Abstract
Lesion or degeneration of the cerebellum can profoundly impair adaptive control of reaching in humans. Computational models have proposed that internal models that help control movements form in the cerebellum and influence planned motor output through the cerebello-thalamo-cortical pathway. However, lesion studies of the cerebellar thalamus have not consistently found impairment in reaching or adaptation of reaching. To elucidate the role of the cerebellar thalamus in humans, we studied a group of essential tremor (ET) patients with deep brain stimulation (DBS) electrodes placed in the cerebellar thalamus. The stimulation can be turned on or off remotely and is thought to reduce tremor by blocking the spread of the pathological output from the cerebellum. We studied the effect of thalamic DBS on the ability to adapt arm movements to novel force fields. Although thalamic DBS resulted in a dramatic and significant reduction of tremor in ET, it also impaired motor adaptation: the larger the stimulation voltage, the greater the reduction in rates of adaptation. We next examined ET patients that had undergone unilateral thalamotomy in the cerebellar thalamus and found that adaptation with the contralateral arm was impaired compared with the ipsilateral arm. Therefore, although both lesion and electrical stimulation of the cerebellar thalamus are highly effective in reducing tremor, they significantly impair the ability of the brain to form internal models of action. Adaptive control of reaching appears to depend on the integrity of the cerebello-thalamo-cortical pathway.
Keywords: Keywords: DBS, essential tremor, internal models, motor control, motor learning, thalamotomy, Vim
Introduction
Our limbs have inertial dynamics that dictate a complex relationship between joint motions and joint torques. In order to reliably produce a simple movement, such as flexion of the elbow, the brain must activate not only elbow flexors but also shoulder flexors that counter the shoulder extension torque produced by acceleration of the elbow. To decelerate the elbow flexion and stop at the target, activation and precise timing of elbow extensors are required. Otherwise, the limb will overshoot the target and oscillate (Vilis and Hore, 1980). Current theories suggest that because of time delays in sensory feedback, the brain implicitly accounts for this physics when it composes motor commands (Shadmehr and Mussa-Ivaldi, 1994). To perform a voluntary movement, the brain appears to perform 2 kinds of computations: 1) given a desired change in the proprioceptively or visually defined sensory state of the limb, it predicts the motor commands that are likely to produce the desired change, and 2) given a planned motor command, it predicts the sensory consequences of that command. These sensorimotor and motor-sensory maps are collectively called “internal models” of action (Wolpert and Ghahramani, 2000).
A fundamental characteristic of internal models is that when they are embedded into a control system, they reduce the reliance of the controller on sensory feedback. As a result, the accuracy of action is thought to be linked to the accuracy of internal models. For example, when internal models of reaching are inaccurate, simulations of reaching show ataxic symptoms (Schweighofer and others, 1998) like those recorded in cerebellar patients (Bastian and others, 1996). Indeed, neuropsychological studies suggest that the cerebellum is crucially involved in the formation of internal models of reaching. For example, patients with lesions in the posterior cerebellum were unable to adapt to changes in visuomotor alignments imposed by prism goggles (Weiner and others, 1983; Martin and others, 1996). Patients with global cerebellar degeneration were profoundly impaired in adapting to the novel dynamics of a force field (Maschke and others, 2004; Smith and Shadmehr, 2005). In contrast, patients with Huntington disease or Parkinson disease showed normal adaptation of reaching in force fields (Krebs and others, 2001; Smith and Shadmehr, 2005) and normal adaptation with prisms (Fernandez-Ruiz and others, 2003).
The dentate nucleus of the cerebellum projects to the ventrolateral thalamus, which in turn projects to the motor areas of the frontal lobe (Sakai and others, 2002). In nonhuman primates, neural correlates of internal models of reaching have been recorded in the frontal motor areas, including the primary motor cortex (Li and others, 2001; Paz and others, 2003), supplementary motor area (Padoa-Schioppa and others, 2004), and premotor cortex (Padoa-Schioppa and others, 2002). In light of results in human patient studies, it seems likely that aspects of the internal models of reaching form in the cerebellum and influence descending motor commands via cerebello-thalamo-cortical pathways.
Current evidence, however, has not led to any consensus about the role of this pathway in motor learning. Martin and others (1996) reported that 2 out of the 3 patients with lesions in the cerebellar thalamus learned to compensate for prism goggles normally, whereas the other patient did not pass criteria for either baseline performance or adaptation. On the other hand, animal lesion research has demonstrated that cerebellar thalamic nucleus is important for the acquisition of certain motor skills. Fabre and Buser (1979) reported that bilateral lesion of the ventrolateral thalamus in cats impaired learning of a reaching task that involved pointing to moving targets. Jeljeli and others (2003) showed that lesion of the ventral thalamic nuclei in rats caused pronounced deficits in their ability to learn to walk on a rotating beam. The inconsistency between human and animal research could be the result of a real interspecies difference in the role thalamus plays in adaptation. For example, the cerebellar nuclei project to the thalamus as well as the spinal motor neurons through brain stem nuclei. It is plausible that in humans, the cerebellum’s contribution to adaptive control of reaching movements is primarily conveyed via brain stem pathways. However, it is difficult to make any conclusion based on the studies so far because of the paucity of available data and inconsistency across patients.
Programable stimulation of the cerebellar thalamus provides a unique opportunity to explore the role of thalamus in human motor adaptation. We studied patients with essential tremor (ET) who had deep brain stimulators (DBS) stereotactically placed in the posterior aspect of their ventrolateral thalamus (VLp), also known as the ventral intermediate nucleus (Vim). ET is characterized by a 4- to 12-Hz “postural tremor” (present during voluntary maintenance of steady posture) that affects both limbs. In advanced stages, this postural tremor is often accompanied with an intention tremor that intensifies as the hand approaches a target (Elble and Koller, 1990). There is growing evidence supporting the hypothesis that the pacemaker for ET is in the inferior olive–cerebellar circuits (for review, see Deuschl and Bergman, 2002). The anomalous oscillation is believed to be then transmitted by the cerebello-thalamo-cortical pathway and manifest as tremor. In ET patients, pathological rhythmic discharges at tremor frequency are seen in all 3 major nuclei of the ventrolateral thalamus: the cerebellar recipient (Vim), the pallidal recipient, and the principal somatosensory nucleus, with Vim having the highest concentration of such tremor-related neurons (Hua and Lenz, 2005). It has been shown that Vim DBS is highly effective for relief of ET (Koller and others, 2000). The success is made possible by accurate and individual localization of the region within Vim that is associated with limb tremor. The locus of Vim DBS implant is determined by the combination of finding Vim’s stereotactic coordinates from MRI, neurophysiological mapping of the nucleus, and intraoperative confirmation of tremor relief with micro- or macrostimulation of the region identified (Garonzik and others, 2002).
The mechanism by which DBS produces its therapeutic effect is still being elucidated. Mathematical modeling of the response of thalamocortical neurons to DBS suggests that with typical settings of the stimulator, axons of thalamic relay neurons within a 2-mm region around the stimulating electrode are driven to fire at the stimulus frequency, whereas cell bodies and the intrinsic activities of these neurons are inhibited (McIntyre and others, 2004). Indeed, positron emission tomography (PET) imaging studies have shown that DBS leads to increased activation, hence blood flow, in the cortical regions that Vim projects to (Ceballos-Baumann and others, 2001; Perlmutter and others, 2002; Haslinger and others, 2003). Thalamic DBS also tends to drive local inhibitory interneurons in the Vim and may potentially drive the cerebellar nuclei antidromically (the dentate, interpositus, and fastigial nuclei all project to VLp [Macchi and Jones, 1997]). The combined effect of thalamic DBS is thought to prevent the tremor-generating signal in the cerebellar nuclei from reaching the cerebral cortex. However, if cerebellar nuclei also convey to the cerebral cortex information related to internal models of reaching, then Vim stimulation might impair adaptive control of reaching.
We found evidence in support of this conjecture. In a reaching task known to induce adaptation, we observed that when DBS was turned on, patients tended to adapt slower than when no stimulation was given. To explore the possibility that this stimulation related adaptation impairment might have been primarily a result of indirect stimulation of cortical motor regions by thalamic DBS (Haslinger and others, 2003), we considered another group of ET patients, those with prior Vim thalamotomy. We found that although tremor was generally small or absent in the arm contralateral to the thalamotomy, adaptation was better with the arm ipsilateral to the thalamotomy. Together, these findings corroborate with our hypothesis that adaptation of reaching requires the integrity of the cerebellar thalamus.
Materials and Methods
Subjects
Twenty ET patients were recruited from the Johns Hopkins Neurosurgery clinic (FAL). Fifteen ET patients had either unilateral (11 patients) or bilateral (4 patients) Vim DBS implants (mean age: 63 years, range: 42–80 years). Thus, a total of 19 unique DBS sides were tested (mean time since procedure: 16 months, range: 1 day to 5 years, see Table 1), and they are considered as separate DBS cases in the data analysis. The other 5 patients had unilateral Vim thalamotomy (mean age: 66 years, range: 51–71 years; mean time since procedure: 7 years, range: 4–12 years). Of these 5 patients, 4 had left Vim thalamotomy and 1 had right Vim thalamotomy. Of these 20 ET patients, 4 were left-handed and 16 right-handed.
