Abstract
The common cuckoo has several host-specific races, each with a distinctive egg that tends to match its host's eggs. Here, we show that the host-race specializing on reed warblers also has a host-specific nestling adaptation. In playback experiments, the nestling cuckoos responded specifically to the reed warbler's distinctive ‘churr’ alarm (given when a predator is near the nest), by reducing begging calls (likely to betray their location) and by displaying their orange-red gape (a preparation for defence). When reed warbler-cuckoos were cross-fostered and raised by two other regular cuckoo hosts (robins or dunnocks), they did not respond to the different alarms of these new foster-parents. Instead, they retained a specific response to reed warbler alarms but, remarkably, increased both calling and gaping. This suggests innate pre-tuning to reed warbler alarms, but with exposure necessary for development of the normal silent gaping response. By contrast, cuckoo chicks of another host-race specializing on redstarts showed no response to either redstart or reed warbler alarms. If host-races are restricted to female cuckoo lineages, then chick-tuning in reed warbler-cuckoos must be under maternal control. Alternatively, some host-races might be cryptic species, not revealed by the neutral genetic markers studied so far.
Keywords: cuckoo, vocal communication, alarm calls, nestling begging, coevolution
1. Introduction
The common cuckoo, Cuculus canorus, is a brood parasite which tricks other species of birds into caring for its eggs and chicks. It occurs in several host-specific races, each laying a distinctive egg type that tends to match its host's eggs (Chance 1940; Brooke & Davies 1988; Moksnes & Røskaft 1995). Two lines of evidence suggest that these host-races are restricted to female cuckoo lineages, with cross-mating by males maintaining the common cuckoo as one species. First, parentage analysis using DNA markers has shown that whereas individual female cuckoos lay all or most of their eggs in the nests of one host species, individual male cuckoos often father offspring in several host species' nests, implying that they had mated with females of more than one host specialization (Marchetti et al. 1998; Skjelseth et al. 2004). Second, there is differentiation between host-races in maternally inherited mitochondrial DNA but not in microsatellite loci of nuclear DNA (Gibbs et al. 2000). These results are consistent with the long-held view that cuckoo egg type is controlled by genes on the female-specific W sex chromosome (Punnett 1933).
Female common cuckoos lay one egg per host nest. Soon after hatching, the cuckoo chick ejects the host eggs or young and so it is raised alone (Jenner 1788). It then stimulates the hosts to supply as much food as for a whole brood of host young by producing unusually rapid begging calls to compensate for its single gape, a deficient visual stimulus for the hosts compared with what they expect from a host brood (Davies et al. 1998; Kilner et al. 1999). This excessive calling, together with a longer nestling period (17‐20 days) compared with the host young (11–14 days), is likely to increase the cuckoo nestling's vulnerability to predators (Haskell 1994; Leech & Leonard 1997; Dearborn 1999). Therefore, it should be especially advantageous for nestling cuckoos to respond to the host parents' alarm calls, which are given to silence their own young when a predator is near the nest (Davies et al. 2004; Platzen & Magrath 2004; Madden et al. 2005).
There is a second reason for why it might pay common cuckoo nestlings to attend to their host parents' alarms. Whereas host nestlings are helpless if attacked, from about a week old the nestling cuckoo has a remarkable defensive display (Jenner 1788; Davies 2000). When approached closely by a human observer, it rears up on its legs, opens its vivid orange-red gape, stretches its neck and then suddenly snaps its head back. This is a shock, even for someone familiar with the performance and could be an effective predator deterrent. If touched, the cuckoo nestling escalates its defence by pecking, heaving its body up and down and by producing foul-smelling, liquid brown faeces. Therefore, the cuckoo nestling might respond to the host's alarm calls not only as a signal to be silent, but also to prepare for defence in case the predator finds it.
We have shown previously that the parental alarms of three of the cuckoo's favourite host species in Britain are very different: reed warblers, Acrocephalus scirpaceus, give a low-pitched, broad frequency ‘churr’; dunnocks, Prunella modularis, give a narrow bandwidth and higher pitched ‘tseep’; while robins, Erithacus rubecula, give a more drawn out and still higher pitched ‘seee’ (Davies et al. 2004). Playback experiments revealed that nestlings of all three host species ceased begging only in response to conspecific alarm calls and cross-fostering experiments suggested that this specific response was not simply an outcome of experience, because when nestlings were raised by another species they did not tune into their foster species' alarms but still retained a selective (but weaker) response to their own species' alarms (Davies et al. 2004; for another example of an innate response see Madden et al. 2005). We argued that neural pre-tuning to their own species' acoustic signals (Marler 1997; Soha & Marler 2000) would enable nestlings to pick out their parents' alarms against a background of irrelevant sounds (calls of other species or noises from vegetation or inanimate objects), and to respond appropriately the first time danger threatened, while exposure to the alarms might fine-tune the response to reduce recognition errors (Davies et al. 2004).
