Abstract
Odor-shock conditioning produces either olfactory preference or aversion in preweanling (12–15 days old) rats, depending on the context. In the mother’s absence, odor-shock conditioning produces amygdala activation and learned odor avoidance. With maternal presence, this same conditioning yields an odor preference without amygdala activation. Maternal presence acts through modulation of pup corticosterone and corticosterone’s regulation of amygdala activity. Over-riding maternal suppression of corticosterone through intra-amygdala corticosterone infusions permits fear conditioning and amygdala activation.
Here we show two circuits for odor-shock conditioning, with maternal presence providing the ‘switch’ by lowering pups’ corticosterone levels. Because pups must learn the diet-dependent maternal odor for interactions with the mother (such as nipple attachment and approach), this system ensures that pups only learn to approach maternal odor. The mother’s ability to modify fear learning circuitry may provide clues to abusive attachment and predisposition for mental illness and altered emotional expression later in life1–3. The validity of an animal model of abusive attachment is strengthened by the wide phylogenetic representation of abusive attachment, which has been documented in chicks, infant dogs, rodents and nonhuman primates4,5. Moreover, these data provide insight into the timing and mechanisms of functional emergence of brain areas during development.
During early life when pups are confined to the nest (the ‘sensitive period’), they exhibit potentiated preference learning and attenuated aversion learning, characterized by odor preferences induced by conditioning with an odor and a 0.5-mA shock6–8. This paradoxical learning does not reflect the pups’ inability to feel pain or threshold differences9, but reflects the inability of odor-shock conditioning to engage the amygdala8,10–12. The sensitive period ends as the pups’ ability to walk emerges and life outside the nest begins (at age 10 d), with a rapid transition to independence by age 21–23 d. In this ‘postsensitive period’, preweanling rats are in a transitional period from dependence to independence. At this stage, the pups need both continued interactions with the mother as well as the engagement of contingency-dependent learning for survival outside the nest. The effects of maternal presence on odor-pain conditioning may ensure that pups continue to only learn approach responses to her odors, whereas in her absence they learn complex contingencies required for survival outside the nest.
Here we present data illustrating that odor-shock learning (0.5-mA shock) in pups accommodates their changing developmental needs. Odor-shock conditioning resulted in an odor preference at an age when pups were confined to the nest (Fig. 1a; 8 d sensitive period; analysis of variance (ANOVA), F3,16 = 3.917, P < 0.005; post-hoc Fisher tests between each group). However, pups between 12 and 15 d old (that is, postsensitive period), an age that represents a transition from nest life to independent life, learned an odor preference while with the mother and an odor aversion while alone (Fig. 1b; ANOVA, F3,28 = 25.563, P < 0.0001; post-hoc Fisher tests between each group). In pups of weaning age (21–23 d old), odor-shock conditioning produced an odor aversion with or without the mother (Fig. 1c; ANOVA, F3,15 = 9.404, P < 0.005; post-hoc Fisher tests between each group). This dual learning system may ensure that pups still only learn to approach the maternal odor, but also learn to avoid odors they encounter outside the nest. The work presented here explored the mechanisms responsible for pups’ dual learning system using a systems-level analysis.
Figure 1.
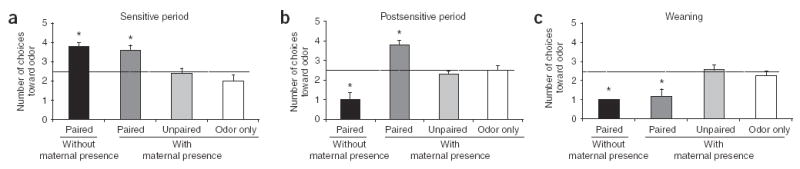
Pup learning from odor-shock conditioning (0.5-mA shock) changes over development and is influenced by maternal (anesthetized) presence. Behavior was examined using a Y-maze test, (a) 8-d-old rats learned to prefer an odor paired with a shock, with or without maternal presence, (b) When conditioned without maternal presence, 12- to 15-d-old pups subjected to paired odor-shock learned an odor aversion. Pups that were conditioned with maternal presence learned an odor preference. (c) Pups of weaning age (21 to 23 d old) learned odor avoidance with or without maternal presence. *P < 0.05. Error bars represent s.e.m.
