Abstract
Insect flight muscle is known for its crystal-quality regularity of contractile protein arrangement within a sarcomere. We have previously shown by X-ray microdiffraction that the crystal-quality regularity in bumble-bee flight muscle is not confined within a sarcomere, but extends over the entire length of a myofibril (>1000 sarcomeres connected in series). Because of this, the whole myofibril may be regarded as a millimetre-long, natural single protein crystal. Using bright X-ray beams from a synchrotron radiation source, we examined how this long-range crystallinity has evolved among winged insects. We analysed >4600 microdiffraction patterns of quick-frozen myofibrils from 50 insect species, covering all the major winged insect orders. The results show that the occurrence of such long-range crystallinity largely coincides with insect orders with asynchronous muscle operation. However, a few of the more skilled fliers among lower-order insects apparently have developed various degrees of structural regularity, suggesting that the demand for skilful flight has driven the lattice structure towards increased regularity.
Keywords: synchrotron radiation, microbeam, single myofibril, single sarcomere, liquid-nitrogen temperature
1. Introduction
In striated muscles, the filaments of contractile proteins (myofilaments) are packed into a regular hexagonal lattice array within a sarcomere, the building block and minimal functional unit of muscle (length, 2–3 μm). In some insect flight muscles, the corresponding contractile protein molecules in the neighbouring filaments are in a high degree of register, so that the whole sarcomere may be regarded as a de facto protein crystal. This degree of register is evident from the conventional fibre X-ray diffraction recordings (i.e. the beam is irradiated perpendicular to the muscle fibre axis: see figure 1b), which generate numerous reflections sharply sampled into arrays of isolated spots, much like in the diffraction patterns from artificially grown protein crystals (Worthington 1961; Holmes et al. 1980; Tregear et al. 1990, 1998; Dickinson et al. 2005).
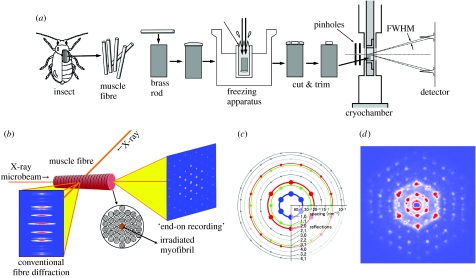
Figure 1.
Methods and principle of end-on cryomicrodiffraction recording from a single myofibril. (a) Method of sample preparation and X-ray recording. FWHM, full width at half maximum. (b) Principle of end-on diffraction recording from a single myofibril within a flight muscle fibre, as contrasted with the conventional method of fibre diffraction. (c) Expected positions of reflections indexed to a hexagonal lattice of myofilaments in insect flight muscle. The coloured circles represent relatively strong reflections, while the grey circles, relatively weak reflections (reproduced from Iwamoto et al. 2002, with permission). (d) One of the best-resolved examples of end-on cryomyocrodiffraction patterns (Aulacophora femoralis).
Figure 2.
Gallery of end-on diffraction patterns from myofibrils. (a–av) Flight and tymbal muscles. Hymenoptera: (a) Xylocopa; (b) Bombus; (c) Formicidae; (d) Ichneumonidae; (e) Arge. Diptera: (f) Tabanus; (g) Eupyrgota; (h) Tipula. Coleoptera: (i) Aulacophora; (j) Oxycetonia; (k) Aiolocaria; (l) Spondylis; (m) Sipalinus; (n) Cicindela. Heteroptera: (o) Nezara; (p) Plautia; (q) Halyomorpha; (r) Physopelta; (s) Lethocerus. Homoptera: (t) Meimuna; (u) Meimuna, tymbal; (v) Terpnosia; (w) Terpnosia, tymbal; (x) Tanna; (y) Orosanga; (z) Bothrogonia; (aa) Aphididae. Thysanoptera: (ab) Thripidae. Psocoptera: (ac) Psococerastis. Lepidoptera: (ad) Odontopera; (ae) Ctenoplusia; (af) Dendrolimus; (ag) Antheraea; (ah) Neogurelca; (ai) Theretra; (aj) Marumba. Trichoptera: (ak) Nemotaulius; (al) Stenopsyche. Mecoptera: (am) Panorpa. Megaloptera: (an) Protohermes. Neuroptera: (ao) Hybris; (ap) Distoleon. Blattaria: (aq) Blattella. Mantodea: (ar) Acromantis. Plecoptera: (as) Perlidae. Orthoptera: (at) Archida; (au) Sinochlora. Odonata: (av) Pseudothemis; (aw) Macromia; (ax) Copera; (ay) Calopteryx. Ephemeroptera: (az) Ephemera. (ba–bc) Leg muscles. (ba) Bombus; (bb) Eupyrgota; (bc) Spondylis. (az) Rabbit psoas. Patterns are displayed in a logarithmic scale after subtraction of the dark current of the charge-coupled device camera (this applies also to figures 1d and 3, and fig.2 in the electronic supplementary material). For full spellings of scientific names see figure 4.