These results raise fascinating questions as to how common cuckoo nestlings might tune into host parent alarms, given that they can be raised by many different host species, with an array of different alarm calls. At one extreme, we could imagine cuckoo nestlings to have less selective pre-tuning compared with the host young, which would enable them to develop a response to a wider range of parental alarms. At the other extreme, each host-race of cuckoo might have evolved not only specific egg-matching appropriate for its particular host species, but also specific chick-tuning for its host's alarm calls. If true, this would raise the problem of how such selectivity might develop in nestlings of both sexes in a system of host-races restricted to female cuckoo lineages.
Here, we first use playback experiments to test whether naturally raised nestling cuckoos of the host-race specializing on reed warblers (henceforth, referred to as reed warbler-cuckoos) respond selectively to reed warbler alarms. Second, experiments in which reed warbler-cuckoos were cross-fostered to be raised either by dunnocks or robins (two other regular cuckoo hosts) test whether the young cuckoos of this host-race have the potential to tune into other host species' alarm calls. Third, we compare these responses with those of another cuckoo host-race specializing on redstarts, Phoenicurus phoenicurus. We examine how the alarm responses of cuckoo nestlings compare with the specific responses previously found in the host nestlings and discuss the implications for cuckoo–host coevolution.
2. Material and methods
(a) Host parent alarm calls
From 2001 to 2004 at various sites in Cambridgeshire, England, we measured the rates of these alarms in reed warblers, dunnocks and robins in response to a human standing 2 m from the nest. We counted the number of calls given during the first minute by the first adult to return to the nest vicinity and compared these rates between parasitized and unparasitized nests.
(b) Playback experiments with reed warbler-cuckoos
This protocol was the same as that previously used to test responses of host nestlings (for details and equipment used, see Davies et al. 2004). We made field recordings in Cambridgeshire, England of the nestling-warning alarm calls of 18 adult reed warblers, 25 dunnocks and 13 robins (response to a human; see above), and of a control, the advertisement call of 21 male chaffinches, Fringilla coelebs (given from tree tops in spring; figure 1). These were edited to give 6 s playback cuts each with five calls (maximum of two cuts from the recording of each adult). In all playback experiments, each cuckoo nestling was given a different playback cut of each call type to avoid pseudoreplication.
Figure 1.
Typical sonograms of the playback stimuli: a chaffinch advertisement control call (‘hreet’), a reed warbler alarm (‘churr’), a dunnock alarm (‘tseep’), a robin alarm (‘seee’) and a redstart alarm (‘hueee’).
Cuckoos, tested at 6–8 days old, came from reed warbler nests in Cambridgeshire. This cuckoo host-race lays greenish, spotted eggs. Because of the individually characteristic egg markings and distances between host nests (Davies & Brooke 1988), it seems probable that the nine nestlings tested (three in 2001, four in 2002, one in 2003 and one in 2004) came from at least seven different female cuckoos. Cuckoo nestlings were removed from the host nest temporarily and replaced with two reed warbler nestlings from part of a nearby brood to prevent host parents from deserting. In the laboratory, cuckoos were placed in a heated old reed warbler nest inside a test box. They were fed from plastic forceps with Nectarblend rearing mix until they stopped begging. Testing began 80 min later, when begging intensity matches that under natural field conditions (Kilner & Davies 1999).
Before each playback experiment, the nestling was stimulated to beg for 5 s, by gently tapping the side of its bill with plastic forceps, to measure baseline begging levels. It then experienced three periods in a fixed order: (i) playback during stimulation to beg (PBS); 18 s playback (three repeats of a 6 s cut) with stimulation by forceps every 3 s; followed immediately by (ii) playback alone (PB); 18 s PB (three more repeats of the same 6 s cut); followed immediately by (iii) stimulation alone (S); 12 s stimulation by forceps every 3 s, as before. The sequence was designed to mimic a natural situation where: one adult is provisioning at the nest when the other adult begins to give alarms at the sight of an approaching predator (PBS); the adult at the nest leaves while alarms continue (PB) and then the chick is stimulated to beg again (S). This protocol allowed us to test the response of a begging cuckoo to alarm calls and its willingness to beg again after recently hearing alarm calls.