Our rationale for assessing how the mother could function as a ‘switch’ between the two learning systems was based on previous data. First, the termination of the sensitive period is coincident with the gradual decline of the pups’ ‘stress hyporesponsive period’ when stressors such as shock begin to produce a surge in corticosterone release11,13. In preweanling pups, odor-shock conditioning requires that corticosterone produce odor aversion learning and basolateral amygdala plasticity11,12. Indeed, giving corticosterone to 7-d-old (that is, sensitive period) pups permits aversion learning and engages the amygdala, whereas depleting 12-d-old (that is, postsensitive period) pups of corticosterone (by adrenalectomy) reinstates the sensitive period11,12. Second, maternal presence suppresses shock-induced corticosterone release in preweanling pups14.
Here we used 12- to 15-d-old (postsensitive period) pups in an odor-shock fear conditioning protocol (0.5-mA shock) similar to one that engages the amygdala in adult rats15. For the paired presentations, pups were administered 11 0.5-mA, 1-s-long tail shocks during the last second of a 30-s-long presentation of a peppermint odor. Controls received either the odor only or unpaired presentations of odor and shock (details in Supplementary Methods online). Pups were conditioned in either the presence or the absence of an anesthetized mother and were tested the next day in a Y-maze (conditioned odor versus the familiar odor of clean bedding). Pups subjected to odor-shock without maternal presence learned to avoid the odor. In contrast, pups subjected to odor-shock with maternal presence developed the paradoxical shock-induced odor preference (Fig. 1b). The olfactory bulb only participated in the conditioning for the ‘paired with maternal presence’ pups that expressed an odor preference (Fig. 2a). This enhanced responding of the olfactory bulb is typical of learning-associated changes in younger (sensitive period) pups and is associated with learning the maternal odor4,13 (ANOVA, F4,21 = 7.798, P < 0.001; post-hoc Fisher tests between each group). We performed auto-radiographic analysis of the basolateral complex (Fig. 2b) and other nuclei (Supplementary Figs. 1 and 2 online) of the amygdala during conditioning. The amygdala’s cortical, medial, basolateral and lateral nuclei only participated in odor-shock conditioning in the mother’s absence (ANOVAs: basolateral, F4,20 = 16.577, P < 0.0001; lateral, F4,20 = 27.940, P < 0.0001; cortical, F4,19 = 10.796, P < 0.0001; medial, F4,20 = 7.425, P < 0.001; post-hoc Fisher tests between each group; details in Supplementary Methods). Next, we reversibly silenced the amygdala with muscimol (0.5 nmol; GABAA receptor antagonist) and found a causal relationship between maternal presence, odor-shock conditioning and amygdala participation. The amygdala silencing disrupted odor aversions learned from odor-shock pairings in the ‘without maternal presence’ condition, suggesting that the amygdala is important in aversion-induced odor-shock conditioning. However, this manipulation did not disrupt paired conditioning in the ‘with maternal presence’ condition (Fig. 2c; ANOVA, F3,24 = 3.667, P < 0.05; post-hoc Fisher tests between each group; placements of infusion cannulae shown in Supplementary Fig. 3 online).
Figure 2.
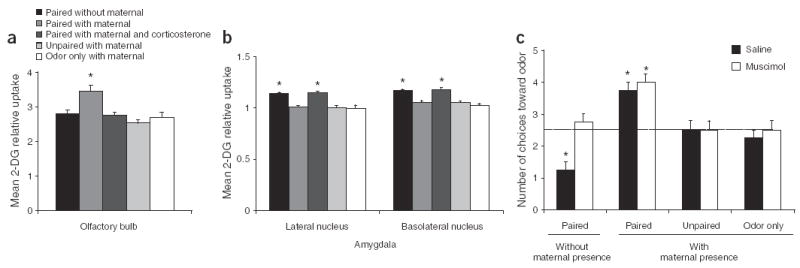
Maternal presence activates a non-amygdala dependent odor-shock circuit and yields odor preference, (a) Olfactory bulb activity during odor-shock acquisition was assessed by relative 14C 2-deoxyglucose (14C 2-DG) uptake. Enhanced uptake was found in pups subjected to paired odor-shocks with maternal presence that expressed an odor preference, (b) Activity in the basolateral and lateral nuclei of the amygdala were enhanced during odor-shock presentation only without maternal presence, as assessed by relative 14C 2-DG uptake. Additional amygdala nuclei and a representative 14C 2-DG/Nissl-stained amygdala section are shown in Supplementary Figs. 1 and 2, respectively. (c) Reversibly silencing the amygdala with the GABA agonist muscimol disrupted the odor aversion learning in pups subjected to odor-shock pairings without maternal presence but had no effect on the pups subjected to odor-shock pairings with maternal presence. *P < 0.05. Error bars represent s.e.m.