Table 1.
DBS subjects information
| Case IDa | DBS setting
|
Time of experiment relative to surgery
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Electrode contacts
|
|||||||||||
| 0 | 1 | 2 | 3 | Case | Voltage (V) | Pulse width (μs) | Frequency (Hz) | No stimulation | DBS on | ΔLIb | |
| 1R | 0 | – | – | – | + | 2 | 270 | 185 | 3 months 10 days | 5 months 10 days | −0.028 |
| 1R | 0 | – | – | – | + | 1.8 | 270 | 185 | 23 months | 23 months | −0.205 |
| 1R | 0 | – | 0 | 0 | + | 1.8 | 270 | 185 | 23.5 months | 23.5 months | −0.112 |
| 2R | 0 | – | – | – | + | 6.7 | 120 | 185 | 6 months | 6 months | −0.300 |
| 3R | + | – | 0 | 0 | 0 | 4.9 | 120 | 185 | 11 months | 11 months | −0.354 |
| 4R | + | – | – | – | 0 | 4.3 | 60 | 185 | 37 months | 37 months | −0.074 |
| 5R | + | – | – | – | 0 | 4.1 | 210 | 185 | −1 dayc | 2 days | −0.342 |
| 5R | + | – | – | – | 0 | 3 | 210 | 185 | −1 dayc | 9 days | −0.192 |
| 5R | + | – | – | – | 0 | 3.8 | 210 | 185 | −1 dayc | 5 months | −0.043 |
| 5R | + | – | – | – | 0 | 2.8 | 210 | 185 | 16 months | 16 months | −0.046 |
| 5L | 0 | 0 | – | + | 0 | 2.5 | 210 | 185 | −1 dayc | 10 days | 0.149 |
| 6R | 0 | – | – | + | 0 | 4.5 | 60 | 145 | 12 months | 12 months | −0.313 |
| 6L | + | – | – | – | 0 | 1.8 | 120 | 185 | −1 dayc | 5 days | 0.028 |
| 7R | + | – | – | – | 0 | 3.2 | 150 | 185 | 9 months | 1 day | −0.187 |
| 7L | 0 | 0 | 0 | – | 0 | 2.5 | 60 | 185 | 1.3 months | 1.3 months | −0.054 |
| 8R | 0 | 0 | 0 | – | 0 | 4 | 60 | 185 | 1.5 months | 1.5 months | −0.049 |
| 9R | – | 0 | 0 | 0 | + | 2 | 60 | 185 | 1.7 months | 1 month | −0.043 |
| 9L | 0 | – | 0 | 0 | + | 3.5 | 90 | 185 | 30 months | 29.5 months | 0.003 |
| 10R | 0 | 0 | – | 0 | + | 3.5 | 150 | 185 | 4.3 months | 4.3 months | 0.126 |
| 10R | 0 | – | – | – | + | 2.9 | 210 | 185 | 6 months | 6 months | 0.100 |
| 11R | – | 0 | 0 | 0 | + | 3.2 | 90 | 185 | 28 months | 28 months | 0.129 |
| 12L | 0 | 0 | 0 | – | + | 3.5 | 90 | 185 | 33 months | 33 months | −0.006 |
| 12L | 0 | – | – | – | + | 2.1 | 90 | 185 | 34 months | 34 months | −0.106 |
| 13R | – | 0 | 0 | 0 | + | 3.6 | 120 | 185 | 24 months | 24 months | −0.156 |
| 14R | 0 | + | – | 0 | 0 | 3.3 | 60 | 185 | 61 months | 61 months | 0.103 |
| 15R | – | + | 0 | 0 | 0 | 3 | 60 | 185 | 8 months | 8 months | −0.121 |
Case ID: number identifies the patient, letter identifies the arm used in the experiment.
ΔLI denotes the average change in learning index between DBS-on and no-stimulation sessions for the last 2 adaptation sets.
Patient was tested the day before surgery.
Twenty-six healthy adults were recruited to serve as control subjects for the 2 patient groups. Nineteen served as controls for the DBS patient group (mean age: 58 years, range: 49–84 years) and 7 as controls for the thalamotomy patient group (mean age: 58 years, range: 50–71 years). Of these 26 subjects, 3 were left-handed and 23 right-handed. No difference in performance or adaptation level was found between the left- and right-handed control subjects. Subjects gave written consent for the experiments, and the experimental procedures were approved by Johns Hopkins Institutional Review Board.
Experimental Design
We examined adaptive control of reaching in force fields. The task that we used has been previously described (Shadmehr and Brashers-Krug, 1997). Briefly, the subject held onto the handle of a robotic arm and reached to targets that were displayed on a video monitor. A sling was used to support the subject’s arm and restrict movements to the horizontal plane. Each reach is called a “trial.” On odd-number trials, the targets appeared at 10 cm from the center of the screen at 1 of 4 angles: 0°, −45°, −90°, or −135° (measured clockwise from the horizontal axis). On even-number trials, the target appeared back at the center of the screen. At the start of each trial, the subject held the cursor at a crosshair (1 cm wide) indicating trial origin for 0.5 s. The crosshair then disappeared and a square box (1 cm wide) representing the target was displayed. At the end of each reach, the subject received color and sound feedback on the speed and duration of his/her reach. A pleasant “burst” sound was played if the trial was completed within 0.5 ± 0.07 s, and the peak movement speed was between 0.20 and 0.55 m/s. Criteria for movement completion and proximity to trial origin and target were relaxed to accommodate for patient’s tremor. At trial start, the target box would be given if the cursor had been held within 1.5 cm from the center of the crosshair for 0.5 s. Movements were considered complete either after movement speed had fallen below 0.03 m/s for 0.5 s or after the cursor had been within 1.5 cm from the target center for 1 s.
Trials were organized into sets of 96 targets. A single session consisted of 4 “null” sets, followed by 4 “adaptation” sets, followed by 3 “washout” sets. During the null sets, the robot arm was passive and the motors were turned off. During the adaptation sets, the robotic arm applied a viscous curl force field at the handle to perturb the subject’s movements. The force applied at the hand, F(t), was proportional in magnitude and perpendicular in direction to the movement velocity of the hand v(t):
| (1) |
where C = [0 13; −13 0] N s/m for the clockwise curl field and C = [0 −13; 13 0] N s/m for the counterclockwise curl field. Also given within the adaptation sets are “catch trials” (probability of 1/6, randomly placed) where the force field was unexpectedly removed for the duration of the trial. During the washout sets, the robot motors were turned off with the intention of washing out the effect of motor adaptation induced by the force field. In total, subjects performed 11 sets of trials or 1056 reaching movements in each session. A complete study consisted of 2 sessions.
DBS Patient Group and DBS Control Group
Fifteen ET patients with DBS implants were trained in the curl fields under 2 conditions: DBS turned on versus DBS turned off. The hand contralateral to the implant was used in each condition. For patients with bilateral implants, the effect of DBS was studied separately for each implant. The implant ipsilateral to the hand performing the reaching task was always turned off in order to eliminate possible interference. Subjects were randomly assigned to have DBS off during the first session or off during the second session. In 10 DBS sides/cases, DBS was turned on during the first session and off during the second session. In 9 DBS sides/cases, the DBS order was off first, on second. In 3 cases among this later group, the patients performed the first session 1 day before their surgeries (see Table 1). To be consistent, when discussing results for the entire DBS patient group, we use the descriptor “no stimulation” in place of “DBS off”. Because we found an effect of stimulation voltage in the group data, we asked 4 DBS patients (patients 1, 5, 10, 12) to return and repeat the study more than once, each time at a different DBS voltage setting. Only data from each patient’s first study are included for group analyses of motor learning. Patient 15 did not complete the washout sets in session 2 and was excluded from the state space analysis (see Supplementary Material online).
For both the patient and the control groups, the counterclockwise field was given in the first session and the clockwise field in the second.
Programing the DBS
Programing of the DBS was performed by a trained physician. The adjustable parameters for DBS are stimulation voltage, pulse width, frequency, polarity at each of the 4 contacts, and polarity at the battery case. The optimal parameter combination for each patient was carefully searched based on reports and observations of stimulation response by both the patient and the physician. Tasks used to evaluate the response include postural hold (arm extension or drinking from a cup), pointing (finger-to-nose pointing), drawing (spiral and line drawing), and writing. The final DBS setting selected was the one that achieved maximum effectiveness on tremor reduction while inducing little, no, or only transient side effects of stimulation such as paresthesia and dysarthria. In some patients, multiple parameter combinations achieved similar therapeutic results. We conducted multiple experiments in 4 such DBS patients, each time under a different stimulation parameter combination to assess the effect of stimulation parameter on motor learning (see Table 1).
