Abstract
Consistent with the epileptogenic and deleterious effects of the potent neurotoxin kainate, the activation of kainate receptors reduces the synaptic inhibition induced by the amino acid γ-aminobutyric acid (GABA). Extrapolating from these data led to the conclusion that kainate receptors are located presynaptically. However, kainate directly depolarizes the inhibitory interneurons, causing them to fire repeatedly. This effect might indirectly decrease the size of inhibitory postsynaptic currents recorded from pyramidal cells and places in doubt the presynaptic location for kainate receptors. Here we show that both effects, membrane depolarization and the reduction of inhibitory potentials, can be dissociated by several means, particularly by the natural agonist of kainate receptors, glutamate. Indeed, when applied at low concentrations, glutamate inhibited GABA release without affecting the firing rate of GABA interneurons. These results indicate that CA1 interneurons contain two populations of kainate receptors, each with different agonist sensitivity and coupled to distinct signaling pathways.
Although the role of N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in synaptic transmission, plasticity, and excitotoxicity has been well established, the kainate receptor is the component of the glutamate-signaling system that has remained most elusive to investigators over the years (1). The lack of specific pharmacological tools has hampered the detection of these receptors in neurons of the brain and the determination of their physiological role. Kainate administration in experimental animals induces seizures and patterns of neuronal damage closely resembling those observed in epileptics and has been widely used as a chemical model for human temporal lobe epilepsy (2, 3). For this and other reasons, it has become important to understand the physiology of these receptors in brain function. The discovery of a specific AMPA receptor antagonist, GYKI53655 (4, 5), has made such studies feasible. Consistent with a role in epilepsy, kainate has been found to depress GABA inhibitory transmission in the rat hippocampus (6–11). GABA is the major inhibitory neurotransmitter in the brain, and its activity is crucial in maintaining neuronal excitability at normal levels.
In addition to the effect of kainate on GABA-mediated synaptic inhibition, a small part of the excitatory input to CA1 inhibitory interneurons seems to be driven by kainate receptors (8, 9). Therefore, bath application of kainate or (RS)-α-amino-3-hydroxy-5-tert-butyl-4-isoxazolepropionic acid (ATPA), the postulated specific agonist of GluR5-containing receptors (7), depolarizes interneurons and increases their firing rate (8, 9). We and others have previously presented evidence supporting the idea that the kainate receptor-mediated depression of GABA transmission results from a reduction in the release probability (6, 10), suggesting the existence of presynaptic kainate receptors at GABA terminals. However, on the basis of the fact that the direct increase of interneuron firing rate might indirectly decrease the size of the inhibitory postsynaptic current (IPSC) (12), the conclusion regarding the existence of presynaptic kainate receptors modulating GABA release has been recently questioned (9).
The understanding of the function of kainate receptors as promoters of excitation in the hippocampus through a disinhibition of principal cells is fundamental in determining their possible role in epilepsy as well as in developing therapeutical strategies. Consequently, we have carried out experiments aimed at clarifying this aspect of kainate receptor functioning. Our results indicate that both effects of kainate, reduction of the inhibitory drive and increase in interneuron firing rate, can be dissociated and are mediated by two different populations of kainate receptors.
Methods
Hippocampal Slices.
Hippocampal slices were prepared from 14- to 16-day-old Wistar rats, as described in detail elsewhere (6, 10). The whole brain containing both hippocampi was positioned on the stage of a vibratome slicer and cut to obtain 350- μm-thick transversal brain slices, which were continuously oxygenated for at least 1 hr before use.
Electrophysiological Recordings.