Each nestling experienced three calls in random order (reed warbler alarm, dunnock alarm and chaffinch control), with 2.5 min between successive playback experiments. Calls were broadcast at a standard sound intensity (60–65 dB measured at the nest, 3 m from the speaker). Audio and video recordings were made and we scored two responses: time (s) gaping per second and the number of begging calls per second. We analysed responses as baseline begging levels minus begging levels during each of the three subsequent periods. A general reduction in begging might be expected simply because of fatigue or habituation to repeated stimulation with no reward, so our analyses focused on differences in response to the playback calls, using repeated measures ANOVA with two factors (playback call type, period of trial) and testing for significant effects within subjects. After testing, cuckoos and temporary replacement host nestlings were returned to their original nests and all were readily accepted back by their hosts/parents.
It would have been preferable to present the various PB treatments blind, but we were unable to do this for logistical reasons. However, three results (see later) suggest there was no experimenter bias. First, there were no differences in pre-test begging levels before the various playbacks. Second, it is difficult to see how any stimulation bias could have caused begging responses to go in different directions for gaping and calling responses. Third, there were clear differences in response to different playbacks even in the PB period, when no tapping stimulation was involved.
(c) Cross-fostering reed warbler-cuckoos
These seven cuckoos (six newly hatched nestlings in 2003, one newly laid egg in 2004) were removed from reed warbler nests in Cambridgeshire. The nestlings were replaced with two reed warbler nestlings from part of a nearby brood. The cuckoos were then transferred to robin or dunnock nests with eggs in the Cambridge University Botanic Garden. The young cuckoos ejected their new fosterer's eggs and were then raised by these new hosts. These cuckoos probably came from six different females. For the female providing two offspring, one was transferred to a dunnock nest and one to a robin nest. Cuckoos were tested at 6–8 days old (as above), with four playback treatments given in random order (alarms of reed warbler, dunnock and robin and the chaffinch control). Five minutes after the end of this experiment, five of the cuckoos were retested with playback alone (same cuts, three repeats of 6 s). After testing, cuckoos were returned to their original host species nests and any temporary replacement host nestlings to their home nests.
(d) Redstart-cuckoos
This host-race, which lays plain blue eggs, was studied in 2004 in South Karelia, eastern Finland, near Taipalsaari (Rutila et al. 2002). We tested six cuckoo nestlings at 6–8 days old in the playback experiment (above), with four playback treatments given in random order (alarms of redstart, reed warbler and dunnock and the chaffinch control). Five minutes after the end of this experiment, all six cuckoos were retested with playback alone (same cuts, three repeats of 6 s). Four other redstart-cuckoos, two on days 3–4 and two on days 9–12, were also tested but only with this playback alone trial (three repeats of a 6 s cut for each of these four playbacks). Redstart alarms (figure 1) were recorded as for the other hosts (see above) at nests on this study site. During testing, redstart hosts were given two redstart nestlings from a nearby brood, and afterwards all nestlings were returned to their original nests. We could not distinguish the eggs of individual female cuckoos. However, the large distances between nests with our cuckoo nestlings (4–300 km) suggest they all came from different females.
(e) Ethical considerations
Playback and cross-fostering experiments were licensed by English Nature and the Finland Regional Environment Centre. No hosts deserted, nestling hunger levels in the experiments were no greater than under natural field conditions, and the growth of the cross-fostered cuckoo nestlings fell within the range of those raised by their normal reed warbler hosts.
3. Results and discussion
(a) Reed warbler alarm rates
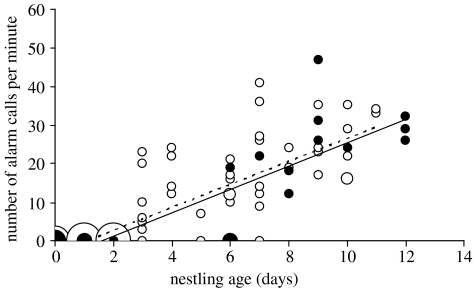
Parent reed warblers did not alarm call at the egg stage (completed clutches, incubation begun), either at unparasitized (n=53) or parasitized nests (n=20). Alarm calling began 3–4 days after hatching, which is the age at which reed warbler chicks start to call regularly while begging (Kilner & Davies 1999). At the nestling stage we visited each nest at 1–5 different ages. To avoid pseudoreplication, we analysed one randomly chosen age per nest for nests with more than one data point. Figure 2 shows that alarm calling rate increased with nestling age, but the rate did not differ between adults with broods of their own and those with a cuckoo chick.
Figure 2.