We have previously demonstrated that, in bumble-bee flight muscle, the myofilaments in neighbouring sarcomeres are also in a high degree of register (Iwamoto et al. 2002). Despite the separation of neighbouring sarcomeres by intervening Z-discs, which may in fact govern the relative alignment, the orientation of the lattice planes is strictly preserved (see the top model of figure 3a), and this spatial relationship apparently extends over the entire length of a myofibril (length, ∼3 mm or ∼1000 sarcomeres and diameter, ∼3 μm). This extraordinary feature was revealed by a novel X-ray diffraction technique, combining the use of X-ray microbeam (a few micrometers in diameter) and the end-on configuration of diffraction recording (figure 1b; the beam is irradiated along the myofibrillar axis). This technique enabled us to shoot the microbeam along a single myofibril within a 3 mm-long flight muscle fibre (or muscle cell, of diameter ∼300 μm, which may contain thousands of myofibrils) without mechanically isolating the myofibril. The diffraction pattern recorded in this way consisted of a set of spot reflections, indexable to a single hexagonal lattice, indicating that the entire myofibril contained only one hexagonal lattice.
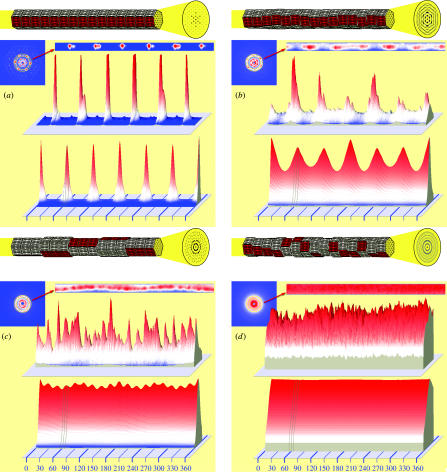
Figure 3.
Analysis of four types of end-on diffraction patterns. (a) Well-registered lattices of Aulacophora femoralis. (b–c) Partially registered lattices of Copera annulata and Neogurelca himachala, respectively. (d) Non-registered lattices from Ctenoplusia albostriata. In each panel, the 2, 0 region of the diffraction pattern is extracted and expressed in a linear strip (top), three-dimensional expression of the intensity (middle), and the angular autocorrelation function (bottom). The triplet of vertical grey lines indicates the angles of 55°, 60° and 65°. The drawing on top of each panel represents the model of lattice register to account for each type of diffraction patterns. The red-coloured facet of each sarcomere depicts its lattice orientation.
Then questions arise as to how this long-range crystallinity has evolved: is this feature seen only in the most advanced insects? Does this feature correlate strictly with phylogeny or is it rather related to muscle functions? What is the regularity in non-flight muscles (e.g. leg muscle)? To address these questions, we have recorded diffraction patterns of myofibrils from flight and non-flight muscles from a variety of species, to cover as many winged insect orders as possible. A major technical advance since our first report (Iwamoto et al. 2002) is the introduction of quick-freezing of fibre specimens (figure 1a), which enables the specimens to remain stable for a long recording time without appreciable radiation damage (Iwamoto et al. 2005). The improvement of the quality of diffraction pattern is evident in the example shown in figure 1d from a Coleopteran Aulacophora. In this diffraction pattern, all the reflection spots predicted previously (Iwamoto et al. 2002; figure 1c) are clearly observed without calculating a 60°-rotary-averaged picture as was done originally.
2. Material and methods
(a) Preparation of rapidly frozen muscle fibre segments
The insects were generally collected at or near the campus of SPring-8. The reared giant waterbug specimen was a gift from Dr Inoda. Thoraces were isolated from these insects and split in halves along the midline to expose the dorsal longitudinal flight (tergosternal) muscle, before they were transferred to a 50% mixture of glycerol and relaxing solution (Iwamoto 1995) with protease inhibitors and stored in a freezer at −20 °C. In Cicindela, its better-developed dorsoventral muscle was used. Skinned rabbit psoas fibres were prepared as described (Iwamoto 1995). On the day of freezing, muscle fibres isolated from the thoraces were transferred to a rigour solution containing 20 mM butanedione monoxime, and then to a rigour solution containing an appropriate cryoprotectant (20% methylpentanediol or 10% dimethylsulphoxide). In a few cases the cryoprotectant was omitted. A short segment (∼2 mm) of a fibre (in the case of minute Thysanoptera, the whole meso-metathorax) was mounted on top of a short brass rod (1×2 mm in cross-section) with a small piece of paper on the top, and was immediately quick-frozen by plunging it into liquid propane (∼90 K) by using a quick-freezing apparatus for electron microscopy (CPC, Leica). The brass rod with a frozen fibre segment was transferred to liquid nitrogen, and the fibre was trimmed to a length of 400 μm using a rotating dicing saw (Disco Corporation, Japan) while immersed in liquid nitrogen, to increase the chances of capturing an undisturbed stretch of myofibril in the microbeam path. The brass rod was then inserted to a cooled brass holder, which was designed so that the fibre axis would coincide with the microbeam path when mounted in a cryochamber (Microstat He, Oxford Instruments, UK) where the specimens were kept at ∼74 K. The stability provided by this temperature allowed hundreds of myofibrillar diffraction patterns to be recorded from a single muscle fibre.