Electrophysiological recordings were performed from visually identified neurons by IR-differential interference contrast microscopy by using a ×40 water immersion objective. All experiments were carried out at room temperature (22–25°C). Slices were continuously perfused with a solution consisting of (in mM) 124 NaCl, 2.69 KCl, 1.25 KH2PO4, 2 MgSO4, 1.8 CaCl2, 26 NaHCO3, and 10 glucose (pH 7.3; 300 mOsm), supplemented with AMPA, NMDA, and metabotropic glutamate receptor (mGluR) antagonists as required. In some instances, the extracellular solution coincided with that used in Frerking et al. (in mM, 119 NaCl/2.5 KCl/2.5 CaCl2/1.3 MgSO4/1 NaH2PO4/26.2 NaHCO3/11 glucose (pH 7.3) (ref. 9). Drugs were applied by gravity, switching between four perfusion lines. To evoke IPSCs, electric pulses were applied by a bipolar electrode made from a θ-glass pipette placed in the stratum oriens within 50–150 μm from the recording site. Tight-seal (>1 GΩ) whole-cell recordings were obtained from the cell body of neurons situated in CA1 pyramidal layer or stratum oriens. Patch electrodes were fabricated from borosilicate glass and had a resistance of 5–10 MΩ when filled with (in mM): 120 CsCl/8 NaCl/1 MgCl2/0.2 CaCl2/10 Hepes/2 EGTA (pH 7.3, 287 mOsm). Under these conditions, the high concentration of chloride in the pipette caused the IPSC to appear as inward currents. Therefore, in current-clamp experiments, K-gluconate substituted for CsCl. In voltage-clamp experiments, 20 mM QX-314 was included in the pipette solution to avoid firing of unclamped cell compartments. Neurons were voltage or current clamped by using an Axopatch 200A amplifier (Axon Instruments). Access resistance (8–30 MΩ) was regularly monitored during recordings, and cells were rejected if it changed more than 15% during the experiment. Data were filtered at 2 kHz, digitized, and stored on a computer by using pCLAMP or axotape software (Axon Instruments, Foster City, CA).
Compounds.
Bicuculline methobromide, kainic acid, Pertussis toxin (PTx), and salts were purchased from Sigma; AMPA, d-2-amino-5-phosphonovaleric acid and SYM2206 were obtained from Tocris Neuramin, Bristol, U.K. Staurosporine, Calphostin-C, and bisyndolylmaleimide were purchased from Calbiochem. QX-314 was from Alomone Laboratories, Jerusalem, Israel. GYKI53655 and LY303070 were kindly provided by D. Leander from Elli Lilly. ATPA was kindly provided by J. Drejer (NeuroSearch, Glostrup, Denmark).
Results
In our experiments, as in other studies, the reduction of evoked IPSC (eIPSC) was generally concomitant with a marked increase in spontaneous IPSC (sIPSC), because kainate receptor agonists potently depolarize interneurons (6, 8–11). To clarify whether the depression of GABAergic transmission is the result of this increase in basal activity, we first investigated whether there are agonists of kainate receptors capable of discriminating between the receptors depolarizing the dendrosomatic compartment and inhibiting the release of GABA.
The effects of diverse kainate receptor agonists on the excitability of identified stratum oriens interneurons and on IPSC recorded from pyramidal cells in the CA1 field of the hippocampus were determined. In all experiments, to avoid the activation of AMPA receptors, we included in the perfusion solution the selective AMPA receptor antagonist GYKI53655 (100 μM) or its active isomer, LY303070, (50 μM). In some experiments, we have also used the new compound SYM2206, which at 100 μM shows selectivity for AMPA over kainate receptors. Selectivity of SYM2206 was assessed in hippocampal cultures. At 100 μM, this compound abolished the AMPA receptor-mediated response evoked by rapid application of kainate in cultured hippocampal neurons, and it completely blocked the EPSC evoked by Schaffer collaterals stimulation. The IC50 for AMPA receptor-mediated responses was estimated to be similar to that found for GYKI53655 (≈1 μM; see ref. 4). SYM2206, however, slightly reduced the kainate receptor-mediated responses (20% at 100 μM) recorded in the presence of GYKI53655 in cultured hippocampal cells (A. V. Paternain and J.L., unpublished results; see also ref. 12). Similarly, NMDA receptors were blocked by adding d-2-amino-5-phosphonovaleric acid (50–200 μM) and when glutamate was applied, mGluR were antagonized by including both MPPG and MCPG at a concentration enough to block almost completely all mGluR subtypes (each at 1–1.5 mM; see ref. 3). Under these conditions, low concentrations of kainate (3 μM) were enough to significantly depress the amplitude of IPSCs evoked by the stimulation of the stratum oriens (eIPSC) (63.2 ± 2.4% of amplitude reduction; mean ± SEM; n = 26). The action of kainate was mimicked by ATPA, a drug that has been postulated to act specifically on GluR5-containing receptors (7). At 10 μM, ATPA caused a reduction of eIPSC amplitude of 58.5 ± 6.2% (n = 8). Similar to the effect of kainate, the depressive activity of ATPA was partially overcome by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (not shown). In contrast, S-AMPA was completely ineffective in reducing the eIPSC amplitude at concentrations high enough to activate AMPA receptors (i.e., 50 μM; −0.1 ± 6.4%, n = 9) but was very potent at concentrations known to activate some types of kainate receptors (14, 15) (92.5 ± 3.5% of amplitude reduction at 500 μM; n = 4).