Alarm calling rate (churrs per minute) of one parent reed warbler in response to a human observer near the nest increased with nestling age (F1,107=204.36, p<0.0001), but did not differ between nests containing broods of reed warbler nestlings (open circles, dashed regression line) and those with a cuckoo nestling (solid circles, solid regression line; F1,107=0.099, p=0.75). Day 0 is day of hatching. The smallest symbol refers to one observation and the area of the larger symbols is directly proportional to the sample size (for largest, n=15). Data from 24 nests with a cuckoo nestling and 86 nests with a reed warbler brood, with one observation per nest.
To analyse the effect of repeated visits to a nest, we calculated the residuals from figure 2 and regressed these against visit number (excluding fourth and fifth visits because of small sample sizes). Residual alarm calling rate declined slightly with visit number (F1,95=4.16, p=0.044), suggesting some habituation to our repeated visits to a nest. However, this effect did not differ between nests containing reed warbler broods or a cuckoo nestling (F1,95=1.11, p=0.29). We conclude that cuckoo nestlings experience the normal alarm calling rate of reed warbler parents who are caring for a brood of their own young.
(b) Playback experiments: reed warbler-cuckoos raised naturally by reed warblers
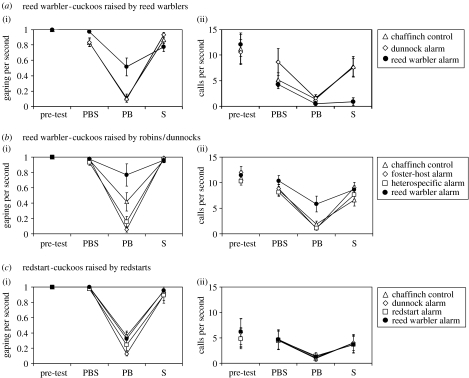
There were no differences in cuckoo baseline gaping or begging call rates before the three playback treatments (figure 3a: continuous gaping in all cases; calling rates F2,14=0.81, p=0.47). During the three test periods, the cuckoos begged strongly only during periods of manual stimulation (figure 3a: PBS and S) and were generally silent during the period of PB. There was also no gaping or calling during quiet periods immediately before or after the experiment. Therefore, they behaved like typical nestlings which, in the absence of specific parental food calls (not tested here), beg only in response to visual or tactile cues signalling the likely arrival of food (Bengtsson & Ryden 1981; Clemmons 1995; Madden et al. 2005). However, there were marked differences between playback treatments in begging during the three test periods (figure 3a: significant period×playback call interaction for both gaping F4,32=9.97, p<0.0001 and for calling F4,28=5.46, p=0.002). There were two main differences. First, whereas the cuckoos readily resumed begging calls after dunnock alarms or chaffinch control calls, they remained almost totally silent after reed warbler alarms (figure 3a, period S, post hoc LSD tests comparing this calling response with that after dunnock or chaffinch, p<0.005; no difference after dunnock versus chaffinch p=0.86). Second, cuckoos gaped silently during reed warbler playbacks but not during those of dunnock or chaffinch (figure 3a: gaping during PB p<0.001 for reed warbler versus both dunnock and chaffinch; no difference in dunnock versus chaffinch response p>0.89). They were also as ready to gape after reed warbler playback as after the other two calls (period S, p=0.103).
Figure 3.
Nestling cuckoo responses to playback of host alarm calls and a control call, measured as: (i) time(s) spent gaping per second and (ii) number of begging calls per second, during a pre-test stimulation period (to measure baseline begging) and then three successive periods of the playback experiment; PBS (playback during stimulation to beg), PB (playback alone) and S (stimulation alone). Mean±1 s.e. are shown for: (a) reed warbler-cuckoos raised by reed warblers (n=9); (b) reed warbler-cuckoos raised by robins or dunnocks (n=7); and (c) redstart-cuckoos raised by redstarts (n=6). The different symbols refer to the different playback calls. In (b), the heterospecific alarm is robin for dunnock-raised cuckoos and dunnock for robin-raised cuckoos.
We conclude that, just like nestling reed warblers (Davies et al. 2004), nestling reed warbler-cuckoos respond specifically to reed warbler alarm calls by reducing calling immediately following host alarms, but not after the other playbacks (period S). Platzen & Magrath (2004) suggest that adults are unlikely to visit the nest until a predator has left the vicinity, so the arrival of an adult after alarms have ended should be an ‘all clear’ signal for the nestlings to beg. However, reluctance to beg immediately following alarms, even during manual stimulation (period S), is adaptive because nestlings eager for food often beg in response to false signals of parental arrival, e.g. nest vibration caused by other animals or by the wind (Budden & Wright 2001; Leonard & Horn 2001; Leonard et al. 2005), and begging calls then could be fatal if a predator was still nearby.