(b) X-ray diffraction recording
X-ray diffraction patterns were recorded at the BL40XU beamline of SPring-8 (Inoue et al. 2001). The data acquisition software was HiPic (Hamamatsu Photonics, Hamamatsu, Japan). The details of microbeam optics and recording procedures are described elsewhere (Iwamoto et al. 2005). In the early experiments, the specimens were scanned manually to seek well-preserved areas. In the more recent experiments, we collected many (≥100) diffraction patterns from each specimen by mechanically scanning the specimen in horizontal and vertical directions with a constant (e.g. 5 μm) step, to exclude subjectivity (see electronic supplementary material fig. 2). Regardless of appearance, all diffraction patterns collected in this way were analysed by means of the three measures of lattice regularity as described later.
(c) Analysis of data
As is detailed in §3d, three objective measures of lattice regularity were calculated from the 2, 0 reflection for each insect species (figures 3 and 4 and electronic supplementary material fig. 1). These were the peak index, standard deviation and sharpness. These were combined to create a single measure of lattice regularity, i.e. the total score.
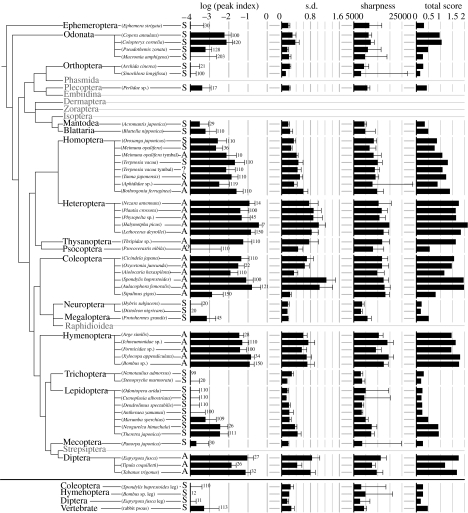
Figure 4.
Summary of quantitative analysis as expressed in the form of phylogenic tree (Wheeler et al. 2001). Except for the total score, the values are expressed as the means+s.d. (the figure by each error bars indicates the number of samples analysed). If the number of samples is small, the data were obtained in the earliest experiments and some human judgement was involved in selecting the patterns to be recorded. This leads to higher indices and total scores (e.g. Halyomorpha) than in the later analyses. Orders that were not studied are printed in grey letters. The letters next to the scientific names represent the muscle types (A, asynchronous; S, synchronous).
The angular autocorrelation function was calculated for the range of scattering angle where the 2, 0 reflection existed (any given radius from the direct beam position in the diffraction pattern) by using the following equation
where x and θ refer to rotary angles in the detector plane (in degrees) and I(x) is the scattering intensity at the particular spot on the pattern.
From the angular autocorrelation function, the ‘peak index’ was calculated as
The peak index refers to the likelihood of having an intensity peak at every 60°.
The standard deviation (s.d.) of scattering intensity describes the extent of intensity fluctuation within the range of scattering angle where the 2, 0 reflection exists, and is described as
where x refers to a rotary angle in the detector plane (in degrees) and I(x) is the scattering intensity after normalizing with respect to the intensity averaged along the entire circumference of the range of scattering angle. For calculation of the autocorrelation function and s.d., the actual integration was done in 1° steps.
The sharpness of the 2, 0 reflection was calculated as the inverse of the full width at half maximum (FWHM) and has a unit of rad−1. Theoretically, the sharpness is also affected by the number of unit cells within the lattice, because the finite widths of the Laue function peaks cannot be ignored if the number of unit cells in the beam is limited. In the case of synchronous muscles (myofibril diameter, ∼1 μm), the unit cell number is limited by the myofibril diameter, while in asynchronous muscles (myofibril diameter, ∼3 μm), it is limited by the microbeam diameter (1.5 μm). If the unit cell size is 50 nm, the difference corresponds to a size of 10 unit cells and accounts for up to 5000 rad−1 in sharpness. Much greater differences are observed between asynchronous and synchronous muscles, and therefore the differences are ascribed to the true difference in the variation of lattice spacing (unit cell size).
As will be described, more regular lattices have greater values of peak index, s.d. and sharpness. From these, the ‘total score’ of lattice regularity was calculated as
The peak index has the greatest contribution in calculating the total score (because this is the amount directly relevant to the hexagonal lattice), but it was not included in calculation if it was 0.0001 or less. These calculations were done for the 1, 1 reflection in the case of rabbit.
3. Results
(a) Myofilament lattice ordering in various insect species
Figure 2 shows a gallery of representative end-on diffraction patterns recorded from the myofibrils from flight and non-flight muscles from a variety of winged insects. The patterns are generally arranged in the order of higher to lower insects.