Thalamotomy Patient Group and Thalamotomy Control Group
We recruited 5 ET patients with Vim thalamotomy (see Table 3) and tested them in 2 sessions in a procedure similar to that of DBS patients. In the morning session, thalamotomy patients trained with the arm ipsilateral to the thalamotomy in the counterclockwise curl field. In the afternoon, they trained with the arm contralateral to the thalamotomy in a clockwise field. Control subjects for the thalamotomy patient group trained with their nondominant arms in the counterclockwise field during the first session and their dominant arms in the clockwise field during the second session.
Table 3.
Thalamotomy subjects information
| Patient | Locus of thalamotomy | Date of surgery |
|---|---|---|
| 1 | Left Vim | 1999 |
| 2 | Left Vim | 1998 |
| 3 | Left Vim | 1991 |
| 4 | Left Vim | 1996 |
| 5 | Right Vim | 1993 |
Performance Measures
For each trial, we measured general movement performance with 4 parameters: path length, movement duration, peak speed, and movement error in terms of angular deviation (defined below) 300 ms after movement onset.
Movement onsets can be easily detected with a speed threshold when the speed profiles of the movements are relatively smooth and single peaked. For ET patients, however, postural tremor can often prevent the hand from holding still at trial origin and add oscillatory irregularities to the movements. Thus a simple speed threshold can lead to false detection of movement onset. We took a number of steps to accurately detect movement onset. The trajectory of each trial was broken down to movement segments that exceeded 0.03 m/s, and only those segments longer than 300 ms were selected. To select the correct movement segment, the starting point of the segment had to be no farther than 1 cm from the origin and the net displacement toward the target for the segment had to be at least 4.5 cm. This precluded erroneous inclusion of looping trajectories resulting from postural tremor while patients attempt to hold still at origin, as well as in trials in which sudden dips in speed occurred on route to target.
To analyze motor adaptation, we focused on the movement error made in the first 300 ms of each reach. We defined angular error as the angle of trajectory deviation from the target direction at a fixed time after movement onset, with the convention that counterclockwise errors were positive. Another frequently used measure of error is displacement in the direction perpendicular to target direction. Results from analysis performed with perpendicular displacement at 250 or 300 ms and angular error at 250 or 300 ms were consistent. We chose to use angular error at 300 ms for this paper.
During the adaptation and washout trials, we measured movement error with respect to errors recorded at the end of the null sets—after subjects had completed nearly 300 practice trials. That is, a baseline movement error for each direction was estimated from the last null set by taking the median angular error of all trials made in that direction. All subsequent analyses on motor adaptation were based on these median-corrected angular error measurements.
Learning Index
To reduce motor errors while unfamiliar forces are applied at the hand, the motor system could adopt either one of 2 strategies: cocontract the muscles to increase the stiffness of the arm or predictively compensate for the force fields by developing an internal model. Both strategies lead to the reduction of trajectory deviations during field trials; however, they result in very different catch trial behaviors. Cocontraction would keep errors small in catch trials just as it does field trial. Internal model, on the other hand, would cause catch trial trajectories to become more deviated in the opposite direction as it evolves to better compensate the external forces (Shadmehr and Mussa-Ivaldi, 1994). Hence, the measure that quantifies learning must capture changes of trajectory errors in both field and catch trials. A learning index (Donchin and others, 2002; Smith and Shadmehr, 2005) is calculated for each set as follows:
| (2) |
where and are the median angular errors for all catch trials and all field trials in the set, respectively. Because and have opposite signs, their difference is the combined angular error of field and catch trials, which corresponds to the net effect of the force field on movement trajectories. This effect depends on the magnitude of the force field as well as compliance of the subject’s arm. By normalizing with the force-field effect, we allow the learning index to be independent of arm compliance. Note that the index is nonnegative. Zero angular error in catch trials yields a zero learning index. When the force field is fully compensated, the learning index attains the maximum 1.0.
We used the average learning index for the second half (third and fourth sets) of the adaptation sets as a measure of the overall level of motor adaptation achieved during each experiment session. We also used the denominator in equation (2) as a measure of each subject’s arm compliance. The average compliance in the second half of the adaptation sets is presented in Table 2.
Table 2.
Performance measures of DBS patients and DBS control subjects
| Performance measure | DBS patients | Controls | Change from controls (%) |
|---|---|---|---|
| Group size | 19 | 19 | — |
| Peak speed (m/s) | 0.29 (0.05) | 0.31 (0.04) | −8 (24*) |
| Path length (cm) | 10.70 (1.34) | 10.06 (0.7) | 6*** (91****) |
| Movement duration (s) | 1.55 (0.33) | 1.11 (0.17) | 39*** (95**) |
| ae at 300 ms (°) | 0.59 (6.85) | −0.18 (4.44) | −433 (53***) |
| Arm compliance (°) | 16.55 | 15.19 | 9 |
Note: With the exception of arm compliance, performance measures in the table are computed using trials from the last null set (before adaptation sets began) of each experimental session. The across-trial mean and standard deviation of each performance measure are averaged across sessions for each subject and then compared between the DBS patient group (n = 19) and the control group (n = 19). The group means of the 2 statistics for each measure are displayed in separate rows with the mean standard deviation shown in parentheses. The columns, from left to right, show mean values for the patient group, mean values for the control group and percent change of the patient group mean from the control group mean. Arm compliance is measured as the average difference between catch trial and field trial angular errors (at 300 ms) during the last 2 adaptation sets, hence given in units of degrees. Standard deviation was not calculated for arm compliance as arm compliance was derived per set rather than per trial. ae: raw angular errors, before corrections for bias. Asterisks indicate significance of the patient group mean difference from controls using 2-sided t-test.
P < 0.05,
P < 0.01,
P < 0.001,
P < 0.0001.
Tremor Analysis
We obtained tremor information by analyzing each patient’s movement trajectories in the task. This approach imposed several constraints. First, tremor recorded by the robot arm was restricted to the horizontal plane. Second, because the trial lengths were short and the frequency resolution of any spectral analysis is the inverse of the data duration, we were not able to measure tremor with a high degree of precision. For most patients, the average recording duration—the sum of time waiting at the origin for target, on route to target, and time at the target—was around 2 s. Trials with large tremor had significantly longer recording durations as more time was spent at the origin waiting for the hand velocity and deviation from origin to decrease below thresholds.
Because of the above limitations, we did not attempt to separately resolve postural tremor—oscillations produced at the origin and target box while patients are attempting to hold still—and “kinetic tremor”—oscillation produced en route to the target. Rather, we measure the amount of tremor present in each trial on the whole. For the first null set of each session, we computed the 1024-point power spectral density (PSD) of each trial’s acceleration profile. The average PSD of the set was then normalized by its integral so that comparisons could be made across subjects. To assess the effects of thalamotomy and DBS on ET, we computed for each subject’s normalized PSD the fractional power occupied by the frequency range from 3 to 10 Hz. Besides being a relevant frequency range for ET, the 3- to 10-Hz band was chosen so that task-related movement power was excluded. The acceleration profile of a point-to-point movement cycles through a peak and a trough much like a sine function does over one period. Because in our task the average time it takes for subjects to make the 10-cm movement is between 0.5 and 1 s, the associated acceleration power will concentrate in the 1- to 2-Hz range. As illustrated in Figure 1A, the large peaks below 3 Hz in all PSD are task related. The same spectral analysis was performed on control subjects, and the averaged PSD between the 2 sessions was used for comparison with patients.
Figure 1.
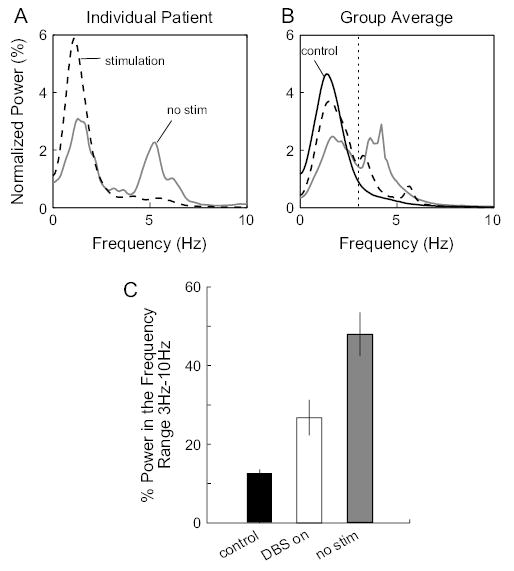
Tremor reduction in DBS patients. (A) Normalized average PSD for trials in the first null set of each experimental session for a DBS patient. With no stimulation, the PSD exhibited a peak centered at 5 Hz. With stimulation, this tremor-associated peak was absent. (B) Group averages of the normalized PSD measured under each stimulation condition were plotted along with the group average PSD for the control subjects (averaged over the 2 sessions). Dotted vertical line marks 3 Hz. The fraction of power in the range of 3–10 Hz (tremor frequency range) was used to quantify tremor amplitude. (C) Average fraction of power in the tremor frequency range for the control group, DBS patients with stimulation, and DBS patients with no stimulation. Error bars are standard errors. Stimulation resulted in significant reduction of tremor power (P = 0.0051).