However, whereas nestling reed warblers reduced both calling and gaping after reed warbler alarms (period S; Davies et al. 2004), the nestling cuckoos reduced calling but continued to gape and they also gaped silently in response to the reed warbler alarm playback (period PB) but not to the other playbacks (figure 3a). We suggest that this difference is adaptive. While the cuckoo's reduction in calling would reduce the chance that a predator locates the nest, the continued gaping might be a preparation for the cuckoo's remarkable defensive display in case the predator finds it.
(c) Playback experiments with cross-fostered reed warbler-cuckoos
We tested whether this specific response develops through exposure to reed warbler alarms by cross-fostering seven reed warbler-cuckoos to the nests of two other frequently used cuckoo–host species. Six were newly hatched cuckoo nestlings transferred within 16 h of hatching from reed warbler nests to either dunnock (n=3) or robin nests (n=3) and one was a newly laid cuckoo egg transferred the day after it was laid (and before incubation) to a dunnock nest. This last cuckoo, an egg with an undeveloped embryo, clearly had no experience of alarms prior to transfer. The six transferred nestlings would not have heard alarms from their original foster-parents, who did not alarm call until nestlings were 3 days old (figure 2). It is also unlikely that they had heard alarm calls of neighbouring parents: in three cases there were no neighbours with nestlings within 400 m, and in the other three the nearest were 60, 125 and 130 m away. After transfer to dunnock or robin nests the cuckoos did not experience reed warbler alarms (nearest pairs 5 km away), but were exposed to normal alarm rates of their new foster-hosts (measured when cuckoos 6–8 days old: for the three robin hosts, 3, 12 and 15 alarms per minute; for the 4 dunnock hosts, 24, 25, 38 and 48 alarms per minute; these rates fall within the range for robins and dunnocks with their own young; see fig. 2 of Davies et al. 2004). If reed warbler-cuckoos can tune into any host's alarms and a selective response develops through exposure, then we predicted that these cross-fostered cuckoos would respond specifically to their new foster-species' alarm calls. At the other extreme, if they were innately pre-tuned only to reed warbler alarms, and their normal response develops without exposure to these alarms, then they should respond in the same way as in figure 3a.
Our playback experiments showed that the response of the cross-fostered cuckoos (figure 3b) differed from that of reed warbler-cuckoos raised naturally by reed warbler hosts (compared with figure 3a: raising experience effect for gaping F1,14=3.81, p=0.071; for calling F1,13=6.77, p=0.022). Cross-fostered cuckoos behaved differently during trials with the different playback calls (figure 3b: period×playback call interaction for both gaping F6,36=15.44, p<0.0001; for calling F6,36=2.94, p=0.02). However, they did not respond selectively to the alarms of their new foster-hosts. Instead, they still responded selectively to reed warbler alarms, but this time in an inappropriate way, by treating them as food calls. All the cross-fostered cuckoos (including the one transferred as an undeveloped egg) begged vigorously in response to reed warbler playbacks, but not to the other playbacks (figure 3b, period PB: gaping response, compared with each of the other two alarms p<0.0001 and with chaffinch control p=0.021; calling response, p<0.02 for comparisons with each of the other playbacks). They also tended to gape (but not call) more during the control chaffinch playback, which was most similar acoustically to the reed warbler alarm (PB chaffinch gaping response compared with reed warbler p=0.021, and with the other calls p<0.081). They were as ready to resume begging calls after the reed warbler playbacks as after the other calls (period S, p=0.72). The cross-fostered cuckoos did not differ from those raised by reed warbler hosts in either mass (t14=0.87, p=0.40) or baseline calling rates prior to the playback (p=0.96). Therefore, these response differences were unlikely to reflect differences in growth or hunger (Kilner & Davies 1999).
These results suggest that reed warbler-cuckoos are pre-tuned specifically to reed warbler alarms, but that exposure to reed warbler vocalizations is necessary for development of the normal silent gaping response. Cross-fostering experiments with dunnock and robin host young suggest they too have an unlearned specific response to their own species' alarm calls which is strengthened by exposure. However, these cross-fostered host young still reduced both calling and gaping, just not as markedly as young raised normally by their own species (Davies et al. 2004). Why cross-fostered cuckoos should increase begging calls in response to reed warbler alarms is curious. Some young song-birds, when raised in isolation from song, beg specifically in response to playback of elements of their own species' song (Whaling et al. 1997; Nelson 2000). Therefore, one possibility is that the cuckoo, too, has this innate response to its host's vocalizations and the alarm ‘churr’ matches elements in reed warbler song or food calls. Perhaps the young cuckoo has to learn that during ‘churr’ alarm calling it gets no food, and this reduces its motivation to beg in response. As cuckoo nestlings become less motivated to beg, they first reduce calling while retaining some gaping (Kilner & Davies 1999). This learning mechanism would, therefore, lead to the silent gaping response shown by reed warbler-cuckoos raised normally by their reed warbler hosts. Cuckoo nestlings do not produce loud begging calls until they are several days old (Kilner & Davies 1999), so such fine-tuning of the alarm response through exposure to host vocalizations early in life is unlikely to be costly to their survival.