The diffraction patterns in the upper rows of figure 2a–ac generally show clear features of a single hexagonal lattice, indicating that these flight muscles belong to the class with long-range crystallinity. The insects which show this feature include Hymenoptera (bees, wasps and ants: figure 2a–e), Diptera (flies and mosquitoes: figure 2f–h), Coleoptera (beetles: figure 2i–n) and Heteroptera (true bugs: figure 2o–s), and these are known almost exclusively to have asynchronous flight muscle operation (i.e. rhythmic contraction of the muscles that control wingbeat timing does not match the rhythm of motor nerve impulses; Cullen 1974; Pringle 1981; Josephson et al. 2000). All examined Hymenopterans, including a primitive sawfly Arge (figure 2d), have generally very well-registered lattices. The same applies to Heteropterans, which includes the extensively studied giant waterbug Lethocerus (figure 2s). A Thysanopteran (thrip), a minuscule insect found in flowers, is also found to have very well-registered lattices (figure 2ab). The lattice register of a Psocopteran (barklouse) Psococerastis seems inferior, but this could be due to the difficulty in handling the small specimen (figure 2ac). These insect orders are phylogenetically close to Heteroptera/Homoptera (Wheeler et al. 2001), and are known to contain asynchronous species (Cullen 1974; Pringle 1981). The extent of lattice register in Coleopterans ranges from very good (Aulacophora: figures 1d, 2i and 3a) to relatively poor (Sipalinus: figure 2m), but this could be due to the difference in the muscle fibre stiffness; the myofibrils in softer muscle fibres are more susceptible to distortion during preparation, resulting in a spuriously low extent of register.
Other features observed in the diffraction patterns from these well-registered lattices are that the reflections are sharp (indicating that the spacing between lattice planes stays nearly constant throughout the myofibril), that the reflections are intense, the lattice spacing is smaller (see electronic supplementary materials) and that higher order reflections (beyond 2, 0) are very often observed.
By contrast, the patterns shown in figure 2ad and beyond generally do not show any clear sign of a hexagonal lattice, but consist of two diffuse concentric rings (Debye–Scherrer rings) corresponding to the 1, 0 and 2, 0 reflections. This indicates that the lattice planes are not preserved, even for a short distance within a myofibril (the top model in figure 3d). Most of the insects which exhibit this type of diffraction patterns are those with synchronous flight muscle operation (i.e. a nerve impulse elicits each wingbeat), including the most primitive of winged insect orders. It is known that the myofibrils in synchronous flight muscles are smaller in diameter than those in asynchronous muscles (Josephson et al. 2000), but this does not explain the total lack of spot-like reflections after considering the beam size (FWHM, 1.5 μm), i.e. still comparable to their myofibril diameter (∼1 μm). The peak intensities are generally lower, and the reflections are broader (indicating that the spacing between lattice planes is more variable within a myofibril) than the reflections from higher insects. Higher order reflections are rarely observed. Insect orders showing this type of diffraction patterns include Mecoptera (scorpionflies: figure 2am), Lepidoptera (butterflies and moths: figures 2ad–ag and 3d), Trichoptera (caddisflies: figure 2ak–al), Neuroptera (antlions, etc.: figure 2ao–ap), Orthoptera (grasshoppers: figure 2at–au) and Ephemeroptera (mayflies: figure 2az).
(b) Insects showing an intermediate degree of lattice register
Despite the general tendency described above, some of the insects with synchronous flight muscle operation have apparently developed various degrees of lattice register. The most notable examples are damselflies Copera and Calopteryx (Odonata, suborder Zygoptera: figures 2ax,ay and 3b). The diffraction patterns from their flight muscle clearly show features of a hexagonal lattice, although the reflections are somewhat spread along the circumference. This type of pattern suggests that the lattice planes are in principle preserved for a long distance, although locally disordered (the top model in figure 3b). Higher order reflections are also observed. Interestingly, the more advanced dragonflies Pseudothemis and Macromia (suborder Anisoptera: figure 2av,aw) have less-registered lattices, although the features of a hexagonal lattice are still evident. A weaker but similar tendency is observed in other insect orders, including Plecoptera (stoneflies: figure 2as), Megaloptera (dobsonflies: figure 2an), Blattaria (cockroaches: figure 2aq) and Mantodea (mantises: figure 2ar). As to the last two orders, the species examined are forest dwellers and fly well.
Lepidopterans, including a Geometrid (Odontoptera, figure 2ad), a Noctuid (Ctenoplusia, figure 2ae), Lasciocampid (Dendrolimus, figure 2af) and a giant Saturniid (Antheraea, figure 2ag) have non-registered lattices. However, the diffraction patterns from three species of Sphingids (hawkmoths, figures 2ah–aj and 3c) consist of numerous spot-like reflections, suggesting that the lattices may be locally registered but not for a long distance (the top model in figure 3c; another possibility is that the lattices are well-ordered but the myofibrils are small in diameter, so that several myofibrils could exist in the microbeam path). Its reflections are sharper than in other synchronous flight muscles, and higher order reflections are observed. Sphingids, including a hummingbird hawkmoth Neogurelca (figure 2ah), are known to feed on nectar while hovering in the air.