Results
We studied the ability of the brain to adapt control of reaching to changes in the dynamics of the environment. Our task is a well-studied paradigm where subjects hold the handle of a robotic arm and reach to visually displayed targets (Shadmehr and Mussa-Ivaldi, 1994). The robot either produced no active forces (null trials) or produced a pattern of forces that depended on hand velocity (force-field trials). We began our study by examining a group of ET patients that had a DBS implant at the anterior aspect of the thalamic cerebellar nucleus (Vim) of the thalamus (n = 15). The basic paradigm involved 2 sessions of testing. In session 1, subjects performed 384 trials in the null sets (baseline training), then 384 trials in a force-field set (adaptation training), and finally 288 trials in the null sets (washout). Session 2 was identical to session 1 except that forces in the field were rotated by 180°. Patients were randomly assigned to 1 of 2 groups: one group had no stimulation in session 1, whereas another group had no stimulation in session 2. Table 1 provides information on stimulation settings and the times at which experiments were conducted relative to the patients’ implant surgery dates.
Stimulation Reduced Tremor in the Initial Null Set
Oscillations of the hand at 4–12 Hz are a typical feature of ET when the arm is held up against gravity. DBS is very effective in treating this tremor (Vaillancourt and others, 2003). Indeed, our patients displayed clear benefits from the DBS during routine neurological examination consisting of tasks such as postural hold (arm extension or drinking from a cup), pointing (finger-to-nose pointing), drawing (spiral and line drawing), and writing. Because we were interested in quantifying the effect of thalamic stimulation on learning control of reaching, we assessed the effect of stimulation on tremor during the same task.
We focused on the effect of DBS on tremor during reaches in the first null set. We measured tremor in each trial by computing a PSD of the hand acceleration profile and then normalized this measure by its integral. We then compared this normalized PSD between the DBS-on condition and the no-stimulation condition. Figure 1A shows this measure for a representative patient. With no stimulation, the PSD was bimodal, showing a task-relevant peak at 1–2 Hz and a tremor-related peak at about 5 Hz. Thalamic stimulation almost completely eliminated the tremor, resulting in an increase of percent power in the task-relevant 1–2 Hz (see Materials and Methods). The group average plot (Fig. 1B) indicated a consistent pattern of tremor reduction in our patients. To quantify this effect, we computed the fraction of power in the 3- to 10-Hz range for each subject (Fig. 1C). Stimulation reduced the fractional power in the tremor frequency range by 44% (paired t-test, P = 0.0051).
After a Period of Practice in the Null Sets, Movement Kinematics Were Comparable between Stimulation Conditions
We found that for almost all patients, in the no-stimulation condition the tremor was largest during the initial null set, but then decreased substantially with time and practice. The initial large tremor may in part have been due to nervousness associated with exposure to a novel task, as ET can be aggravated by stress (Gengo and others, 1986). With practice and familiarity, patients may have been able to assume a more relaxed posture and mental state.
Figure 2 provides examples of reaching movements of a DBS patient during early and late null sets with and without stimulation. Figure 2A shows that with no stimulation, the patient’s movements in the first null set exhibited significant tremor both while the hand was waiting at the origin and while the hand was moving. In the later null sets during the same no-stimulation session (Fig. 2B), the patient’s tremor was mostly confined to the waiting period and its magnitude was greatly reduced so that the total movement time was shortened almost by half. Surprisingly, tremor in late null set with no stimulation was comparable with tremor with DBS turned on (Fig. 2C,D). Indeed, across all patients, we found that by the last null set tremor magnitude (in terms of fraction of power in the 3- to 10-Hz range) in the no-stimulation condition had been reduced from the first null set by an average of 32%. This compares with the 44% tremor reduction by DBS (from the first null set of the no-stimulation condition to the first null set in the DBS-on condition). Therefore, regardless of the stimulation condition, tremor had substantially decreased by the last null set of each experimental session.
Figure 2.
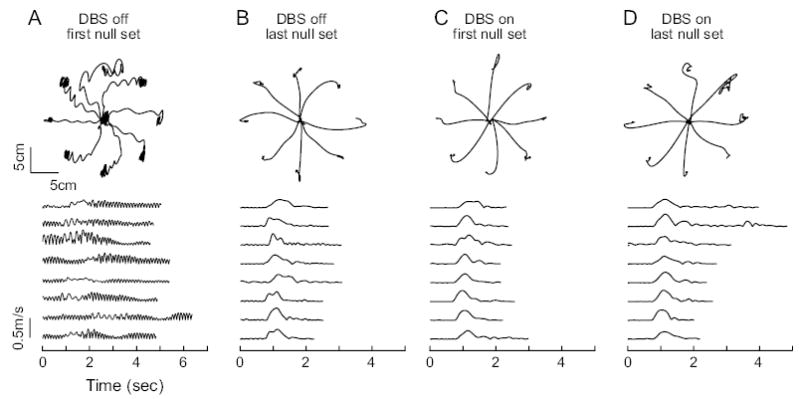
Example of reach trajectories from a DBS patient. Top row: paths of the first movements made in each direction during selected sets. Bottom row: speed profiles of the movements in the top row, corresponding to directions 0°, 45°, . . . , 315° (from top to bottom). (A) Trajectories from the first null set of the no-stimulation session. (B) Trajectories from the last null set of the no-stimulation session. (C) Trajectories from the first null set of the DBS-on session. (D) Trajectories from the last null set of the DBS-on session. With DBS on, the first null set began with dramatically less tremor than the no-stimulation condition. However, in the no-stimulation condition, with the support of the sling at the elbow and increasing familiarity with the task, tremor subsided to levels comparable with DBS on.
Once the tremor had subsided, did DBS affect other aspects of reaching? We focused on trials made in the last null set of each session and used 4 parameters to characterize movement trajectories: path length, angular errors at 300 ms after movement onset, peak speed, and movement duration. The mean and standard deviation values of these parameters were used to compare both across stimulation condition and subject group. Surprisingly, we found that with stimulation there was no significant within-subject change in the mean value of any of the 4 kinematic parameters. DBS also did not change patient’s arm compliance. In fact, performance with DBS turned on showed a significant increase in standard deviations of path length (24%, 2-sided paired t-test, P = 0.0085) and peak speed (8%, P = 0.013). Thus, although DBS effectively suppressed tremor, it did not improve the average movement kinematics and actually resulted in increased trial-to-trial variability of the movements. As compared with control subjects, ET patients had increased mean path length (6%) and movement duration (39%) (Table 2). Performance by patients also showed significantly increased intertrial variability in all parameters.
Stimulation Impaired Reaching Adaptation to Force Fields
Adapting to altered dynamics of reaching requires changes in motor commands that initiate the reach (Thoroughman and Shadmehr, 1999). These changes are due to feedforward mechanisms because in catch trials where the dynamics are unexpectedly removed, the limb overcompensates, resulting in aftereffects. Figure 3A shows the average size of aftereffects achieved by a control subject and a DBS patient toward the end of trainings in the adaptation sets. For the control subject, aftereffects from the 2 experimental sessions were comparable, indicating similar amounts of adaptation. The DBS patient, however, showed significantly larger aftereffects in the no-stimulation session than in the DBS session.
Figure 3.
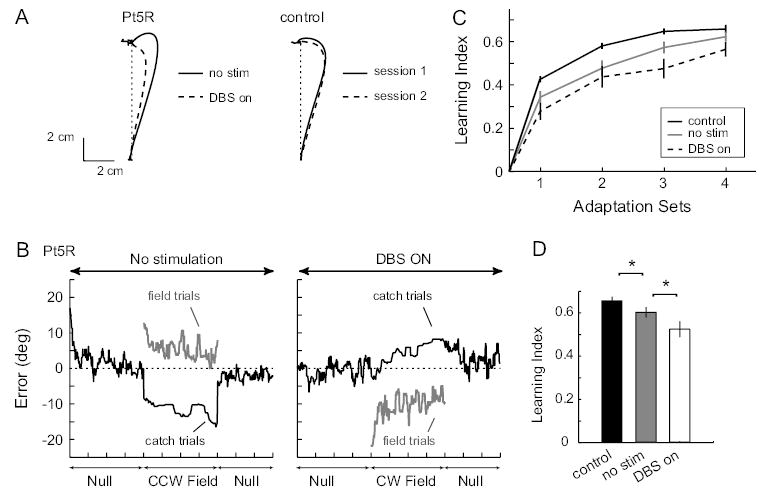
Effect of DBS on motor adaptation. (A) Average catch trial trajectories of a DBS patient and a control subject. Trials were taken from the last adaptation set of each session and were rotated to a canonical direction before averaging. Trials from the session where a clockwise force field was given are inverted for ease of visual comparison. (B) Performance of a DBS patient with stimulation (left) and with out stimulation (right). Shown here are moving averages (window size = 15) of angular error for all trials in each session. The patient achieved significantly higher catch trial errors and lower field trial errors with DBS off than DBS on. (C) Average learning index for each adaptation set is shown for the control group and each stimulation condition for the patient group. Error bars are standard errors. (D) Summary of performance: the average learning index over the last 2 adaptation sets for each subject group. With no stimulation, patients showed reduced learning index compared with control subjects (P = 0.025). With DBS turned on, an additional reduction in learning was observed (P = 0.024).