(d) Redstart-cuckoos
Reed warbler alarms are not unusually stimulating for any nestling bird, because neither dunnock nor robin nestlings respond to them (Davies et al. 2004). Nevertheless, to make sure that a specific response to reed warbler alarms was not a general property of all cuckoo host-races, we tested nestlings of another cuckoo host-race specializing on redstarts. Redstart-cuckoos begged in response to manual stimulation, but showed no differential response to playbacks (figure 3c: period×playback call interaction for gaping F6,30=1.03, p=0.43; for calling F6,30=0.83, p=0.56). They did not differ significantly from the reed warbler-cuckoos in either mass (t13=0.27, p=0.80) or baseline begging prior to playback (continuous gaping in all cases; calling rate t13=1.66, p=0.12). Their different response is thus unlikely to reflect differences in growth or hunger.
Their lack of response to redstart alarms (a distinctive ‘hueee’; figure 1) is interesting, because redstart nestlings also did not respond to these (J. R. Madden 2004, unpublished work). As in some other species nesting in rigid crevices, parental alarms may warn mates rather than nestlings, with nestling begging switched on by food calls rather than switched off by alarms (Madden et al. 2005). That redstart-cuckoos also lack a specific response to reed warbler alarms is further supported by comparing the responses in the playback alone trial (§2) of our two groups of cuckoos who had not experienced reed warbler alarms in nature. All five reed warbler-cuckoos raised by robins or dunnocks which were tested in the playback alone trial begged vigorously (gaping and calling) to reed warbler alarms, whereas none of the 10 redstart-cuckoos did so (p<0.01, Fisher's exact test).
4. General discussion
We conclude that the host-race of the common cuckoo that specializes on reed warblers has host-specific adaptations not only at the egg stage (well-matched eggs), but also at the chick stage (well-tuned nestlings). We suggest the response to the host parents' alarm call is especially advantageous for a cuckoo chick because its conspicuous vocal begging performance, necessary to stimulate adequate host provisioning, is likely to increase its vulnerability to predation. However, parent alarms do not always function to warn nestlings; in some species they may distract predators or alert mates (Madden et al. 2005 and references therein). In the other host-race we tested (redstart-cuckoos), where the host's own chicks did not respond to their parent's alarms, the cuckoo nestlings likewise did not respond. Clearly, more host-races need to be studied to determine whether host-specific adaptations are as frequent in cuckoo nestlings as in cuckoo eggs.
Our most surprising result is that the reed warbler-cuckoo's specific response to reed warbler alarms was evident even in the cross-fostered cuckoos and, furthermore, it involved the opposite response to the normal response, namely an increase in begging calls. This suggests that these cuckoos are pre-tuned to respond to their host species' calls but need exposure to distinguish alarm calls from food calls. Equally striking, was the failure of the cross-fostered cuckoos to tune into their new foster host's alarms. This selective pre-tuning was also evident in dunnock and robin chicks, where cross-fostering also failed to induce responses to a foster species' alarms (Davies et al. 2004).
How might reed warbler-cuckoos acquire an unlearned, specific response to reed warbler alarms? In theory, host-specific nestling adaptations could easily evolve in parasitic cuckoos that specialize on one host species, or several similar, closely related hosts (e.g. mimetic begging calls, Langmore et al. 2003). However, it is harder to see how these might evolve in a species such as the common cuckoo, which parasitizes many different host species, each with their own distinctive traits. If host-races are restricted to female cuckoo lineages, with cross-mating by males maintaining C. canorus as one species (Marchetti et al. 1998; Gibbs et al. 2000), then a mother would pass on her egg characteristics to her daughter if genes for egg type were either entirely on the female-specific W sex chromosome (Punnett 1933), or regulated by genes on this chromosome. However, this mechanism would not result in alarm-tuning inheritance in both sons (the homogametic sex in birds, ZZ) and daughters (WZ), assuming our sample of 16 reed warbler-cuckoos was highly likely to include both sexes. Maternal control through genomic imprinting of an autosomal gene would also not solve the problem. Imagine a female cuckoo that is RR, namely homozygous for responding to reed warbler alarms. If she mates with a male from a different host-race who is DD, and the maternal allele is imprinted, then all her offspring (RD) will respond to reed warbler alarms. However, if her RD daughters then produce offspring that also express only the maternal allele at this locus, then half of the grand-offspring will express D and so be pre-disposed to respond to the wrong host alarm.
