0.019 (0.0024; 11) | 225.1 (45.8; 7) | 0.021 (0.0041; 8) | 0.0068 (0.0018; 8) | 7.1 (0.6; 13) | 0.054 (0.0033; 12) | 14.4 (0.69; 12) | 14.3 (1.41; 12) | 18.0 (4.4; 6) | 0.85 (0.20; 6) |
See definition in table 1.
Excluding one outlier (Dixon's test for outliers, p<0.01).
Variable was log-transformed for analysis.
Means (per female) of the regression residuals of individual clutches versus female length measured at the end of incubation; correcting each clutch individually for the respective female size yields the highest possible precision, as female size increased nonlinearly during adulthood.
3. Results
(a) Juvenile growth
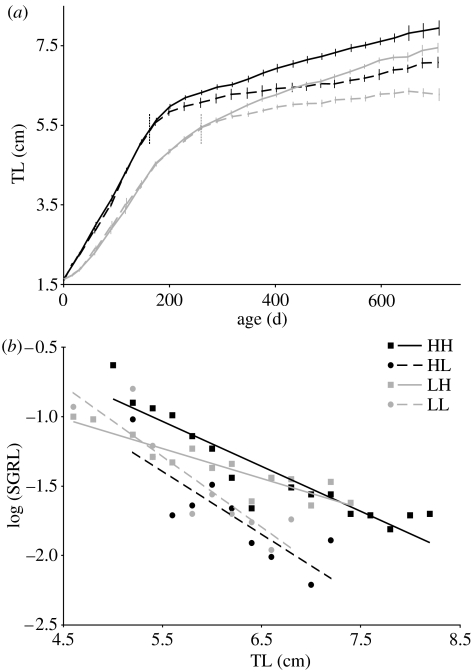
Juvenile growth was almost linear until an age of about 170 d (except a short phase of decelerated growth directly after independence in Ljuv fishes) and diverged markedly between treatments before maturation (figure 1a). As expected, juvenile growth (‘SGRL’, see table 1) was significantly faster in Hjuv than in Ljuv females (nested ANCOVA, JUV: F1,55.8=195.82, p<0.001; female(JUV): F44,386=1.14, p=0.26; TL: F1,386=512.04, p<0.001). The juvenile growth trajectories between females in the two treatment groups did not overlap at all.
Figure 1.
Growth patterns of females raised with high–high (HH), high–low (HL), low–high (LH) and low–low (LL) food rations. (a) Means (±s.e.) of repeated measurements of total length (TL); vertical stippled lines indicate the mean age of first spawning of Hjuv and Ljuv females. (b) Specific growth rates of length (SGRL; log-transformed) in relation to female total length; means per treatment of 2 mm size classes are shown; slopes of LH and HH females differed (test for parallelism after Kleinbaum & Kupper 1978, pp. 99–103; t27,0.975=−2.22, p<0.05), while slopes of HL and LL did not (t16,0.975=0.57, p>0.1).
(b) Adult growth
Around the mean age of maturation, growth slowed down in all females (figure 1a). In addition, there was already a marked effect of changed rations after four weeks (table 2a). LH females grew faster and HL females decelerated growth, compared to females whose ration did not change (table 2b). Over the entire adult period, specific growth rates were higher in females fed the high adult ration compared to females kept on the low ration, while the juvenile treatment had no significant effect. This was the case both when comparing mean adult SGRL between 4 weekly measurements (table 2), and when analysing SGRL over the entire range of body sizes (juvenile treatment: JUV: F1,51.2=2.52, p<0.12; female(JUV): F42,515=4.80, p<0.001; TL: F1,515=119.61, p<0.001; adult treatment: AD: F1,75.9=66.99, p<0.001; female(AD): F42,515=1.87, p=0.001; TL: F1,515=119.61, p<0.001; nested ANCOVAs; mean growth rates versus size are shown in figure 1b). At the end of the experiment, LH females were still significantly smaller than HH females (Mann-Whitney U-test, U=6.0, p=0.005, n1,2=8, 8).