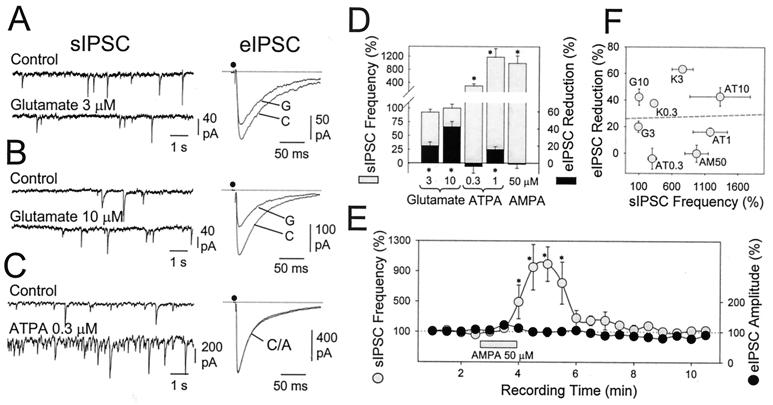
All of the above agonists also increased the frequency of sIPSC in pyramidal cells. However, the endogenous agonist of these receptors, glutamate, when applied at low concentrations (10 μM) was able to significantly inhibit the eIPSC (42.2 ± 6.1%; n = 11; P < 0.001; Fig. 1B), an action which was prevented by 100 μM CNQX (5.9 ± 12.3% reduction, n = 4). However, despite this activity, glutamate did not increase the frequency of sIPSC (100 ± 7%; n = 4), indicating that at this concentration, glutamate acts weakly on kainate receptors that depolarize interneurons. Glutamate was active at even lower concentration (3 μM, 19.9 ± 4.2% of depression; n = 4; P < 0.001), without altering the frequency (93 ± 5%; n = 7) of spontaneous events in the cell under recording (Fig. 1A). In these same neurons, kainate (0.3 μM) positively increased the frequency of sIPSC (447 ± 103%; n = 6), depressing the eIPSC amplitude by 32.1 ± 4.6% (n = 3) (see Fig. 1F).
Figure 1.
Dissociation of kainate receptor-induced depression of eIPSC and increased spontaneous activity. (A) At 3 μM the endogenous agonist of kainate receptors, glutamate, did not increase the frequency of spontaneous inhibitory events (sIPSC) in pyramidal neurons (left-hand records are 10-sec raw recordings), while it significantly reduced the amplitude of eIPSC, induced by electric shocks applied to the stratum oriens (right-hand records are averages of 12 responses). At 10 μM, glutamate further reduced the amplitude of eIPSC without altering the frequency of sIPSC (B). (C) The agonist of GluR5-containing receptors, ATPA, notably increased sIPSC frequency (Left) without affecting the amplitude of eIPSC (Right). (D) Effect of different concentration of glutamate, ATPA and AMPA, on both the frequency of sIPSC (shaded bars) and eIPSC amplitude (black bars). Bars = mean ± SEM of 4–11 experiments. *, P < 0.001, Student's t test. (E) Time course of the action of AMPA (in the continuous presence of 100 μM GYKI53655) on both the sIPSC frequency and the eIPSC amplitude. Each point represents the mean value (±SEM; n = 6) of consecutive 30-sec periods. (*, P < 0.005, U-Mann–Whitney test). (F) The frequency of sIPSC during kainate receptor agonist application (normalized to the control, 100%) is plotted vs. the degree of eIPSC reduction obtained at each concentration of agonist, irrespective of the type and concentration of agonist used [glutamate (G), ATPA (AT), AMPA (AM), and kainate (K); concentrations are in micromolar). Note the lack of correlation (r2 < 0.01) between these two parameters.
Spontaneous activity is composed of IPSC induced by action potential firing together with miniature IPSC (mIPSC). According to previous observations (6, 10), the frequency of mIPSC should be reduced on presynaptic kainate receptor activation. However, the decrease of eIPSC amplitude selectively caused by glutamate was not accompanied by a large change in sIPSC frequency. This result seems to be inconsistent with our previous data. However, it should be noted that the frequency of sIPSCs [i.e., in the absence of tetrodotoxin (TTX)] was usually much larger than that of mIPSC (in the presence of TTX). In our experiments, we calculated that mIPSC represented 17–20% of the total sIPSC population. Therefore, the fractional decrease in mIPSC frequency expected in these experiments was overridden by the presence of more frequent action-potential-dependent IPSC. In addition, because action-potential-driven IPSC are much larger than mIPSC, the number of these small currents tends to be underestimated in these calculations. Thus, the frequency of events calculated in these experiments should preferentially reflect those driven by interneuron firing.