Figure 3B shows for the same DBS subject the time course of angular errors (trajectory deviation at 300 ms into the movement) during each experimental session. For a system that learns to predict the dynamics of the task, we would expect to see decreasing field trial errors along with increasing catch trial errors (aftereffects). This patient exhibited the expected error pattern both with and without thalamic stimulation. However, training without stimulation led to significantly larger after-effects (as seen in Fig. 3A) and smaller force-field errors than training with stimulation. We used the ratio of catch trial errors to the difference between catch and field errors as a learning index (Donchin and others, 2002; Smith and Shadmehr, 2005). As errors in catch trials increase and errors in field trials decrease this index increases from 0 to 1, with unity value reflecting complete adaptation. Figure 3C plots the distribution of this index for each subject group. Without stimulation, patients were impaired in adaptation with respect to controls. However, stimulation further degraded this performance. Figure 3D quantifies this effect by averaging performance in the last 2 training sets. When no stimulation was applied, ET patients showed on average an 8% reduction in learning index compared with controls (P = 0.025). However, with stimulation, the patients showed an additional 13% reduction in the learning index (P = 0.024 when comparing DBS on and no stimulation; 20% reduction comparing DBS on with control, P = 0.0007).
Acquisition of internal models involves error-dependent trial-to-trial changes in motor commands. For adaptation to take place, error experienced in a given movement to a given target needs to influence subsequent motor commands for that movement direction; this corrective influence may “spill over” to other movement directions as well, resulting in generalization of adaptation. We can quantify this pattern of direction-dependent trial-to-trial adaptation via an error generalization function (Thoroughman and Shadmehr, 2000; Donchin and others, 2003; Smith and Shadmehr, 2005). The rate of adaptation also depends on the strength of motor memory retention. It is possible that patients do not adapt as well because the trace of motor memory somehow decays faster. In Supplementary Material, we characterized these properties of adaptation for our subject populations with an autoregressive linear state space model that has been previously applied to study both healthy subjects and movement disorder patients (Thoroughman and Shadmehr, 2000; Donchin and others, 2003; Smith and Shadmehr, 2005). Our goal was to use the model to identify components of the adaptive computation that were affected by either ET itself or stimulation, which led to the overall reduction in learning we observed with learning index. We found that neither ET nor thalamic stimulation significantly affected the general shape of the error generalization function or motor memory retention. Rather, they significantly reduced the strength of generalization in several key movement directions relative to the direction in which error was experienced. In particular, at the movement direction where error was experienced, ET patients without stimulation showed over 30% reduction in error sensitivity compared with controls. Thalamic stimulation led to an additional 37% reduction in this sensitivity to errors.
Adaptation Impairment Was Correlated with Stimulation Voltage
Thalamic stimulation does not simply switch off a subcortical–cortical neuronal relay. Rather, variation of stimulation parameters (voltage amplitude, frequency, pulse duration, and electrode selection) produces a complex pattern of activity in the thalamocortical circuitry. A recent study found that although increased stimulation voltage was consistently associated with increased tremor relief, pulse duration had only a small effect and frequency change had no significant effect (O’Suilleabhain and others, 2003). If the degree of tremor reduction depends on parameter settings, then do deficits in motor learning also depend on parameters of stimulation? Figure 4A,B illustrate the performance of 2 patients with 2 different stimulation voltage settings. With the DBS off, both subjects demonstrated motor adaptation (the exact levels of adaptation vary from patient to patient). When DBS was turned on, performance of the subject with higher voltage (Fig. 4A) was significantly reduced, whereas performance of subject with lower voltage (Fig. 4B) remained similar to that of the off state. Figure 4C plots the relationship between the magnitude of within-subject percent change in the learning index and the stimulation voltage. We found a significant correlation (Pearson’s correlation, r = −0.67, P = 0.0018; Spearman rank correlation, r = −0.62, P = 0.0044) between stimulation voltage and the degree of impairment in motor adaptation. The voltage sensitivity was somewhat stronger when the electrode configuration was in bipolar mode (stimulating with respect to 1 of the 4 electrodes, Fig. 4C) than in unipolar mode (stimulating with respect to the battery case, Fig. 4D).
Figure 4.
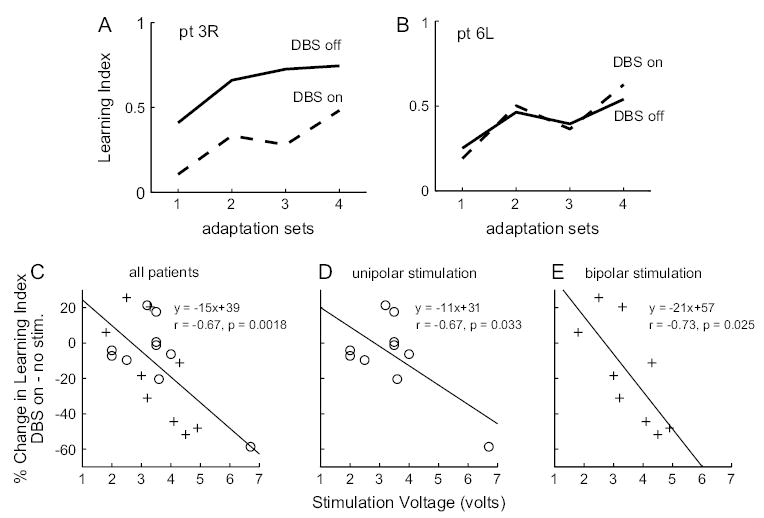
Relationship between stimulation voltage and adaptation impairment. (A) Performance by a patient whose stimulation voltage for the DBS was set at 4.9 V. (B) Performance by a patient whose stimulation voltage was set at 1.8 V. (C) Percent change in learning index (DBS on − no stim.) as a function of stimulation voltage for all 19 DBS cases. Circles indicate patients with unipolar stimulation, and pluses indicate patients with bipolar stimulation. (D, E) Relationship between stimulation voltage and percent change in learning index fit separately for patients with unipolar and bipolar stimulations.
In contrast, we did not observe a correlation between learning impairment and pulse width of DBS (frequency of stimulation was identical in all but one of our patients). The partial correlation between percent change in learning index and stimulation voltage, controlling for stimulation frequency, pulse width, stimulation mode (bipolar or unipolar), number of cathodes activated, number of all activated contacts, time of the study relative to each patient’s implant surgery, and time lag between the DBS-on session and no-stimulation session (see Table 1), was r = −0.75 (P = 0.005, df = 10, 2 tailed). This indicates that in our study, voltage was the only parameter in the above 8 factors that plays a significant role in motor adaptation impairment. We further performed stepwise regression to examine the effect of interaction between stimulation voltage and pulse width, which is related to total current output from the DBS and found no significant improvement of fit between learning index reduction and voltage.
Although each of the linear regressions in Figure 4C, D, and E reveals strong correlation between percent reduction in learning index and stimulation voltage, the intercepts of the regressions are 39%, 31%, and 57% for the combined, unipolar stimulation, and bipolar stimulation groups, respectively, predicting a facilitation of adaptation at 0 V stimulation. However, when DBS is programed to stimulate at 0 V, we should not expect any change in the level of adaptation between DBS on and no stimulation. The intersession learning index change for control subjects was −1 ± 11% (mean and standard deviation), rendering it unlikely that there exists some forward interference or facilitation of performance from session 1 to session 2. We speculate that the relationship between adaptation impairment and voltage may be better characterized by a nonlinear function. One possibility is a sigmoid-type function that gradually decreases from 0% reduction near 0 V, then decreases more steeply beyond 3 V, and finally saturates somewhere beyond 7 V. It is also possible that the relationship between adaptation reduction and voltage is nonmonotonic. At low stimulation voltage, patients may adapt better than the no-stimulation condition given that the abnormal tremor signal is a source of noise that can be disruptive to normal neuronal processing. Our finding that, on average, ET patients adapt less than control subjects (Fig. 3) when no stimulation is given lends support to this hypothesis. Given limited patient population, it is difficult to conclude the true relationship between adaptation reduction and voltage. It is clear, however, that at stimulation voltage beyond 4 V, adaptation is greatly reduced.
Vim Thalamotomy Impaired Reach Adaptation in Force Fields
Was the impairment of adaptation due to the fact that Vim thalamic stimulation indirectly stimulated motor regions of the cerebral cortex? To explore this question, we recruited 5 ET patients who had undergone unilateral Vim thalamotomy (Table 3) and tested them in the same paradigm as the DBS patients. The important difference was that in one session the patient used the arm ipsilateral to the thalamotomy and in the other session the contralateral arm.