(c) Reproductive schedules
Hjuv fishes spawned for the first time earlier (U-test, U=139.5, p=0.006, n=23, 23) but at a larger size than Ljuv fishes (U=147, p<0.01; figure 2a). Only 9 females raised young successfully when spawning for the first time, while the remaining females swallowed their eggs within 5 d after spawning. The probability of raising the first clutch successfully did not depend on juvenile treatment (Fisher-exact test, p=0.192, n=27, excluding females without access to males). However, the interval between the first breeding attempt and first successful raising of young was longer in Hjuv than in Ljuv females (U=22, p=0.008, n=9, 14). This interval still tended to be longer in Hjuv females when only HH and LL females were compared (figure 2a, U=5.5, p=0.082, n=5, 6) suggesting that this result is not primarily caused by a change of the food regime in half of the females.
Figure 2.
Reproductive traits of females raised with different food rations (female means±s.e. are shown except in (a)). (a) Age and size at two developmental stages of females receiving high (black) and low (grey) food; data for first raising are shown only for females receiving the same ration throughout life (HH and LL); medians and quartiles for age and size are shown. (b) Rate of raising successful broods over the reproductive lifespan. (c) Weight of young (means per female of brood means) at the end of brood care. (d) Total biomass of young produced during the reproductive lifespan of females that raised young successfully at least once.
The reproductive lifespan of females was not affected by juvenile or adult treatment (table 2, excluding females that died from diseases or after male aggression). However, females that grew up with little food raised broods at a faster rate than Hjuv females, irrespective of adult treatment (figure 2b, table 2). Similarly, spawning rates tended to be higher in females raised with little food compared to Hjuv females, while the adult treatment did not affect spawning rates (table 2).
(d) Offspring production
Both adult and juvenile treatment influenced clutch size (Had>Lad and Hjuv>Ljuv, table 2). However, due to the different food rations, females differed in size between treatments. Overall, clutch size increased with female size at clutch production (regression analysis, d.f.=1,53, R2=0.53, p<0.001). The treatment effects on clutch size vanished when including female size as a covariate (table 2a).
Remarkably, at the end of brood care independent young of females raised in poor conditions were longer (Taborsky in press) and heavier (figure 2c, table 2) than young of Hjuv females, regardless of which adult treatment their mothers received. Female size was not related to offspring mass (regression analysis, d.f.=1,27, R2<0.001, p=0.92), and was therefore not included as a covariate.
Size differences of independent young might result from potential differences in incubation duration between treatments. However, the results remained unaltered when correcting for the total brood care duration (table 2a). Moreover, the first incubation phase was shorter for offspring of mothers receiving low food as adults, while the length of the second incubation phase did not differ between treatments (table 2).
(e) Reproductive success
Females receiving the high-food ration as adults (Had) produced more young and a higher biomass over their reproductive lifespan (cf. ‘reproductive success’ in table 1) than Lad females (figure 2d, table 2). Remarkably, there were no significant interactions between treatments when analysing their effects on reproductive success (table 2) and, accordingly, the reproductive success of females kept under same adult but different juvenile conditions did not differ significantly (number of young: HH versus LH: U=8.0, p=0.093, n1,2=5, 8; HL versus LL: U=8.0, p=0.48, n1,2=4, 6; biomass of young: HH versus LH: U=11.0, p=0.22; HL versus LL: U=9.0, p=0.61, Mann-Whitney U-tests).