A similar but inverse situation was found for ATPA. At 1 μM, this compound depolarized stratum oriens interneurons (11.8 ± 2.1 mV; n = 4), notably increasing their spontaneous firing and subsequently the sIPSC frequency in pyramidal cells (1179 ± 261%; n = 7; P < 0.001) (Fig. 1D). However, at this concentration, ATPA reduced the amplitude of eIPSC only slightly, although significantly (16.1 ± 3.2%; n = 11; P < 0.001). At a lower concentration (0.3 μM), ATPA still increased the frequency of sIPSC (303 ± 65.6%, n = 5; P < 0.001) essentially without blocking GABA release (4 ± 7.8% increase of eIPSC amplitude, n = 9) (Fig. 1 C and D). The action of AMPA was similar to ATPA in that at a low concentration (i.e., 50 μM), it drastically increased the number of sIPSC (973 ± 178%; n = 5) while being essentially devoid of any depressive activity on GABA release (Fig. 1 D and E). Thus, agonist sensitivity for sIPSC frequency (i.e., interneuron depolarization) and eIPSC amplitude (i.e., GABA release) is different, as further illustrated by the lack of correlation between the increase in sIPSC rate and the degree of eIPSC depression induced by different kainate receptor agonists (Fig. 1F). These results suggest a different nature for the receptors mediating each effect.
The possibility that the reduction in IPSC amplitude is caused by a presynaptic action, and that it does not depend on the secondary activation of receptors other than the kainate subtype (GABAB; adenosine, muscarinic, etc.), was addressed specifically in previous papers (6, 10). However, we wanted to rule out further the participation of any of these receptors in the present experiments as well as to ascertain whether the selective action of glutamate on eIPSC originated from a presynaptic mechanism. For this reason, we measured the action of glutamate on the frequency of sIPSC and on the amplitude of eIPSC under the blockade of AMPA receptors (GYKI53655, 100 μM), NMDA receptors (200 μM d-2-amino-5-phosphonovaleric acid), and mGluRs (MCPG + MPPG, 1.5 mM each), but also including naloxone (100 μM), 2-OH-saclofen (50–150 μM), atropine sulfate (50 μM), and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) (0.1 μM) to block opioid, GABAB, muscarinic, and adenosine receptors, respectively. Under these conditions, glutamate (10 μM) was even more effective in reducing the amplitude of eIPSC (53.5 ± 6.0%, n = 6), having no effect on the frequency of sIPSC. Consequently, these results rule out the possibility that the action of glutamate on GABA release was because of the release of an unknown substance, activating secondarily one of those receptors.
To determine whether the specific effect of glutamate involved a presynaptic locus, we calculated the coefficient of variation (CV) of the eIPSC in 18 neurons treated with 3 or 10 μM glutamate. As expected for a change in quantal content rather than in quantal size, the variation of the mean eIPSC amplitude observed during the exposure to glutamate was proportional in all but one case to the change in CV−2 (not shown), indicating a presynaptic locus for the observed reduction in eIPSC amplitude (see ref. 6 and references therein).
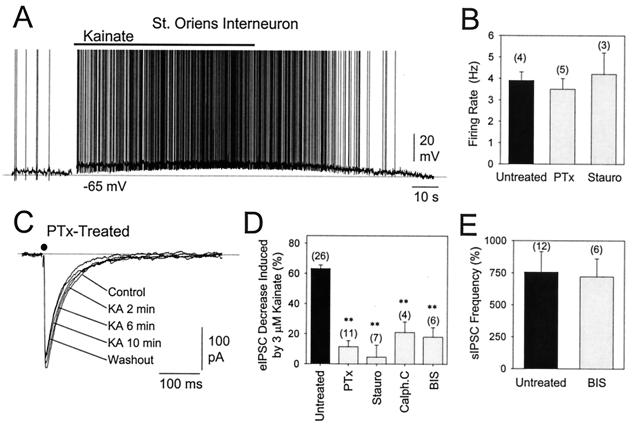
To substantiate further that the effect of kainate receptor activation on eIPSC amplitude and membrane depolarization was indeed mediated by different receptors, we took advantage of the fact that activation of a G-protein and the activity of protein kinases (i.e., PKC) is necessary for the former to occur (10). Thus, we studied the effect of kainate after incubating hippocampal slices with either PTx (5 μg/ml, 3–5 h at 37°C), staurosporine (0.5 μM), or with the specific PKC inhibitors Calphostin C (0.5 μM) and bisyndolylmaleimide (0.1 μM). In all cases, the action of kainate on the modulation of eIPSC amplitude was severely impaired, often being barely detectable (Fig. 2 C and D). On the other hand, kainate and ATPA were still able to depolarize interneurons in the presence of either inhibitor (Fig. 2A), and no differences in the degree of depolarization or in the increase in sIPSC frequency (Fig. 2E) were observed when neurons were compared from treated and untreated slices. Interestingly, all stratum oriens interneurons recorded (n = 12) were sensitive to ATPA (1–10 μM). On average, 3 μM kainate depolarized interneurons slightly less than 1 μM ATPA (8.6 ± 1.8 mV (n = 7) and 12 ± 1.8 mV (n = 5), respectively). In four stratum oriens interneurons, the firing rate elicited by 3 μM kainate was measured to be 3.9 ± 0.4 Hz. This kainate-induced activity was not altered in PTx- (3.5 ± 0.5 Hz; n = 5) or in staurosporine-treated (4.2 ± 1.0 Hz; n = 3) slices (see Fig. 2B). These results strongly indicate that, unlike the control of GABA release, the induction of depolarization mediated by kainate receptor activation does not require coupling to a second messenger cascade.