Diffraction patterns similar to those of hawkmoths are found in cicadas, Meimuna (figure 2t,u), Terpnosia (figure 2v,w) and Tanna (figure 2x). These are members of Homoptera (cicadas, leafhoppers, aphids, etc.). This order is often regarded as a suborder of Hemiptera along with Heteroptera, and whether the flight muscle of each species is synchronous or asynchronous depends on what family the species belongs to (Cullen 1974). Cicadas have synchronous flight muscle (Cullen 1974), but the tymbal muscle (used to produce sound) of Meimuna is suggested to be asynchronous (Josephson & Young 1981). However, the patterns from both muscles are similar and typical hexagonal patterns are rarely observed. Of the three species examined, Terpnosia has better-registered lattices and a few diffraction patterns show features of a hexagonal lattice (figure 2v).
As for smaller Homopterans, a synchronous (Cullen 1974) Ricaniid planthopper Orosanga (figure 2y) has intermediately registered lattices, while asynchronous species (Cullen 1974), a Cicadellid leafhopper Bothrogonia and an aphid, have well-registered lattices (figure 2z,aa, respectively).
(c) Diffraction patterns from non-flight muscles
Diffraction patterns were recorded from the leg muscles of some of the insects that have asynchronous flight muscles (figure 2ba–bc). The patterns show that the lattices are not registered, much like in the flight muscles of lower insects. It is therefore likely that the highly registered lattices are a structure specific to flight muscles.
Finally, an end-on diffraction patterns from vertebrate skeletal muscle (rabbit psoas) is shown for comparison (figure 2bd). Its pattern is similar to those from synchronous flight muscles, except that the strongest reflections are indexed to the 1, 0 and 1, 1 planes of the hexagonal lattice. As in the majority of synchronous flight muscles, the pattern consists of Debye–Scherrer rings, but on close examination, some of the patterns show very weak signs of a hexagonal lattice (see §3d). Therefore, the myofibrils in vertebrate skeletal muscle may have a limited extent of consistency in lattice plane orientations.
(d) Quantification of the extent of lattice register
The descriptions so far are based on visual inspections of the patterns. To access the extent of lattice register more quantitatively, we employ three quantitative indices as listed below. These indices include:
Angular autocorrelation function f(θ) for the 2, 0 reflection. This quantity refers to the likelihood of finding intensities at two angular positions θ degrees apart (figure 3 shows some examples). If the reflection is indexable to a hexagonal lattice, it should have a peak at every 60°, and it is naturally expected that f(60°) is greater than the values 5° apart, i.e. f(55°) and f(65°). Here, we define a quantity , which we refer to as ‘peak index’ (a more detailed expression is given in §2). A positive peak index indicates that the pattern shows signs of a hexagonal lattice.
Standard deviation (s.d.) of intensities measured along the circumference of the 2, 0 reflection. This s.d. value is a measure of the intensity fluctuation along the circumference. This value can be large if the reflection consists of sharp, isolated peaks (regardless of whether a peak appears at every 60°), but it is zero for an ideal Debye–Scherrer ring.
The width of the 2, 0 reflection measured across the circumference of reflection (FWHM). This value is small if the spacing between the lattice planes is constant throughout the myofibril, and is large if the spacing has variations along the myofibril. Therefore, the inverse of the FWHM (i.e. sharpness) can serve as an additional measure of lattice regularity. (No corrections were made for the myofibril diameter. See §2 for details.)
These three indices for lattice register were determined for all insects examined and rabbit skeletal muscle, and are summarized in figure 4. It can be seen that these indices are positively correlated to each other (see electronic supplementary material). This means that the diffraction patterns showing clearer signs of a hexagonal lattice tend to show greater intensity fluctuation along the circumference of the 2, 0 reflection and have sharper peaks.
Finally, a total score of lattice regularity was calculated for each species (the rightmost column of figure 4). This quantity is the length of the vector that would be generated by plotting the three indices on the three (x, y and z) axes of the Cartesian coordinates, respectively (for detailed method of calculation see §2). The comparison of the total scores confirms the earlier qualitative descriptions: the insects belonging to orders Heteroptera, Thysanoptera, Coleoptera, Hymenoptera and Diptera mark high scores (>1). Insects showing an intermediate degree of lattice register (damselfly, cicada and hawkmoth) mark scores of around one. Figure 4 also shows that some of the insects with synchronous flight muscle have some degree of lattice regularity (score ∼0.5). These include orders Plecoptera, Megaloptera, Blattaria and Mantodea. Rabbit skeletal muscle attains a similar extent of lattice regularity.
4. Discussion
In this paper, we have analysed the extent of myofilament lattice register in the quick-frozen flight muscle fibres of insects representing most of the winged insect orders, by means of end-on X-ray microdiffraction. The results show that the occurrence of the long-range crystallinity coincides very well with asynchronous flight muscles, while synchronous flight muscles generally lack such long-range crystallinity. The good inter-sarcomeric lattice register in asynchronous flight muscles may be a consequence of their mechanical characteristics. For the asynchronous operation, the stretch activation capability (delayed rise of tension after stretch: Machin & Pringle 1960; Jewell & Rüegg 1966; Pringle 1978) is essential. Apparently, strong mechanical linkages that stabilize the relative positions of myosin heads and actin monomers help this process, as has been suggested from the chemical cross-linking experiment on rabbit skeletal muscle (Tawada & Kawai 1990). In asynchronous insect flight muscles, these linkages are provided by the stiff C-(connecting) filaments (Thorson & White 1983), which connect the myosin filaments to the Z-disc (this separates the neighbouring sarcomeres). The Z-disc is known to be built so as to organize both actin filaments and C-filaments into a hexagonal lattice, and to transmit the lattice planes to the next sarcomere (although with a parallel shift in the lattice planes; Ashhurst 1967; Saide & Ullrick 1973; Deatherage et al. 1989). Such a structural design was probably adopted each time an ancestral asynchronous species emerged, and all the progenies succeeded the same design, regardless of their wingbeat frequencies (some large insects beat their wings at frequencies so low that they would not require asynchrony; see Pringle 1981; Josephson et al. 2000).