(f) Life-history trade-offs
The correlations between growth and reproduction and between number and size of offspring (see table 1 for definitions) were similar for females with the same juvenile treatment, but differed markedly between adult treatments. In HH females (figure 3a; Kendall's τ=−0.80, p=0.05, n=5), and HL females (τ=−1.0, p=0.042, n=4) clutch mass decreased with increasing growth rate, while in LH and LL females clutch mass increased slightly with growth rate but these correlations were not significant. In females raised with the low-food ration the size of offspring decreased significantly with increasing clutch size (Pearson correlation coefficients; LH: r=−0.71, p=0.003, n=15; LL: r=−0.72, p<0.001, n=19), while the correlations for HH and HL were only weakly negative and not significant (figure 3b).
Figure 3.
Relationships between (a) growth and mean clutch biomass at end of brood care and (b) clutch size and mean offspring size per brood (see table 1 for explanation of variables); lines represent least-square trendlines for each treatment group.
4. Discussion
In S. pleurospilus, the rate of clutch production, offspring size and two major life-history trade-offs were determined by the growth conditions mothers encountered as juveniles. In contrast, there was no long-lasting effect of juvenile environment on the growth rates of adults, which were flexibly adjusted to ambient food conditions. While it is known that an individual's ecology during early development can influence certain life-history traits and fitness later in life (reviewed in Schlichting & Pigliucci 1998; Lindström 1999), these results demonstrate that juvenile ecology can determine suites of reproductive traits and key life-history trade-offs over the entire adult life in long-lived, iteroparous animals. The existence of such suites may results from a feedback loop triggered by size-dependent mortality.
In my experiment, females were switched to the adult treatment after their first breeding attempt, i.e. at a certain developmental stage rather than at a certain age. This procedure was chosen to reflect the behaviour of the fishes under natural conditions. When starting to breed, both sexes move to deeper water, where males start to defend breeding territories. In the experiment, first spawning occurred at a mean size of 5.7 cm, which coincides with the size when S. pleurospilus perform the habitat switch in Lake Tanganyika (B. Taborsky, unpublished data). As the first breeding attempt was the only visible sign of maturation in females, the adult treatment began immediately afterwards. Hence the juvenile treatment phase covered the entire phase of ‘early development’ (the time from birth to developmental maturity, Lindström 1999), plus the period of ovary maturation of their first clutch, which is short (about 2–3 weeks in Tropheini, Yanagisawa & Nishida 1991; B. Taborsky, unpublished data) relative to the entire treatment period (mean 257 d).
The manipulation of food rations during the juvenile period resulted in strongly diverging growth trajectories. In accordance with many general life-history models I expected slowly growing fishes to start reproducing as early as possible, while the fast growing group should delay reproduction (reaction norm of size and age at maturation has positive slope). In contrast, first spawning occurred at a smaller size but later age in Ljuv females. Such reaction norms with negative slope have been frequently found in empirical studies (reviewed by Day & Rowe 2002). A general life-history model presented by Day and Rowe predicted a positive slope of the age–size reaction norm when no restrictive assumptions were made. This slope changed and became negative, however, when the authors introduced a minimum size threshold for maturation to the model. If such a size threshold exists in S. pleurospilus, which is suggested by strongly right-skewed length (skewness 0.93) and weight (skewness 1.77) distributions at maturation, then this could explain the results for age and size at first spawning in S. pleurospilus. Hjuv females grew further over the minimum size threshold than Ljuv females, but still matured at an earlier age than Ljuv females.
In S. pleurospilus, the first substantial reproductive investment is made when females raise the first clutch successfully. A large clutch volume of yolk-rich eggs is produced and females starve during most of the incubation period, while in unsuccessful breeding attempts females largely recover the energy contained in eggs by consuming them. Hjuv females took longer from first spawning until raising their first young. They started to raise young at a larger size but slightly later age than Ljuv females. Hence the onset of successful reproduction of the females is in line with the prediction of delayed maturation. Delayed maturation may be an adaptation to environments with size-dependent mortality risk, where faster growth strongly enhances survival chances (cf. model predictions by Stearns & Koella 1986; Taborsky et al. 2003).