Figure 2.
Effect of kainate-receptor activation in slices treated with PTx or PKC inhibitors. (A) Depolarizing effect of 3 μM kainate on a stratum oriens interneuron, in a slice treated with PTx. (B) PTx or staurosporine treatment has no action on the 3-μM kainate-induced increase of firing rate of stratum oriens interneurons. (C) IPSC recorded from CA1 pyramidal neurons and evoked by single stimuli applied to the stratum oriens in a slice treated with PTx (5 μg/ml) for 3–5 h at 37°C before recording. eIPSC are the average of 11 consecutive responses (0.2 Hz) starting at the indicated times. (D) The decrease of eIPSC amplitude induced by kainate (untreated, n = 26) was abolished in slices treated with PTx (5 μg/ml) or with each of the following PKC inhibitors applied at a concentration of 10-fold the reported IC50 value: staurosporine (Stauro, 0.5 μM), Calphostin C (Calph. C, 0.5 μM), or bisyndolylmaleimide (BIS, 0.1 μM). (E) PKC inhibition did not modify the kainate-induced increase in sIPSC. Values are mean ± SEM of the number of neurons indicated in parentheses. **, P < 0.001, Student's t test.
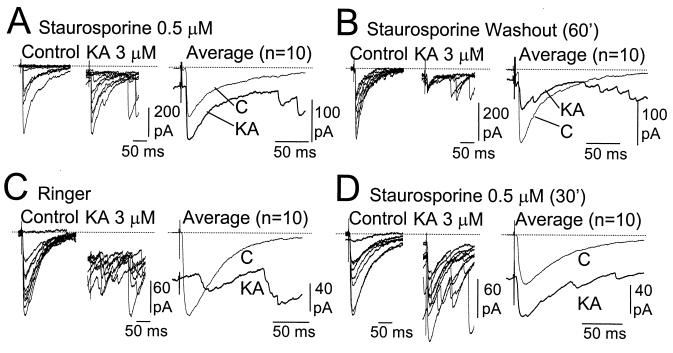
We then tried to determine whether the impairment of the kainate-induced depression of GABA release by inhibition of PKC could be reversed by washing the inhibitor out. For this reason, we monitored the same neuron for long recording periods. In the CA1 pyramidal neuron shown in Fig. 3A, recorded from a slice treated with staurosporine, exposure to kainate increased rather than decreased the mean eIPSC amplitude. However, after a 60-min staurosporine washout period, the application of kainate was very effective in reducing the eIPSC amplitude recorded from the same neuron (Fig. 3B). We also tried the inverse experiment. After demonstrating the inhibitory action of kainate on the eIPSC recorded from a pyramidal cell of a control slice (Fig. 3C), staurosporine was added to the perfusion solution. As can be seen in Fig. 3D, a 30-min incubation in staurosporine depressed the action of kainate on eIPSCs. We were able to replicate these results in four different slices.
Figure 3.