Because ET is generally a bilateral disease, one expects to find significant tremor in the arm ipsilateral to the thalamotomy as compared with the contralateral arm. Figure 5A plots our measure of tremor during reaches in the null field for a representative patient and for the entire group. For the patient, the hand ipsilateral to the thalamotomy exhibited a clear peak in PSD at 5 Hz, whereas no such peak was evident in the contralateral hand. As expected, the fraction of power in the 3- to 10-Hz range was lower on average when the patients used the arm contralateral to the thalamotomy than the ipsilateral arm (Fig. 5B). In terms of movement kinematics, thalamotomy did not significantly affect either the mean or the standard deviation of peak speed, path length, movement duration, or angular error of movements made in the last null set. Additionally, thalamotomy had no significant effect on arm compliance measured during adaptation sets. Compared with control subjects, thalamotomy patients showed significant reduction in peak speed (23%) and increase in movement duration (24%) (Table 4). Patients also showed reduction of standard deviation for peak speed (24%), though numerically it was not different from that of the control subjects for DBS patients (Table 2); thus, this reduction in intertrial peak speed variability may be an artifact of the small sample size. To test this, we compared the data of all ET patients (n = 24: 5 thalamotomy subjects and 19 DBS cases) with the data of all control subjects (n = 26) (Table 4). We found that ET patients showed significantly increased intertrial variability in path length (65%), movement duration (85%), and angular errors (44%) but not in peak speed. ET patients, on average, moved significantly slower than control subjects—they achieved 12% smaller peak speed, and their movement path lengths and durations were 5% and 35% longer, respectively. Our measures of movement kinematics indicated that ET patients moved slower than healthy control subjects and their trajectories tended to be more variable across trials.
Figure 5.
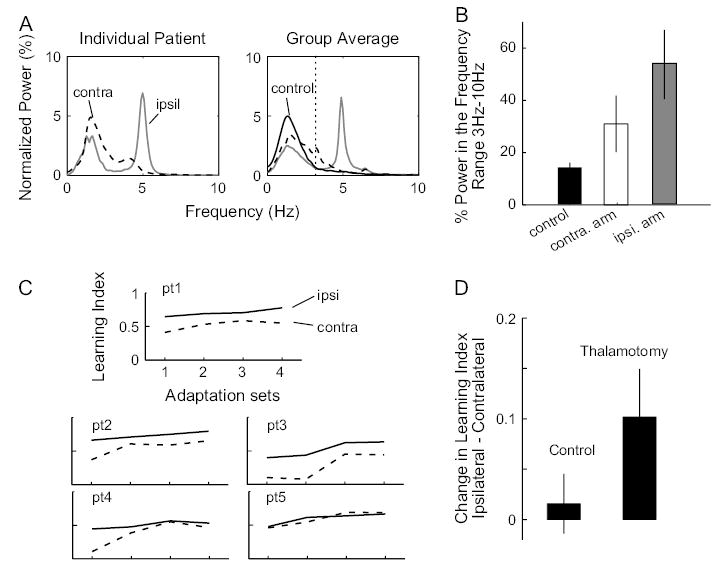
Effect of thalamotomy on tremor and motor adaptation. (A) Left: normalized average PSD for all trials in the first null set of each session for a thalamotomy patient. PSD for the untreated arm, ipsilateral to the thalamotomy, has a tremor-associated peak at 5 Hz. In the treated arm, contralateral to thalamotomy, this peak is greatly reduced. Right: group averages of normalized PSD for the patients’ ipsilateral arms and contralateral arms, as well as the control subjects’ (average of the 2 arms). Dotted vertical line marks 3 Hz, as in Figure 1A. (B) Group average of fractional power in the tremor frequency range (3–10 Hz) for the control subjects and the ipsilateral and the contralateral arms of the patients. Error bars are standard errors. (C) Performance of each thalamotomy patient quantified by learning index. Solid line indicates performance of the ipsilateral arm and dotted line that of the contralateral arm. (D) Average between-arm change in learning index for the patient group (ipsilateral – contralateral) and the control group (session 1 – session 2). Only learning indices from the last 2 adaptation sets are used. Thalamotomy patients show a significant decrease in adaptation in the contralateral arm (P = 0.038).
Table 4.
Performance measures of thalamotomy patients and thalamotomy control subjects
| Performance measure | Thalamotomy patients | Thalamotomy controls | Change from controls (%) | All ET patients | All controls | Change from controls (%) |
|---|---|---|---|---|---|---|
| Group size | 5 | 7 | — | 24 | 26 | — |
| Peak speed (m/s) | 0.26 (0.04) | 0.34 (0.06) | −23.4** (−23.8**) | 0.28 (0.05) | 0.32 (0.05) | −12** (10) |
| Path length (cm) | 10.43 (1.40) | 10.49 (1.13) | −0.5 (22.7) | 10.65 (1.35) | 10.18 (0.82) | 5** (65***) |
| Movement duration (s) | 1.52 (0.36) | 1.23 (0.22) | 24.1* (65.0) | 1.54 (0.34) | 1.14 (0.18) | 35***** (85***) |
| ae at 300 ms (°) | 0.23 (6.14) | −0.08 (5.18) | −388 (12.9) | 0.52 (6.70) | −0.15 (4.64) | −441 (44*****) |
| Arm compliance (°) | 16.83 | 16.66 | 1.0 | 16.61 | 15.59 | 7 |
Note: This table follows the same convention as Table 2. All ET patients—combining DBS and thalamotomy patients; all controls—combining the respective control subject groups.
P < 0.05,
P < 0.01,
P < 0.001,
P < 0.0001,
P < 0.00001.
Figure 5C plots the learning index for all the thalamotomy patients. Switching from contralateral to ipsilateral arm produced a significant improvement in performance (1-sided paired t-test of the learning index over the last 2 training sets, comparing ipsilateral with contralateral arm P = 0.038). Therefore, the thalamotomy patients as a group were significantly better in learning the task when they used the arm that exhibited more tremor (i.e., the arm ipsilateral to the thalamotomy).
Discussion
We tested the hypothesis that the cerebello-thalamo-cortical pathway plays a crucial role in adaptation of reaching movements by studying ET patients in whom this pathway was disrupted by Vim DBS or thalamotomy. We found that although both DBS and thalamotomy effectively reduced tremor during posture and reaching, they significantly impaired the rates of adaptation. In addition, we observed a significant correlation across the patients between stimulation voltage and the amount of adaptation impairment induced by stimulation. Patients with larger stimulation voltage tended to show greater adaptation impairment. The cerebellum has long been associated with motor adaptation. A number of psychophysical patient studies have found that damage to the cerebellum can profoundly impair the ability to adapt to novel kinematics or dynamics of reaching (Weiner and others, 1983; Martin and others, 1996; Maschke and others, 2004; Smith and Shadmehr, 2005). It is thought that the cerebellum has the ability to rapidly form internal models and “correct” the motor commands that are planned by the cortical motor areas by supplying information that predicts and compensates for constraints of the task (Conrad and others, 1974; Vilis and Hore, 1980). Alternatively, the cerebellum may compute signals that are crucial for forming an internal model (such as motor errors) and convey these signals to the cortical motor areas where motor memories form. In humans, the cerebellum directs most of its output to the cerebellar thalamus and only a small number of fibers to the red nucleus (Nolte and Angevine, 2000); thus, from the anatomical standpoint, the cerebello-thalamo-cortical pathway should play a significant role in human motor adaptation, particularly reaching adaptation. However, until now, there has been very little empirical evidence directly supporting the importance of the cerebellar thalamus in human reaching adaptation (Martin and others, 1996). In the present study, we found evidence for this hypothesis using a within-subject design. We found that reversible disruption of the cerebellar thalamus produced adaptation deficits.
Additionally, we showed that during the no-stimulation condition ET patients with DBS implants had an intermediate amount of adaptation impairment between stimulator-on and healthy controls. This suggests an underlying adaptation deficit associated with ET, a finding that is consistent with the current understanding that ET results from abnormal oscillatory activities in the inferior olive–cerebellum neural network (Elble, 2000; Deuschl and Bergman, 2002). Animal models of ET have shown enhancement of olivary rhythmicity with injection of β-carboline drugs, which produces a tremor that resembles ET (Elble, 1998). Clinically, it has been observed that ET can disappear after lesions of the cerebellum (Dupuis and others, 1989), the pons (Urushitani and others, 1996; Nagaratnam and Kalasabail, 1997), or the thalamus (Duncan and others, 1988). PET studies of ET have shown hyperactivity in the cerebellum (Jenkins and others, 1993), the inferior olive, as well as the thalamus (Hallett and Dubinsky, 1993). These works, along with the well-established surgical success of Vim DBS and thalamotomy for the suppression of ET, support the theory that tremor-related oscillations originate in the olivocerebellar circuits and propagate to the motor cortex by the cerebello-thalamo-cortical pathway. Taken together, it seems that in the untreated state of ET, functional disturbance of the cerebello-thalamo-cortical pathway caused by tremor-related oscillations compromises the relay and processing of information pertaining to reach adaptation. Thalamic lesion or stimulation disrupts the transmission of this oscillation and relieves ET but can further impair motor adaptation.