We suggest three other possibilities. First, a mother cuckoo's W chromosome genes might code for a host-race specific factor in her eggs which activates the appropriate alarm response circuitry in her developing offspring. Second, non-genetic maternal effects, arising from differences in experience between females of the different host-races, might also activate specific host-race responses (see Greene 1989; McCaffery et al. 1998; Agrawal et al. 1999). Third, some host-races may have evolved into cryptic species, with selection maintaining adaptive differences in both eggs and nestlings, despite some gene flow through male promiscuity (Parker & Partridge 1998). Such differences might not be revealed by the neutral genetic markers studied so far (Gibbs et al. 2000).
Acknowledgments
We thank the Natural Environment Research Council for funding; the Director, J. S. Parker, for permission to work in the Cambridge University Botanic Garden; the National Trust, A. Colston and M. Lester for permission to work on Wicken Fen and C. J. R. Thorne and the Wicken Fen Ringing Group for facilities there; and the following for help with finding nests, with the experiments or with discussion: A. and J. Davies, H. L. Gibbs, J. Haikola, A. Harris, C. A. Hinde, A. Jeffrey, K. Koskela, R. M. Kilner, H. Markland, D. W. Mock, A. P. Radford, J. M. Reid, the Rutila family, N. Seddon and M. D. Sorenson.
Footnotes
Present address: BirdLife International, Wellbrook Court, Girton Road, Cambridge CB3 0NA, UK.
References
- Agrawal A.A, Laforsch C, Tollrian R. Transgenerational induction of defences in animals and plants. Nature. 1999;401:60–63. 10.1038/43425 [Google Scholar]
- Bengtsson H, Ryden O. Development of parent–young interactions in asynchronously hatched broods of altricial birds. Z. Tierpsychol. 1981;56:255–272. [Google Scholar]
- Brooke M. de L, Davies N.B. Egg mimicry by cuckoos, Cuculus canorus, in relation to discrimination by hosts. Nature. 1988;335:630–632. 10.1038/335630a0 [Google Scholar]
- Budden A.E, Wright J. Falling on deaf ears: the adaptive significance of begging in the absence of a parent. Behav. Ecol. Sociobiol. 2001;49:474–481. 10.1007/s002650100323 [Google Scholar]
- Chance E.P. Country Life; London: 1940. The truth about the cuckoo. [Google Scholar]
- Clemmons J.R. Vocalizations and other stimuli that elicit gaping in nestling black-capped chickadees (Parus atricapillus) Auk. 1995;112:603–612. [Google Scholar]
- Davies N.B. Poyser; London: 2000. Cuckoos, cowbirds and other cheats. [Google Scholar]
- Davies N.B, Brooke M. de L. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 1988;36:262–284. [Google Scholar]
- Davies N.B, Kilner R.M, Noble D.G. Nestling cuckoos Cuculus canorus exploit hosts with begging calls that mimic a brood. Proc. R. Soc. B. 1998;265:673–678. 10.1098/rspb.1998.0346 [Google Scholar]
- Davies N.B, Madden J.R, Butchart S.H.M. Learning fine-tunes a specific response of nestlings to the parental alarm calls of their own species. Proc. R. Soc. B. 2004;271:2297–2304. doi: 10.1098/rspb.2004.2835. 10.1098/rspb.2004.2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearborn D.C. Brown-headed cowbird nestling vocalizations and risk of nest predation. Auk. 1999;116:448–457. [Google Scholar]
- Gibbs H.L, Sorenson M.D, Marchetti K, Brooke M. de L, Davies N.B, Nakamura H. Genetic evidence for female host-specific races of the common cuckoo. Nature. 2000;407:183–186. doi: 10.1038/35025058. 10.1038/35025058 [DOI] [PubMed] [Google Scholar]
- Greene E. A diet-induced developmental polymorphism in a caterpillar. Science. 1989;243:643–646. doi: 10.1126/science.243.4891.643. [DOI] [PubMed] [Google Scholar]
- Haskell D. Experimental evidence that nestling begging behaviour incurs a cost due to nest predation. Proc. R. Soc. B. 1994;257:161–164. [Google Scholar]