Reversibility of the impairment of kainate-induced GABA release by inhibition of PKC. (A) Kainate failed to reduce the amplitude of eIPSC recorded from CA1 neurons in slices previously treated with staurosporine. Single responses are shown superimposed (Left) and averaged (Right). This neuron could be maintained stable during the period that staurosporine was washed out (60 min). In this situation, the application of kainate induced a marked reduction in IPSC amplitude (B). (C) In a control slice, kainate increased spontaneous activity and reduced the amplitude of eIPSC. (D) After incubation of this same slice with staurosporine for 30 min, the effect of kainate on the eIPSC amplitude recorded from the same cell was largely prevented. Note that the increase in sIPSC persisted. These appeared as late components in the average records, because 10 trials were not enough to average them out. Note also that the change of the holding current (i.e., postsynaptic depolarization) occurred in every case of kainate application. These results were obtained in four occasions.
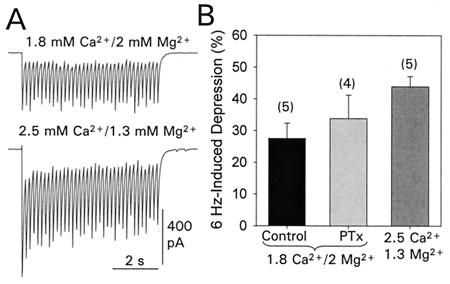
In view of the recent report showing that sustained activation of fast-spiking neocortical neurons caused a steady-state depression that is frequency dependent and reflects a presynaptic function (16), we evaluated the degree of frequency-dependent depression of eIPSC in our recording conditions. Previously, the rapid and profound (i.e., >90%) reduction of eIPSC amplitude observed on repetitive stimulation (e.g., a 6-sec train at 6 Hz) of hippocampal inhibitory pathways had been taken to account for the depression of the eIPSC by bath-applied kainate (9). However, we did not observe such a marked depression of eIPSC amplitude on repetitive stimulation (Fig. 4). A 6-Hz stimulation pattern produced an averaged reduction in the eIPSC amplitude of 27 ± 5% (n = 5). This value significantly differs from the value reported by Frerking et al. (9). The extracellular concentration of divalent cations (Ca2+ and Mg2+) used in that and our study were different, and we checked for this as the source of discrepancy. Raising the extracellular concentration of Ca2+ to 2.5 mM and setting the Mg2+ concentration to 1.3 mM [the concentrations used in the study by Frerking et al. (9)] enhanced the synaptically induced responses (Fig. 4A), and also the frequency-induced depression was significantly increased (43.9 ± 3.2%, n = 5). However, such an amount of depression was still significantly lower than the value reported in the mentioned study. In contrast, our measurements are similar to the data described in experiments involving sustained 20-Hz activation of neocortical inhibitory synapses (16). The reason for this frequency-induced depression of synaptic transmission is not well understood, but we could discard involvement of a PTx-sensitive G-protein, because we observed a similar degree of depression (33.8 ± 7.4%, n = 4) in slices treated with PTx (Fig. 4B).
Figure 4.
Frequency-induced depression of eIPSCs. (A) IPSC activated by a 6-Hz train show a moderated amplitude depression (Upper). Increasing the extracellular Ca2+ augmented the evoked release, as evidenced by the larger amplitude responses recorded form the same cell. Under these Ca2+/Mg2+ conditions, the synaptic depression was significantly augmented (Lower). Records are the average of six consecutive trains. (B) A summary of the degree of synaptic depression observed under normal recording conditions (1.8 Ca2+/2 Mg2+) in control slices (black bar) and in slices treated with PTx (gray bar) as well as under conditions described by Frerking et al. (9) (darker gray bar; 2.5 Ca2+/1.3 Mg2+). The degree of depression was estimated as the difference in amplitude between the first IPSC and the mean amplitude of the three final responses of the train. Bars = mean ± SEM of the number of experiments indicated in brackets.
Discussion
Present results demonstrate that glutamate, the endogenous kainate receptor agonist, ATPA, an agonist of GluR5-containing receptors, and AMPA, an activator of some types of kainate receptors, are all able to dissociate the increase in the sIPSC observed on kainate-receptor activation from its effects on GABA release. Furthermore, the effect on GABA release but not on the depolarizing action of kainate was sensitive to the blockade of either G-proteins or PKC. These results unequivocally indicate the existence of two populations of kainate receptors in hippocampal interneurons with different agonist sensitivity and furthermore document that they use different signaling mechanisms.