What is the nature of the information contained in the cerebellar outflow to the thalamus? One possibility is that the cerebellum forms internal models that compensate for specific dynamics of the task (forces produced by the robot) and correct the motor cortical commands. That is, the site of plasticity is in the cerebellum. Alternatively, the cerebellum may be involved in generating certain critical components of the internal model to be used by cortical motor areas. In particular, the cerebellum is well situated for computing motor errors. The intermediate zone of the cerebellar cortex receive afferents about the limbs from both the motor cortex and the spinal cord, allowing it to compare the desired motor output with the results of motor action. Both hypotheses on the cerebellum’s role in internal model formation can explain the gross impairments in movement control and motor adaptation seen in cerebellar patients (Martin and others, 1996; Smith and Shadmehr, 2005). However, because motor error is a crucial training signal for adaptation of internal models, these two possible functional roles of the cerebellum cannot be distinguished with the current experiments.
Recently, Diedrichsen and others (2005) showed with an functional magnetic resonance imaging study that when reaching motor errors were generated by force field, visual rotation, or target jump and resulted in similar patterns of online feedback correction, the cerebellum became activated regardless of the nature of the error and whether the error led to adaptation. This suggests that the cerebellum may be involved in error correction even when no new internal model is forming and supports the possibility that internal models form in motor cortical regions but depend on information supplied by the cerebellum through the thalamus. On the other hand, it has been shown that patients with cerebellar degeneration show somewhat preserved online error feedback correction when given force perturbations (Smith and others, 2000), whereas they are profoundly impaired in tasks that involve trial-to-trial error-driven learning (Smith and Shadmehr, 2005). These studies on cerebellar degeneration patients suggest that their ability to generate motor errors and to compensate accordingly is not completely abolished; rather, it is the ability to use these errors to drive adaptive changes to motor command that is abolished. Thus, although it is clear that the cerebellum plays a critical role in motor plasticity, we do not yet understand the relative contributions of the cerebellum, the thalamus, and the motor cortices in reaching motor control and adaptation.
How does thalamic stimulation affect the brain? High-frequency stimulation produces a complex pattern of excitation and inhibition, and its influence can reach beyond the stimulating nucleus. That is, thalamic stimulation is likely to affect downstream and upstream neurons via orthodromic and antidromic stimulation of the nearby axons (Perlmutter and others, 2002; Anderson and others, 2003; Hashimoto and others, 2003; Haslinger and others, 2003; McIntyre and others, 2004). Indeed, imaging studies have demonstrated increased activity in the thalamus, M1, and supplementary motor area in resting ET patients with DBS on versus off (Perlmutter and others, 2002; Haslinger and others, 2003). Although no significant changes were found in the cerebellar nuclei, it is possible that thalamic stimulation might artificially generate action potentials in the cerebellar thalamic axons, which could travel antidromically to the cerebellar nuclei without causing large changes in synaptic activity. Thus, thalamic stimulation is likely to disrupt neuronal activity in 3 locations: the motor cortex, the thalamus, and the cerebellar nuclei.
Given this, an alternate interpretation for our DBS study is that adaptation impairment associated with thalamic stimulation was not due to the disruption of the cerebellar thalamus. Rather, it was a result of indirect stimulation of the motor cortical regions via the thalamocortical neurons in Vim. However, we found that thalamotomy and stimulation affected adaptation similarly. Therefore, this suggests that impaired adaptation cannot be exclusively attributed to indirect stimulation of the motor cortex or the cerebellar nuclei.
Our finding that DBS impairs motor adaptation is consistent with recent reports showing that stimulation of the subthalamic nucleus in Parkinson disease impairs performance in certain cognitive or declarative memory tasks. Halbig and others (2004) compared the DBS-on and -off conditions and found that stimulation impaired recall in a declarative memory task. Hershey and others (2004) found that subthalamic stimulation in Parkinson disease impaired performance in a task that required spatial working memory. It seems that stimulation, whether in the subthalamic nuclei or in the cerebellar thalamus, has the potential to produce certain side effects in addition to its known therapeutic actions.
Previously known side effects associated with Vim DBS and thalamotomy in ET patients include paresthesia, dysarthria, persistent and transient arm ataxia, and gait disturbance (Mohadjer and others, 1990; Shahzadi and others, 1995; Schuurman and others, 2000; Dowsey-Limousin, 2002). For patients who have DBS, these side effects can often be reversed by turning the stimulator off. Still, many patients who experience side effects choose to leave the stimulator on during the day because the benefit of tremor suppression far outweighs the side effects. Comparative studies of the effects of thalamic DBS and thalamotomy on ET and 2 other movement disorders–associated severe tremor (Parkinson disease, multiple sclerosis) have shown that although the 2 surgical therapies are equally effective for tremor suppression, DBS tends to give fewer side effects and greater improvement in function as measured by patient’s ability to perform daily life activities, self-assessment of surgical outcome, and neuropsychological evaluations (Schuurman and others, 2000). For patients with bilateral drug-resistant tremor, bilateral thalamotomy is no longer used in clinical practice, whereas bilateral thalamic stimulation is a viable therapy. In the present study, we found that although thalamotomy produced motor adaptation deficits, DBS impaired adaptation in a voltage-dependent fashion. This means that at low stimulation voltage, DBS has the potential to eliminate tremor without affecting motor adaptation, further suggesting that DBS may be advantageous over thalamotomy.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institutes of Health (NIH) (NS037422). HC was supported by a National Research Service Award fellowship from the NIH. SEH was supported by a postdoctoral grant from the Parkinson’s Disease Foundation and the National Parkinson Foundation and a grant from the American Parkinson’s Disease Association. MAS was supported by a grant from the Hereditary Disease Society of America. We thank Drs H. Chris Lawson and Stephen Grill for their help with patient recruitment for the study. We also thank Drs Steve Wise and Amy Bastian for their helpful comments on the manuscript.
Footnotes
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
References
- Anderson ME, Postupna N, Ruffo M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol. 2003;89:1150–1160. doi: 10.1152/jn.00475.2002. [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol. 1996;76:492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Boecker H, Fogel W, Alesch F, Bartenstein P, Conrad B, Diederich N, von Falkenhayn I, Moringlane JR, Schwaiger M, Tronnier VM. Thalamic stimulation for essential tremor activates motor and deactivates vestibular cortex. Neurology. 2001;56:1347–1354. doi: 10.1212/wnl.56.10.1347. [DOI] [PubMed] [Google Scholar]
- Conrad B, Matsunami K, Meyer-Lohmann J, Wiesendanger M, Brooks VB. Cortical load compensation during voluntary elbow movements. Brain Res. 1974;71:507–514. doi: 10.1016/0006-8993(74)90994-9. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Bergman H. Pathophysiology of nonparkinsonian tremors. Mov Disord. 2002;17(Suppl 3):S41–S48. doi: 10.1002/mds.10141. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Hshambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci. 2005;25:9919–9931. doi: 10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin O, Francis JT, Shadmehr R. Quantifying generalization from trial-by-trial behavior of adaptive systems that learn with basis functions: theory and experiments in human motor control. J Neurosci. 2003;23:9032–9045. doi: 10.1523/JNEUROSCI.23-27-09032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin O, Sawaki L, Madupu G, Cohen LG, Shadmehr R. Mechanisms influencing acquisition and recall of motor memories. J Neurophysiol. 2002;88:2114–2123. doi: 10.1152/jn.2002.88.4.2114. [DOI] [PubMed] [Google Scholar]
- Dowsey-Limousin P. Postoperative management of Vim DBS for tremor. Mov Disord. 2002;17(Suppl 3):S208–S211. doi: 10.1002/mds.10165. [DOI] [PubMed] [Google Scholar]
- Duncan R, Bone I, Melville ID. Essential tremor cured by infarction adjacent to the thalamus. J Neurol Neurosurg Psychiatry. 1988;51:591–592. doi: 10.1136/jnnp.51.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis MJ, Delwaide PJ, Boucquey D, Gonsette RE. Homolateral disappearance of essential tremor after cerebellar stroke. Mov Disord. 1989;4:183–187. doi: 10.1002/mds.870040210. [DOI] [PubMed] [Google Scholar]
- Elble R, Koller W. 1990. Tremor. Baltimore, MD: Johns Hopkins University Press.