In addition, the myofibrils in asynchronous flight muscle are generally well separated from each other by a large volume of mitochondria. This configuration probably helps ATP to diffuse into myofibrils evenly during high-frequency wingbeats. However, this means that each myofibril must support its own force without the aid of neighbouring myofibrils, and any discontinuity along its length (such as lattice plane transition) could lead to mechanical weakness.
The C-filaments also help to keep the sarcomere length, and consequently the lattice spacing, fairly constant, resulting in sharper reflections. On the other hand, synchronous flight muscles are more or less similar to vertebrate skeletal muscle in both structure and function, in that they can shorten appreciably upon activation, and therefore the C-filaments are apparently missing. Projectin, a titin (connectin)-family protein (Hu et al. 1990), is known to be the major constituent of the C-filament, connecting the Z-disc and the A-band (the region of a sarcomere in which the myosin and actin filaments overlap: Saide 1981; Saide et al. 1989; Lakey et al. 1993; Kulke et al. 2001). In synchronous flight muscles and non-flight muscles of insect, projectin does exist, but apparently it does not connect the Z-disc and the A-band; in these muscles, projectin is known to be localized to the A-band (Vigoreaux et al. 1991; Ayme-Southgate et al. 2000), and extends to the I-band (the region of a sarcomere which contains the actin filaments alone) but does not reach the Z-disc (Shimamura et al. 2003).
In this respect, it is of interest to know how the sarcomeres are constructed in the flight muscles with intermediately registered lattices. The flight muscles of dragonflies can shorten more than 10%, just like vertebrate skeletal muscle (Fitzhugh & Marden 1997). The amplitude of shortening during flight of a Sphingid (Manduca sexta) is ∼12% (Tu & Daniel 2004). Its length–tension curve (Tu & Daniel 2004) is as steep as in bumble-bee, although the amplitude of shortening of the latter is ∼3% (Josephson & Ellington 1997). The localization of projectin should be examined, as well as muscle fibre stiffness and the ability of stretch activation. Mechanical studies are important, because they are expected to reveal the relation between the occurrence of intermediately registered lattices and the way of life of these insects, which heavily depend on flight (Odonates and hawkmoths). Possibly better registered lattices are better suited for transmitting force with precision timing needed for high-frequency wingbeat, and such an evolutionary pressure could give rise to better registered lattices whenever skilful flight is needed.
It is believed that the asynchronous operation has independently evolved 7–10 times (Josephson et al. 2000), and so should the long-range crystallinity. On the other hand, intermediately registered lattices are sporadically seen among synchronous flight muscles. These intermediately registered lattices seem to be more weakly linked to phylogenic groups. It has been suggested that the asynchrony in flight muscle is necessitated by the reduction in the body size and a consequent increase in wingbeat frequency (Pringle 1981), because high-frequency calcium release and reuptake in the myoplasm would be energetically costly. This explanation does not necessarily apply to those with intermediately registered lattices, because neither hawkmoths nor damselflies are small. More extensive studies on these marginal groups, including more species of Homoptera, Psocoptera (both orders contain synchronous and asynchronous species) and synchronous Siricid wasps (horntails: Pringle 1981), will provide more clues to the evolution of the long-range crystallinity and its relation to asynchrony.
Finally, it is worth noting that a limited extent of lattice register was observed in vertebrate skeletal muscle. It is known that in vertebrate skeletal muscle the hexagonal filament lattice is converted to a square lattice at the Z-disc (Knappeis & Carlsen 1962). The observed lattice register suggests that there exists some weak mechanism to restore the lattice orientation in the neighbouring sarcomere. More work is needed to clarify the nature of this mechanism, including the use of more crystalline teleost muscle (Harford & Squire 1986).
To summarize, the long-range crystallinity as revealed by X-ray cryomicrodiffraction recordings has so far been found almost exclusively in asynchronous flight muscles, and their occurrence seems to be correlated well with the phylogenic classification. The lattices in the leg muscle of these insects are not in register. Although most of the insects with synchronous flight muscle show little lattice register, some exhibit lattices with intermediate register. Its occurrence could be more directly related to the function of muscle than phylogeny, as these lattices are often found in skilful fliers.