An argument against the existence of presynaptic kainate receptors regulating GABA release comes from the observation that repetitive (i.e., 6-Hz) stimulation of the inhibitory pathways severely depressed the eIPSC (9). We have evaluated this possibility under our experimental conditions and never found a marked depression of eIPSC amplitude on repetitive stimulation. In our experiments, a repetitive stimulation pattern produced an averaged reduction similar to that recently found in experiments involving sustained activation of neocortical inhibitory synapses (16) but significantly different from that reported by Frerking et al. (9). At present, we have no explanation for this discrepancy but, under our experimental conditions, such a frequency-dependent depression of IPSC is clearly insufficient to account for the reduction of eIPSC observed during bath application of kainate. The results from the present experiments contradict the conclusion that kainate-evoked depression of IPSC is a mere consequence of the repetitive firing evoked by the activation of the interneuronal somatodendritic receptors, rather than a direct action of presynaptic kainate receptors (9). Our results demonstrate that low concentrations of glutamate depress the GABAergic eIPSC in the absence of repetitive presynaptic firing. If the increase in spontaneous activity were responsible for the depression of eIPSC, then some correlation between the increment of interneuron activity and the eIPSC amplitude reduction would be expected, irrespective of the agonist used. However, in support of our conclusions, this was not the case (Fig. 1F). The treatment of hippocampal slices with PTx or staurosporine to disrupt the second messenger cascades involved in the IPSC depression induced by kainate (10) also dissociated between the two phenomena: the depolarizing activity of kainate receptors on interneurons persisted in the absence of kainate-induced modulation of GABAergic transmission.
Therefore, the mechanism of frequency-dependent depression of GABA release seems to be entirely irrelevant to depression eIPSC under conditions not involving rapid and synchronous activation of inhibitory axons. Although the reason for this needs further clarification, it is reasonable to think that if the frequency-dependent depression develops on kainate application, it will occur only in axons originating from intact interneurons, which may be a small fraction of the number of fibers activated by each electrical stimulus in the slice preparation. Regardless of the explanation, it can be concluded that under our experimental conditions, these two kainate receptor-induced phenomena are largely independent.
The different sensitivity of presynaptic and somatic receptors to different agonists indicates that they differ in terms of their molecular composition. The GluR5 subunit of kainate receptors is predominantly expressed in hippocampal interneurons (17), but we have recently found that an important population of interneurons also express GluR6 and that these two subunits can assemble to form heteromeric receptors with new pharmacological properties. For instance, they are gated by both AMPA and ATPA (18). Consequently, the functional diversity of kainate receptors is greater than anticipated, and a detailed pharmacological characterization of all these receptors would be necessary before a conclusion could be drawn on what type of receptor is mediating each function.
Interestingly, the sensitivity to glutamate of kainate receptors responsible for regulating GABA release was greater than that of those inducing the somatodendritic depolarization. This is what would be expected for a receptor placed at a site not able to sense the synaptic glutamate bolus. In the present experiments, the actual concentration of glutamate that the kainate receptor senses is not known, considering that the activity of specific transporters may rapidly decrease the extracellular concentration of glutamate (e.g., ref. 19). However, it is unlikely that under our experimental conditions (room temperature and continuous supply of glutamate to the bath), the glutamate concentration underwent a significant reduction in the extracellular space (see ref. 20). In any case, we have previously shown that the dose-response curve for glutamate-induced depression of GABA release is bell shaped and presents a maximum at around 100 μM (21). Although the concentration that glutamate reaches outside the synapses is largely unknown (22), recent estimates indicate that glutamate concentration transiently peaks at 160–190 μM in the extrasynaptic space (23) Therefore, these and even smaller concentrations may be sufficient for the activation of kainate receptors placed at a distance from the release site, given their apparent higher sensitivity to glutamate. Thus, the two requirements for this phenomenon to occur, a sufficient concentration of glutamate beyond the synapse and a high receptor sensitivity, are accomplished. In keeping with this idea, recent experiments have examined the effect of synaptically released glutamate on monosynaptic inhibitory transmission in the hippocampus and found that a brief burst of activity in glutamatergic afferents reduced GABAergic transmission in a manner that depends on kainate receptor activation (24). Further experiments have indicated that such a modulation of GABA release depends on a PTx-sensitive G-protein and PKC activation (25), very much in agreement with previous and present results.
During the review process of this paper, a report by Frerking et al. (26) appeared that examines the mechanisms underlying the kainate-induced depression of the eIPSC. In agreement with our conclusion, they now show that a direct frequency-dependent depression of the IPSC does not contribute significantly to the effect of kainate on the eIPSC. However, these authors conclude that kainate acts to depress the eIPSC mainly by two major effects: an increase of spontaneous GABA release and the subsequent activation of presynaptic GABAB receptors, and a decrease in postsynaptic responsiveness to GABA. Our results are inconsistent with these conclusions because, in our experiments, the eIPSC was depressed by glutamate in the absence of increased spontaneous activity (i.e., in the absence of massive release of GABA) and vice versa. On the other hand, in support of our view is the observation that kainate-depressed Ca2+-dependent GABA release from isolated presynaptic terminals (27, 28).