- Elble RJ. Animal models of action tremor. Mov Disord. 1998;13(Suppl 3):35–39. doi: 10.1002/mds.870131306. [DOI] [PubMed] [Google Scholar]
- Elble RJ. Origins of tremor. Lancet. 2000;355:1113–1114. doi: 10.1016/S0140-6736(00)02054-7. [DOI] [PubMed] [Google Scholar]
- Fabre M, Buser P. [Visually guided movement in the cat: difference in the effects of a bilateral lesion of the thalamic nucleus ventralis lateralis performed either before or after training] C R Seances Acad Sci D. 1979;288:417–420. [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Diaz R, Hall-Haro C, Vergara P, Mischner J, Nunez L, Drucker-Colin R, Ochoa A, Alonso ME. Normal prism adaptation but reduced after-effect in basal ganglia disorders using a throwing task. Eur J Neurosci. 2003;18:689–694. doi: 10.1046/j.1460-9568.2003.02785.x. [DOI] [PubMed] [Google Scholar]
- Garonzik IM, Hua SE, Ohara S, Lenz FA. Intraoperative microelectrode and semi-microelectrode recording during the physiological localization of the thalamic nucleus ventral intermediate. Mov Disord. 2002;17(Suppl 3):S135–S144. doi: 10.1002/mds.10155. [DOI] [PubMed] [Google Scholar]
- Gengo FM, Kalonaros GC, McHugh WB. Attenuation of response to mental stress in patients with essential tremor treated with metoprolol. Arch Neurol. 1986;43:687–689. doi: 10.1001/archneur.1986.00520070045016. [DOI] [PubMed] [Google Scholar]
- Halbig TD, Gruber D, Kopp UA, Scherer P, Schneider GH, Trottenberg T, Arnold G, Kupsch A. Subthalamic stimulation differentially modulates declarative and nondeclarative memory. Neuroreport. 2004;15:539–543. doi: 10.1097/00001756-200403010-00031. [DOI] [PubMed] [Google Scholar]
- Hallett M, Dubinsky RM. Glucose metabolism in the brain of patients with essential tremor. J Neurol Sci. 1993;114:45–48. doi: 10.1016/0022-510x(93)90047-3. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslinger B, Boecker H, Buchel C, Vesper J, Tronnier VM, Pfister R, Alesch F, Moringlane JR, Krauss JK, Conrad B, Schwaiger M, Ceballos-Baumann AO. Differential modulation of subcortical target and cortex during deep brain stimulation. Neuroimage. 2003;18:517–524. doi: 10.1016/s1053-8119(02)00043-5. [DOI] [PubMed] [Google Scholar]
- Hershey T, Revilla FJ, Wernle A, Gibson PS, Dowling JL, Perlmutter JS. Stimulation of STN impairs aspects of cognitive control in PD. Neurology. 2004;62:1110–1114. doi: 10.1212/01.wnl.0000118202.19098.10. [DOI] [PubMed] [Google Scholar]
- Hua SE, Lenz FA. Posture-related oscillations in human cerebellar thalamus in essential tremor are enabled by voluntary motor circuits. J Neurophysiol. 2005;93:117–127. doi: 10.1152/jn.00527.2004. [DOI] [PubMed] [Google Scholar]
- Jeljeli M, Strazielle C, Caston J, Lalonde R. Effects of ventrolateral-ventromedial thalamic lesions on motor coordination and spatial orientation in rats. Neurosci Res. 2003;47:309–316. doi: 10.1016/s0168-0102(03)00224-4. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Bain PG, Colebatch JG, Thompson PD, Findley LJ, Frackowiak RS, Marsden CD, Brooks DJ. A positron emission tomography study of essential tremor: evidence for overactivity of cerebellar connections. Ann Neurol. 1993;34:82–90. doi: 10.1002/ana.410340115. [DOI] [PubMed] [Google Scholar]
- Koller WC, Pahwa PR, Lyons KE, Wilkinson SB. Deep brain stimulation of the Vim nucleus of the thalamus for the treatment of tremor. Neurology. 2000;55:S29–S33. [PubMed] [Google Scholar]
- Krebs HI, Hogan N, Hening W, Adamovich SV, Poizner H. Procedural motor learning in Parkinson’s disease. Exp Brain Res. 2001;141:425–437. doi: 10.1007/s002210100871. [DOI] [PubMed] [Google Scholar]
- Li CS, Padoa-Schioppa C, Bizzi E. Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron. 2001;30:593–607. doi: 10.1016/s0896-6273(01)00301-4. [DOI] [PubMed] [Google Scholar]
- Macchi G, Jones EG. Toward an agreement on terminology of nuclear and subnuclear divisions of the motor thalamus. J Neurosurg. 1997;86:670–685. doi: 10.3171/jns.1997.86.4.0670. [DOI] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain. 1996;119(Pt 4):1183–1198. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- Maschke M, Gomez CM, Ebner TJ, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol. 2004;91:230–238. doi: 10.1152/jn.00557.2003. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol. 2004;91:1457–1469. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- Mohadjer M, Goerke H, Milios E, Etou A, Mundinger F. Long-term results of stereotaxy in the treatment of essential tremor. Stereotactic Funct Neurosurg. 1990;54/55:125–129. doi: 10.1159/000100201. [DOI] [PubMed] [Google Scholar]
- Nagaratnam N, Kalasabail G. Contralateral abolition of essential tremor following a pontine stroke. J Neurol Sci. 1997;149:195–196. doi: 10.1016/s0022-510x(97)05397-5. [DOI] [PubMed] [Google Scholar]
- Nolte J, Angevine J. 2000. The human brain in photographs and diagrams, 2nd ed. St. Louis, MO: Mosby, Inc.
- O’Suilleabhain PE, Frawley W, Giller C, Dewey RB., Jr Tremor response to polarity, voltage, pulsewidth and frequency of thalamic stimulation. Neurology. 2003;60:786–790. doi: 10.1212/01.wnl.0000044156.56643.74. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Li CS, Bizzi E. Neuronal correlates of kinematics-to-dynamics transformation in the supplementary motor area. Neuron. 2002;36:751–765. doi: 10.1016/s0896-6273(02)01028-0. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Li CS, Bizzi E. Neuronal activity in the supplementary motor area of monkeys adapting to a new dynamic environment. J Neurophysiol. 2004;91:449–473. doi: 10.1152/jn.00876.2002. [DOI] [PubMed] [Google Scholar]
- Paz R, Boraud T, Natan C, Bergman H, Vaadia E. Preparatory activity in motor cortex reflects learning of local visuomotor skills. Nat Neurosci. 2003;6:882–890. doi: 10.1038/nn1097. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW, Bastian AJ, Zackowski K, Hershey T, Miyawaki E, Koller W, Videen TO. Blood flow responses to deep brain stimulation of thalamus. Neurology. 2002;58:1388–1394. doi: 10.1212/wnl.58.9.1388. [DOI] [PubMed] [Google Scholar]
- Sakai ST, Inase M, Tanji J. The relationship between MI and SMA afferents and cerebellar and pallidal efferents in the macaque monkey. Somatosens Mot Res. 2002;19:139–148. doi: 10.1080/08990220220131533. [DOI] [PubMed] [Google Scholar]
- Schuurman PR, Bosch DA, Bossuyt PM, Bonsel GJ, van Someren EJ, de Bie RM, Merkus MP, Speelman JD. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342:461–468. doi: 10.1056/NEJM200002173420703. [DOI] [PubMed] [Google Scholar]
- Schweighofer N, Arbib MA, Kawato M. Role of the cerebellum in reaching movements in humans. I. Distributed inverse dynamics control. Eur J Neurosci. 1998;10:86–94. doi: 10.1046/j.1460-9568.1998.00006.x. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Brashers-Krug T. Functional stages in the formation of human long-term motor memory. J Neurosci. 1997;17:409–419. doi: 10.1523/JNEUROSCI.17-01-00409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzadi S, Tasker RR, Lozano A. Thalamotomy for essential and cerebellar tremor. Stereotactic Funct Neurosurg. 1995;65:11–17. doi: 10.1159/000098890. [DOI] [PubMed] [Google Scholar]
- Smith MA, Brandt J, Shadmehr R. Motor disorder in Huntington’s disease begins as a dysfunction in error feedback control. Nature. 2000;403:544–549. doi: 10.1038/35000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. Electromyographic correlates of learning an internal model of reaching movements. J Neurosci. 1999;19:8573–8588. doi: 10.1523/JNEUROSCI.19-19-08573.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. Learning of action through adaptive combination of motor primitives. Nature. 2000;407:742–747. doi: 10.1038/35037588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushitani M, Inoue H, Kawamura K, Kageyama T, Fujisawa M, Nishinaka K, Udaka F, Kameyama M. [Disappearance of essential neck tremor after pontine base infarction] No To Shinkei. 1996;48:753–756. [PubMed] [Google Scholar]
- Vaillancourt D, Sturman M, Verhagen M, Bakay R, Corcos D. Deep brain stimulation of the VIM thalamic nucleus modifies several features of essential tremor. Neurology. 2003;61:919–925. doi: 10.1212/01.wnl.0000086371.78447.d2. [DOI] [PubMed] [Google Scholar]
- Vilis T, Hore J. Central neural mechanisms contributing to cerebellar tremor produced by limb perturbations. J Neurophysiol. 1980;43:279–291. doi: 10.1152/jn.1980.43.2.279. [DOI] [PubMed] [Google Scholar]
- Weiner MJ, Hallett M, Funkenstein HH. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology. 1983;33:766–772. doi: 10.1212/wnl.33.6.766. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci. 2000;3(Suppl):1212–1217. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