Acknowledgments
We express thanks to T. Inoda for the gift of the reared Lethocerus specimen, and J. Wakayama and T. Tamura for their help at the beamline. This work was conducted under approval of SPring-8 Proposal Review Committee (proposal Nos. 2003A0186; 2003B0189; 2004A0584; 2004B0473). Supported by Grant-in-Aid, grant no. 15500294, Ministry of Education, Culture, Sports, Science and Technology and Special Coordination Funds of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Supplementary Material
References
- Ashhurst D.E. Z-line of the flight muscle of Belostomatid water bugs. J. Mol. Biol. 1967;27:385–389. doi: 10.1016/0022-2836(67)90027-7. 10.1016/0022-2836(67)90027-7 [DOI] [PubMed] [Google Scholar]
- Ayme-Southgate A, Southgate R, McEliece M.K. Drosophila projectin: a look at protein structure and sarcomeric assembly. Adv. Exp. Med. Biol. 2000;481:251–264. doi: 10.1007/978-1-4615-4267-4_15. [DOI] [PubMed] [Google Scholar]
- Cullen M.J. The distribution of asynchronous muscle in insects with particular reference to the Hemiptera: an electron microscope study. J. Entomol. A. 1974;49:17–41. [Google Scholar]
- Deatherage J.F, Cheng N, Bullard B. Arrangement of filaments and cross-links in the bee flight muscle Z-disk by image analysis of oblique sections. J. Cell Biol. 1989;108:1775–1782. doi: 10.1083/jcb.108.5.1775. 10.1083/jcb.108.5.1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson M, Farman G, Frye M, Bekyarova T, Gore D, Maughan D, Irving T. Molecular dynamics of cyclically contracting insect flight muscle in vivo. Nature. 2005;433:330–333. doi: 10.1038/nature03230. 10.1038/nature03230 [DOI] [PubMed] [Google Scholar]
- Fitzhugh G.H, Marden J.H. Maturational changes in troponin T expression, Ca2+-sensitivity and twitch contraction kinetics in dragonfly flight muscle. J. Exp. Biol. 1997;200:1473–1482. doi: 10.1242/jeb.200.10.1473. [DOI] [PubMed] [Google Scholar]
- Harford J, Squire J. ‘Crystalline’ myosin cross-bridge array in relaxed bony fish muscle. Low-angle X-ray diffraction from plaice muscle and its interpretation. Biophys. J. 1986;50:145–155. doi: 10.1016/S0006-3495(86)83447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K.C, Tregear R.T, Barrington-Leigh J. Interpretation of the low angle X-ray diffraction from insect flight muscle in rigor. Proc. R. Soc. B. 1980;207:13–33. [Google Scholar]
- Hu D.H, Matsuno A, Terakado K, Matsuura T, Kimura S, Maruyama K. Projectin is an invertebrate connectin (titin): isolation from crayfish claw muscle and insect flight muscle. J. Muscle Res. Cell Motil. 1990;11:497–511. doi: 10.1007/BF01745217. 10.1007/BF01745217 [DOI] [PubMed] [Google Scholar]
- Inoue K, Oka T, Suzuki T, Yagi N, Takeshita K, Goto S, Ishikawa T. Present status of high flux beamline (BL40XU) at SPring-8. Nucl. Instrum. Meth. Phys. Res. A. 2001;467/8:674–677. 10.1016/S0168-9002(01)00443-0 [Google Scholar]
- Iwamoto H. Strain sensitivity and turnover rate of low force cross-bridges in contracting skeletal muscle fibres in the presence of phosphate. Biophys. J. 1995;68:243–250. doi: 10.1016/S0006-3495(95)80180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto H, Nishikawa Y, Wakayama J, Fujisawa T. Direct X-ray observation of a single hexagonal myofilament lattice in native myofibrils of striated muscle. Biophys. J. 2002;83:1074–1081. doi: 10.1016/S0006-3495(02)75231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto H, Inoue K, Fujisawa T, Yagi N. X-ray microdiffraction and conventional diffraction from frozen-hydrated biological specimens. J. Synchrotron Radiat. 2005;12:479–483. doi: 10.1107/S090904950501352X. 10.1107/S090904950501352X [DOI] [PubMed] [Google Scholar]
- Jewell B.R, Rüegg J.C. Oscillatory contraction of insect fibrillar muscle after glycerol extraction. Proc. R. Soc. B. 1966;164:428–459. [Google Scholar]
- Josephson R.K, Ellington C.P. Power output from a flight muscle of the bumblebee Bombus terrestris. I. Some features of the dorso-ventral flight muscle. J. Exp. Biol. 1997;200:1215–1226. doi: 10.1242/jeb.200.8.1215. [DOI] [PubMed] [Google Scholar]
- Josephson R.K, Young D. Synchronous and asynchronous muscles in cicadas. J. Exp. Biol. 1981;91:219–237. [Google Scholar]
- Josephson R.K, Malamud J.G, Stokes D.R. Asynchronous muscle: a primer. J. Exp. Biol. 2000;203:2713–2722. doi: 10.1242/jeb.203.18.2713. [DOI] [PubMed] [Google Scholar]