Fishes often respond to short-term changes in food rations by flexibly adjusting growth rates (reviewed in Metcalfe & Monaghan 2001; Ali et al. 2003). In this study, rations were changed after a period of 6–12 months. Still, LH females accelerated growth immediately after the food switch, while HL females almost ceased growing, which shows that growth remains flexible in these fishes, probably throughout life. Increasing the growth rate may enhance the fitness of females switching from a poor to rich habitat in two ways. (i) Generally, larger females are more fecund, and in absolute terms LH females indeed produced larger clutches than LL females. (ii) Even small size increments should decrease mortality risk under natural conditions (Sogard 1997; Taborsky et al. 2003), where S. pleurospilus are mainly predated by gape-size limited predators, i.e. other fishes. Although LH females clearly accelerated their growth after the switch to the high-food ration, they did not show compensatory growth (sensu Ali et al. 2003) as they grew slower than same-sized HH females (cf. figure 1b). According to Ali et al. (2003), compensatory growth occurs when growth-depressed animals grow significantly faster than control animals that have not experienced growth depression.
In contrast to growth rates, several important reproductive traits were affected by juvenile but not by adult treatment. Ljuv females produced successful clutches at a faster rate than Hjuv females, suggesting adjustment of reproductive strategies to natural mortality risk. Ljuv females, which are still small when they become adult, would face a higher predation risk, favouring fast reproductive rates (e.g. Taborsky et al. 2003) and high reproductive investment (e.g. Roff 1992).
Ljuv females produced heavier young at independence, while adult treatment did not influence offspring size. This result cannot be explained by differences in the pattern or total duration of brood care. In principle, it would be possible that the weight differences of young could have been caused by differential levels of food competition in tanks containing different numbers of offspring. If food limitation indeed had determined the size of young, a strong negative correlation between number and size of young would be expected when food is limited most (i.e. in the HL group, where females and clutches are relatively large, but food is scarce), while it should be flat when most food relative to fish biomass is available, i.e. in the LH group. However, the opposite was the case (cf. figure 3b). Direct observations also suggested that the food of young consisted mainly of detritus and algae, which were plentiful in all tanks, and that the young were, on the whole, not dependent on the food cubes provided for the mother (B. Taborsky, personal observation).
Apparently, Ljuv females provided more energy for their offspring right from the start. They produced eggs with a higher dry weight, resulting in young already being significantly larger for their age after the first incubation phase (Taborsky in press), during which they consume only yolk reserves. It appears as if females tailor offspring size to the environmental conditions they themselves encountered during ontogeny. Several studies have shown that larger offspring have survival advantages under adverse growth conditions, while under good conditions small young do equally well (Hutchings 1991; Mousseau & Fox 1998; Einum & Fleming 1999) or even better (Kaplan 1992). Over a much shorter time scale guppies (Poecilia reticulata) also adjusted offspring size to past food conditions. When food availability was manipulated during two successive between-brood intervals, offspring size after the second interval depended on the ration of the first but not of the second interval (Reznick & Yang 1993). In contrast, the ration in two subsequent inter-spawning intervals had no effect on egg size in sticklebacks, Gasterosteus aculeatus, but the length of the second interspawning-interval was influenced by the ration received during the first interval (Ali & Wootton 1999).
Notably, the overall reproductive success depended only on the energy supply during adulthood. Females receiving the high-food ration as adults produced more young and a higher total clutch biomass than Lad females, while the reproductive success of HH and LH, and of HL and LL did not differ, respectively. LH females combined the rapid production of large young (juvenile treatment effect) with a slight fecundity advantage (compared to LL; adult treatment effect), which was achieved by accelerating growth. As they did not do significantly worse than HH females, their strategy may represent an adaptive, plastic response to the food manipulations. Still, total numbers of young and clutch biomass of LH females were slightly lower than those of HH females, so I cannot exclude that with a larger sample size the difference may have been statistically significant. HL females, on the other hand, reproduced slowly and had small young like HH females, but produced smaller clutches. Despite superior juvenile conditions they did not perform better than LL females, apparently because of energy limitations caused by a mismatch of large size achieved as juveniles and the small ration received as adults. Larger individuals are most severely affected if food supply becomes short (Wikelski & Thom 2000; Bateson et al. 2004).