Due to the important role of corticosterone in infant rat learning and in the mother’s ability to reduce shock-induced corticosterone release in pups, we assessed corticosterone’s effect on learning and amygdala activity10,11. Corticosterone levels during conditioning were significantly higher in pups subjected to paired conditioning without maternal presence than in those with maternal presence, including shock and nonshock groups (Fig. 3a; ANOVA F3,16 = 11.794, P < 0.0005; post-hoc Fisher tests between each group). Maternal effects on corticosterone and fear learning were further supported through systemic and intra-amygdala infusions of corticosterone. In pups subjected to paired odor-shock with maternal presence, the systemic administration of corticosterone (3 mg) 30 min before conditioning enabled odor aversion learning, as well as the incorporation of the amygdala into the learning circuit12,13 (Fig. 3b; behavior ANOVA, F2,12 = 11-400, P < 0.005; post-hoc Fisher tests between each group; olfactory bulb and amygdala data are included in Figs. 2a and 2b, respectively, along with the associated statistics). Furthermore, in these pups (odor-shock with maternal presence), direct infusion of corticosterone (50 ng) into the amygdala during conditioning resulted in the learning of an odor aversion12 (Fig. 3c; ANOVA, F2,20 = 35.362, P < 0.0001; post-hoc Fisher tests between each group; placement of infusion cannulae are shown in Supplementary Fig. 4 online).
Figure 3.
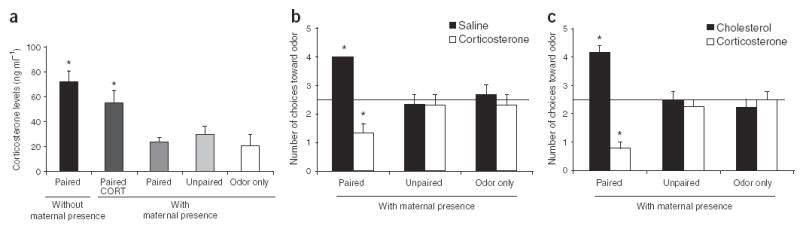
Assessment of the association between corticosterone, learning and the amygdala, (a) Radioimmunoassay (RIA) corticosterone levels were low in pups receiving shock with maternal presence, but high in those receiving shock without maternal presence, (b) Pups subject to paired odor-shock with maternal presence were given systemic corticosterone 30 min before conditioning. These pups showed odor aversion learning, (c) Intra-amygdala corticosterone permitted these pups to learn an odor aversion. *P < 0.05. Error bars represent s.e.m.
In summary, our data suggest that preweanling pups have two odor-shock learning circuits, with maternal presence providing suppression of stress-induced corticosterone release and engaging the odor-shock circuit for odor preference learning supporting infant-mother attachment. These data provide insight into the timing and mechanisms of functional emergence of the amygdala and suggests ways in which the functional maturation of brain development may be disrupted by stress.
Supplementary Material
Acknowledgments
Supported by grants to R.M.S. from the US National Institute of Child Health and Human Development (HD33402), the US National Science Foundation (IOB0544406) and the Oklahoma Center for the Advancement of Science and Technology; and by funds to S.M. from the University of Oklahoma.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Teicher MH, et al. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 2.Heim C, Nemeroff CB. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 3.Pollak SD, Kistler DJ. Proc Natl Acad Scl USA. 2002;99:9072–9076. doi: 10.1073/pnas.142165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan PM. Ann NY Acad Sci. 2003;1008:122–131. doi: 10.1196/annals.1301.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maestripieri D, Lindell SG, Ayala A, Gold PW, Higley JD. Neurosci Biobehav Rev. 2005;29:51–57. doi: 10.1016/j.neubiorev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Haroutunian V, Campbell BA. Science. 1979;205:927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- 7.Camp LL, Rudy JW. Dev Psychobiol. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan PM, et al. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barr GA. NIDA Res Monogr. 1995;158:172–201. [PubMed] [Google Scholar]
- 10.Roth TL, Sullivan PM. Biol Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Levine S. Eur J Pharmacol. 2000;405:149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- 12.Moriceau S, Wilson DA, Levine S, Sullivan RM. J Neurosci. 26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriceau S, Sullivan RM. Behav Neurosci. 2004;118:274–281. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanton ME, Levine S. Dev Psychobiol. 1990;23:411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- 15.Fanselow MS, LeDoux JE. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