- Jenner E. Observations on the natural history of the cuckoo. Phil. Trans. R. Soc. 1788;78:219–237. [Google Scholar]
- Kilner R.M, Davies N.B. How selfish is a cuckoo chick? Anim. Behav. 1999;58:797–808. doi: 10.1006/anbe.1999.1197. 10.1006/anbe.1999.1197 [DOI] [PubMed] [Google Scholar]
- Kilner R.M, Noble D.G, Davies N.B. Signals of need in parent–offspring communication and their exploitation by the common cuckoo. Nature. 1999;397:667–672. 10.1038/17746 [Google Scholar]
- Langmore N.E, Hunt S, Kilner R.M. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature. 2003;422:157–160. doi: 10.1038/nature01460. 10.1038/nature01460 [DOI] [PubMed] [Google Scholar]
- Leech S.M, Leonard M.L. Begging and the risk of predation in nestling birds. Behav. Ecol. 1997;8:644–646. [Google Scholar]
- Leonard M.L, Horn A.G. Begging in the absence of parents by nestling tree swallows. Behav. Ecol. 2001;12:501–505. 10.1093/beheco/12.4.501 [Google Scholar]
- Leonard M.L, Horn A.G, Mukhida A. False alarms and begging in nestling birds. Anim. Behav. 2005;69:701–708. 10.1016/j.anbehav.2004.05.022 [Google Scholar]
- Madden J.R, Kilner R.M, Davies N.B. Nestling responses to adult food and alarm calls: I. Species-specific responses in two cowbird hosts. Anim. Behav. 2005;70:619–627. 10.1016/j.anbehav.2004.11.019 [Google Scholar]
- Marchetti K, Nakamura H, Gibbs H.L. Host race formation in the common cuckoo. Science. 1998;282:471–472. doi: 10.1126/science.282.5388.471. 10.1126/science.282.5388.471 [DOI] [PubMed] [Google Scholar]
- Marler P. Three models of song learning: evidence from behaviour. J. Neurobiol. 1997;33:501–516. 10.1002/(SICI)1097-4695(19971105)33:5%3C501::AID-NEU2%3E3.0.CO;2-8 [PubMed] [Google Scholar]
- McCaffery A.R, Simpson S.J, Islam M.S, Roessingh P. A gregarizing factor present in the egg pod foam of the desert locust Schistocerca gregaria. J. Exp. Biol. 1998;201:347–363. doi: 10.1242/jeb.201.3.347. [DOI] [PubMed] [Google Scholar]
- Moksnes A, Røskaft E. Egg-morphs and host preference in the common cuckoo Cuculus canorus: an analysis of cuckoo and host eggs from European museum collections. J. Zool. Lond. 1995;236:625–648. [Google Scholar]
- Nelson D.A. A preference for own-subspecies' song guides vocal learning in a song bird. Proc. Natl Acad. Sci. USA. 2000;97:13 348–13 353. doi: 10.1073/pnas.240457797. 10.1073/pnas.240457797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G.A, Partridge L. Sexual conflict and speciation. Phil. Trans. R. Soc. B. 1998;353:261–274. doi: 10.1098/rstb.1998.0208. 10.1098/rstb.1998.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzen D, Magrath R.D. Parental alarm calls suppress nestling vocalization. Proc. R. Soc. B. 2004;271:1271–1276. doi: 10.1098/rspb.2004.2716. 10.1098/rspb.2004.2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnett R.C. Inheritance of egg-colour in the parasitic cuckoos. Nature. 1933;132:892. [Google Scholar]
- Rutila J, Latja R, Koskela K. The common cuckoo Cuculus canorus and its cavity nesting host, the redstart Phoenicurus phoenicurus: a peculiar cuckoo–host system? J. Avian Biol. 2002;33:414–419. 10.1034/j.1600-048X.2002.02937.x [Google Scholar]
- Skjelseth S, Moksnes A, Røskaft E, Gibbs H.L, Taborsky M, Taborsky B, Honza M, Kleven O. Parentage and host preference in the common cuckoo Cuculus canorus. J. Avian Biol. 2004;35:21–24. 10.1111/j.0908-8857.2004.03219.x [Google Scholar]
- Soha J.A, Marler P. A species-specific acoustic cue for selective song learning in the white-crowned sparrow. Anim. Behav. 2000;60:297–306. doi: 10.1006/anbe.2000.1499. 10.1006/anbe.2000.1499 [DOI] [PubMed] [Google Scholar]
- Whaling C.S, Solis M.M, Doupe A.J, Soha J.A, Marler P. Acoustic and neural basis for innate recognition of song. Proc. Natl Acad. Sci. USA. 1997;94:12 694–12 698. doi: 10.1073/pnas.94.23.12694. 10.1073/pnas.94.23.12694 [DOI] [PMC free article] [PubMed] [Google Scholar]