The expected negative relationship between growth and reproduction was present only in the groups raised with high food, while number and size of young correlated negatively in the groups raised with little food. Absence of negative correlations in the remaining groups does not imply that the respective trade-offs did not affect these fishes (Reznick 1985; van Noordwijk & de Jong 1986). The direction of phenotypic correlations between life-history traits depends on the relative variation of resource acquisition and allocation (van Noordwijk & de Jong 1986). The variation of resource acquisition was probably lower among individuals receiving the same food rations while the trade-off was measured (i.e. as adults) than among those receiving different rations. Hence the observed variation in trade-offs was probably caused by variation in allocation patterns. The fact that allocation depended on juvenile but not adult conditions again points towards an early determined strategy that is affected relatively little later in life. Allocation pathways may be triggered initially by nutritional conditions and then become fixed as shown for a number of morphological, physiological and behavioural traits (reviewed in Bateson 2001).
In conclusion, the results reported here suggest that the juvenile growth history exhibits a life-long effect on parental reproductive schedules, investment in offspring and key life-history trade-offs. Early individual history may thereby contribute substantially to life-history variation found in natural populations.
Acknowledgments
I am grateful to M. Taborsky for constructive comments and help at each stage of the study, Ian Hamilton and two anonymous referees for valuable comments on an earlier draft of this manuscript, and Z. Bachar, D. Bonfils, R. Eggler C. Grüter, N. Hirt, S. Immler, S. Lehner, R. Schürch and P. Vonlanthen for help with logistics and data collection. The author was financed by the Austrian Science Fund (FWF grant P14327-B06) and the Forschungsstiftung, University of Bern (48/2003). This study was conducted under licence No. 46/01-03, Kantonales Veterinäramt, Bern, Switzerland.
References
- Ali M, Wootton R.J. Effect of variable food levels on reproductive performance of breeding female three-spined sticklebacks. J. Fish. Biol. 1999;55:1040–1053. 10.1111/j.1095-8649.1999.tb00739.x [Google Scholar]
- Ali M, Nicieza A, Wootton R.J. Compensatory growth in fishes: a response to growth depression. Fish Fish. 2003;4:147–190. [Google Scholar]
- Arendt J.D. Allocation of cells to proliferation vs. differentiation and its consequences for growth and development. J. Exp. Zool. 2000;288:219–234. 10.1002/1097-010X(20001015)288:3%3C219::AID-JEZ3%3E3.0.CO;2-C [PubMed] [Google Scholar]
- Aune A, Imsland A.K, Pittman K. Growth of juvenile halibut, Hippoglossus hippoglossus (L.), under a constant and switched temperature regime. Aquacult. Res. 1997;28:931–939. 10.1046/j.1365-2109.1997.00924.x [Google Scholar]
- Bateson P. Fetal experience and good adult design. Int. J. Epidemol. 2001;30:928–934. doi: 10.1093/ije/30.5.928. 10.1093/ije/30.5.928 [DOI] [PubMed] [Google Scholar]
- Bateson P, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. 10.1038/nature02725 [DOI] [PubMed] [Google Scholar]
- Berrigan D, Koella J.C. The evolution of reaction norms—simple models for age and size at maturity. J. Evol. Biol. 1994;7:549–566. 10.1046/j.1420-9101.1994.7050549.x [Google Scholar]