- Knappeis G.G, Carlsen F. The ultrastructure of the Z disc in skeletal muscle. J. Cell Biol. 1962;13:323–335. doi: 10.1083/jcb.13.2.323. 10.1083/jcb.13.2.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulke M, Neagoe C, Kolmerer B, Minajeva A, Hinssen H, Bullard B, Linke W.A. Kettin, a major source of myofibrillar stiffness in Drosophila indirect flight muscle. J. Cell Biol. 2001;154:1045–1057. doi: 10.1083/jcb.200104016. 10.1083/jcb.200104016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakey A, Labeit S, Gautel M, Ferguson C, Barlow D.P, Leonard K, Bullard B. Kettin, a large modular protein in the Z-disc of insect muscles. EMBO J. 1993;12:2863–2871. doi: 10.1002/j.1460-2075.1993.tb05948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin K.E, Pringle J.W. The physiology of insect fibrillar muscle. III. The effect of sinusoidal changes of length on a beetle flight muscle. Proc. R. Soc. B. 1960;152:311–330. doi: 10.1098/rspb.1960.0041. [DOI] [PubMed] [Google Scholar]
- Pringle J.W.S. The Croonean lecture, 1977: stretch activation of muscle: function and mechanism. Proc. R. Soc. B. 1978;201:107–130. doi: 10.1098/rspb.1978.0035. [DOI] [PubMed] [Google Scholar]
- Pringle J.W.S. The Bidder lecture, 1980: the evolution of fibrillar muscle in insects. J. Exp. Biol. 1981;94:1–14. [Google Scholar]
- Saide J.D. Identification of a connecting filament protein in insect fibrillar flight muscle. J. Mol. Biol. 1981;153:661–679. doi: 10.1016/0022-2836(81)90412-5. 10.1016/0022-2836(81)90412-5 [DOI] [PubMed] [Google Scholar]
- Saide J.D, Ullrick W.C. Fine structure of the honeybee Z-disc. J. Mol. Biol. 1973;79:329–337. doi: 10.1016/0022-2836(73)90009-0. 10.1016/0022-2836(73)90009-0 [DOI] [PubMed] [Google Scholar]
- Saide J.D, Chin-Bow S, Hogan-Sheldon J, Busquets-Turner L, Vigoreaux J.O, Valgeirsdottir K, Pardue M.L. Characterization of components of Z-bands in the fibrillar flight muscle of Drosophila melanogaster. J. Cell Biol. 1989;109:2157–2167. doi: 10.1083/jcb.109.5.2157. 10.1083/jcb.109.5.2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura J, Maruyama K, Kimura S. Localization of projectin in locust flight muscle. Comp. Biochem. Physiol. B. 2003;136:419–423. doi: 10.1016/s1096-4959(03)00252-5. 10.1016/S1096-4959(03)00252-5 [DOI] [PubMed] [Google Scholar]
- Tawada K, Kawai M. Covalent cross-linking of single fibres from rabbit psoas increases oscillatory power. Biophys. J. 1990;57:643–647. doi: 10.1016/S0006-3495(90)82582-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorson J, White D.C.S. Role of cross-bridge distortion in the small-signal mechanical dynamics of insect and rabbit striated muscle. J. Physiol. 1983;343:59–84. doi: 10.1113/jphysiol.1983.sp014881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregear R.T, Wakabayashi K, Tanaka H, Iwamoto H, Reedy M.C, Reedy M.K, Sugi H, Amemiya Y. X-ray diffraction and electron microscopy from Lethocerus flight muscle partially relaxed by adenylylimidodiphosphate and ethylene glycol. J. Mol. Biol. 1990;214:129–141. doi: 10.1016/0022-2836(90)90152-C. 10.1016/0022-2836(90)90152-C [DOI] [PubMed] [Google Scholar]
- Tregear R.T, Edwards R.J, Irving T.C, Poole K.J.V, Reedy M.C, Schmitz H, Towns-Andrews E, Reedy M.K. X-ray diffraction indicates that active cross-bridges bind to actin target zones in insect flight muscle. Biophys. J. 1998;74:1439–1451. doi: 10.1016/S0006-3495(98)77856-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu M.S, Daniel T.L. Cardiac-like behavior of an insect flight muscle. J. Exp. Biol. 2004;207:2455–2464. doi: 10.1242/jeb.01039. 10.1242/jeb.01039 [DOI] [PubMed] [Google Scholar]
- Vigoreaux J.O, Saide J.D, Pardue M.L. Structurally different Drosophila striated muscles utilize distinct variants of Z-band-associated proteins. J. Muscle Res. Cell Motil. 1991;12:340–354. doi: 10.1007/BF01738589. 10.1007/BF01738589 [DOI] [PubMed] [Google Scholar]
- Wheeler W.C, Whiting M, Wheeler Q.D, Carpenter J.M. The phylogeny of the extant hexapod orders. Cladistics. 2001;17:113–169. doi: 10.1111/j.1096-0031.2001.tb00115.x. 10.1111/j.1096-0031.2001.tb00115.x [DOI] [PubMed] [Google Scholar]
- Worthington C.R. X-ray diffraction studies on the large-scale molecular structure of insect muscle. J. Mol. Biol. 1961;3:618–633. doi: 10.1016/s0022-2836(61)80025-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.