- Cavalier-Smith T. R- and K-tactics in the evolution of protist developmental systems: cell and genome size, phenotype diversifying selection, and cell cycle patterns. BioSystems. 1980;12:43–59. doi: 10.1016/0303-2647(80)90037-4. 10.1016/0303-2647(80)90037-4 [DOI] [PubMed] [Google Scholar]
- Clark C.W, Mangel M. Oxford series in ecology and evolution. Oxford University Press; Oxford, UK: 2000. Dynamic state variable models in ecology. [Google Scholar]
- Day T, Rowe L. Developmental thresholds and the evolution of reaction norms for age and size at life-history transitions. Am. Nat. 2002;159:338–350. doi: 10.1086/338989. 10.1086/338989 [DOI] [PubMed] [Google Scholar]
- Desai M, Hales C.N. Role of fetal and infant growth in programming metabolism in later life. Biol. Rev. 1997;72:329–348. doi: 10.1017/s0006323196005026. 10.1017/S0006323196005026 [DOI] [PubMed] [Google Scholar]
- Einum S, Fleming I.A. Maternal effects of egg size in brown trout (Salmo trutta): norms of reaction to environmental quality. Proc. R. Soc. B. 1999;266:2095–2100. 10.1098/rspb.1999.0893 [Google Scholar]
- Gotthard K. Increased risk of predation as a cost of high growth rate: an experimental test in a butterfly. J. Anim. Ecol. 2000;69:896–902. doi: 10.1046/j.1365-2656.2000.00432.x. 10.1046/j.1365-2656.2000.00432.x [DOI] [PubMed] [Google Scholar]
- Houston A.I, McNamara J.M. Cambridge University Press; Cambridge, UK: 1999. Models of adaptive behaviour. [Google Scholar]
- Huck U.W, Labov J.B, Lisk R.D. Food-restricting 1st generation juvenile female hamsters (Mesocricetus auratus) affects sex-ratio and growth of 3rd generation offspring. Biol. Reprod. 1987;37:612–617. doi: 10.1095/biolreprod37.3.612. 10.1095/biolreprod37.3.612 [DOI] [PubMed] [Google Scholar]
- Hutchings J.A. Fitness consequences of variation in egg size and food abundance in Brook Trout Salvelinus fontinalis. Evolution. 1991;45:1162–1168. doi: 10.1111/j.1558-5646.1991.tb04382.x. [DOI] [PubMed] [Google Scholar]
- Kaplan R.H. Greater maternal investment can decrease offspring survival in the frog Bombina orientalis. Ecology. 1992;73:280–288. [Google Scholar]
- Kleinbaum D.G, Kupper L.L. Duxbury Press; Boston, MA: 1978. Applied regression analysis and other multivariable methods. [Google Scholar]
- Lessells C.M, Boag P.T. Unrepeatable repeatabilities—a common mistake. Auk. 1987;104:116–121. [Google Scholar]
- Lindström J. Early development and fitness in birds and mammals. Trends Ecol. Evol. 1999;14:343–348. doi: 10.1016/s0169-5347(99)01639-0. [DOI] [PubMed] [Google Scholar]
- Lummaa V, Clutton-Brock T. Early development, survival and reproduction in humans. Trends Ecol. Evol. 2002;17:141–147. 10.1016/S0169-5347(01)02414-4 [Google Scholar]
- McCormick M.I, Hoey A.S. Larval growth history determines juvenile growth and survival in a tropical marine fish. Oikos. 2004;106:225–242. 10.1111/j.0030-1299.2004.13131.x [Google Scholar]
- Metcalfe N.B, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. 10.1016/S0169-5347(01)02124-3 [DOI] [PubMed] [Google Scholar]
- Mousseau T.A, Fox C.W. The adaptive significance of maternal effects. Trends Ecol. Evol. 1998;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. 10.1016/S0169-5347(98)01472-4 [DOI] [PubMed] [Google Scholar]
- Reznick D.N. Costs of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. [Google Scholar]
- Reznick D.N. Plasticity in age and size at maturity in male guppies (Poecilia reticulata)—an experimental evaluation of alternative models of development. J. Evol. Biol. 1990;3:185–203. 10.1046/j.1420-9101.1990.3030185.x [Google Scholar]
- Reznick D.N, Yang A.P. The influence of fluctuating resources on life-history patterns of allocation and plasticity in female guppies. Ecology. 1993;74:2011–2019. [Google Scholar]
- Reznick D.N, Callahan H, Llauredo R. Maternal effects on offspring quality in poeciliid fishes. Am. Zool. 1996;36:147–156. [Google Scholar]
- Roff D.A. Chapman & Hall; New York: 1992. The evolution of life histories. [Google Scholar]
- Schlichting C.D, Pigliucci M. Sinauer Associates; Sunderland, MA: 1998. Phenotypic evolution: a reaction norm perspective. [Google Scholar]
- Schürch R, Taborsky B. The functional significance of buccal feeding in the mouthbrooding cichlid Tropheus moorii. Behaviour. 2005;142:265–281. [Google Scholar]
- Siems D.P, Sikes R.S. Tradeoffs between growth and reproduction in response to temporal variation in food supply. Environ. Biol. Fish. 1998;53:319–329. 10.1023/A:1007407925835 [Google Scholar]
- Sinervo B, Doughty P. Interactive effects of offspring size and timing of reproduction on offspring reproduction: experimental, maternal, and quantitative genetic aspects. Evolution. 1996;50:1314–1327. doi: 10.1111/j.1558-5646.1996.tb02371.x. [DOI] [PubMed] [Google Scholar]
- Sogard S.M. Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull. Mar. Sci. 1997;60:1129–1157. [Google Scholar]
- Stearns S.C.The evolution of life histories1992Oxford University Press; Oxford, UK [Google Scholar]
- Stearns S.C, Koella J.C. The evolution of phenotypic plasticity in life-history traits: predictions of reaction norms for age and size at maturity. Evolution. 1986;40:893–913. doi: 10.1111/j.1558-5646.1986.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Taborsky B. Size-dependent distribution in littoral fish: optimization or competitive exclusion? In: Almada V.C, Oliveira R.F, Goncalves E.J, editors. Behaviour and conservation of littoral fishes. ISPA; Lisboa: 1999. pp. 351–376. [Google Scholar]
- Taborsky B. Mothers determine offspring size in response to own filial growth conditions. Biol. Lett. In press doi: 10.1098/rsbl.2005.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taborsky B, Dieckmann U, Heino M. Unexpected discontinuities in life-history evolution under size-dependent mortality. Proc. R. Soc. B. 2003;270:713–721. doi: 10.1098/rspb.2002.2255. 10.1098/rspb.2002.2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto G. Adaptive plasticity in ontogenetic niche shifts stabilizes consumer-resource dynamics. Am. Nat. 2003;162:93–109. doi: 10.1086/375540. 10.1086/375540 [DOI] [PubMed] [Google Scholar]
- van Noordwijk A.J, de Jong G. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 1986;128:137–142. 10.1086/284547 [Google Scholar]
- Werner E.E, Gilliam J.F. The ontogenetic niche and species interactions in size structured populations. Annu. Rev. Ecol. Syst. 1984;15:393–425. 10.1146/annurev.es.15.110184.002141 [Google Scholar]
- Wikelski M, Thom C. Marine iguanas shrink to survive El Nino—changes in bone metabolism enable these adult lizards to reversibly alter their length. Nature. 2000;403:37–38. doi: 10.1038/47396. 10.1038/47396 [DOI] [PubMed] [Google Scholar]
- Wootton R.J. Chapman & Hall; London: 1990. Ecology of teleost fishes. [Google Scholar]
- Yanagisawa Y, Nishida M. The social and mating system of the maternal mouthbrooder Tropheus moorii (Cichlidae) in Lake Tanganyika. Jpn. J. Ichthyol. 1991;38:271–282. [Google Scholar]