In conclusion, here we present data demonstrating that kainate-receptor activation induces the depression of GABA release in a manner independent of presynaptic neuronal activity. Ultimately, our results indicate the existence of two separate populations of kainate receptors. One population localizes to the somatodendritic compartment (8, 9) of (at least) stratum oriens interneurons, and its activation involves the opening of cationic channels and the subsequent membrane depolarization. In contrast, the other population functions as metabotropic receptors, which trigger a second messenger cascade that would be located at the presynaptic aspect of inhibitory synapses. Although the molecular composition of these kainate receptors in neurons remains to be unequivocally determined, our data indicate that kainate receptors with different signaling mechanisms coexist in hippocampal interneurons.
Acknowledgments
We thank Dr. J. Drejer for providing us with ATPA, Dr. M. Sefton for editorial assistance, and D. Guinea for technical help. We thank Dr. A.V. Paternain for the determination of SYM2206 selectivity. We thank Elli Lilly & Co. (Indianapolis, IN) for the generous gift of GYKI53655 and LY303070. J.C.L-G. is the recipient of a long-term fellowship awarded by the European Molecular Biology Organization. This work was supported in part by grants to J.L. from the Direcciòn General de Enseñanza Superior e Investigación Cientifica (DGESIC) (PM-0008/96; EU-96–0007) and the Community of Madrid (08.5/0042/1998).
Abbreviations
- NMDA
N-methyl-d-aspartate
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- IPSC
inhibitory postsynaptic current
- sIPSC
spontaneous IPSC
- eIPSC
evoked IPSC
- mIPSC
miniature IPSC
- PTx
Pertussis toxin
- ATPA
(RS)-α-amino-3-hydroxy-5-tert-butyl-4-isoxazolepropionic acid
- mGluR
metabotropic glutamate receptors
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lerma J. Neuron. 1997;19:1155–1158. doi: 10.1016/s0896-6273(00)80407-9. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Ari Y. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 3.Nadler J. Life Sci. 1981;29:2031–2042. doi: 10.1016/0024-3205(81)90659-7. [DOI] [PubMed] [Google Scholar]
- 4.Paternain A V, Morales M, Lerma J. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- 5.Wilding T J, Huettner J E. Mol Pharmacol. 1995;47:582–587. [PubMed] [Google Scholar]
- 6.Rodríguez-Moreno A, Herreras O, Lerma J. Neuron. 1997;19:893–901. doi: 10.1016/s0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- 7.Clarke V R J, Ballyk B A, Hoo K H, Mandelzys A, Pellizari A, Bath C P, Thomas J, Sharpe E F, Davies C H, Ornstein P L, et al. Nature (London) 1997;389:599–602. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- 8.Cossart R, Esclapez M, Hirsch J C, Bernard C, Ben-Ari Y. Nat Neurosci. 1998;1:470–478. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- 9.Frerking M, Malenka R C, Nicoll R A. Nat Neurosci. 1998;1:479–486. doi: 10.1038/2194. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Moreno A, Lerma J. Neuron. 1998;20:1211–1218. doi: 10.1016/s0896-6273(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 11.Bureau I, Bischoff S, Heinemann S F, Mulle C. J Neurosci. 1999;15:653–663. doi: 10.1523/JNEUROSCI.19-02-00653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li P, Wilding T J, Kim S J, Calejesan A, Huettner J E, Zhou M. Nature (London) 1999;397:161–164. doi: 10.1038/16469. [DOI] [PubMed] [Google Scholar]
- 13.Bushell T, Jane D E, Tse H-W, Watkins J C, Garthwaite J, Collingridge G L. Br J Pharmacol. 1996;117:1457–1462. doi: 10.1111/j.1476-5381.1996.tb15306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg P H. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- 15.Huettner J E. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- 16.Galarreta M, Hestrin S. Nat Neurosci. 1998;1:587–594. doi: 10.1038/2822. [DOI] [PubMed] [Google Scholar]
- 17.Bahn S, Volk B, Wisden W. J Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paternain A V, Herrera M T, Nieto M A, Lerma J. J Neurosci. 2000;20:196–205. doi: 10.1523/JNEUROSCI.20-01-00196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garthwaite J. Br J Pharmacol. 1985;85:297–307. doi: 10.1111/j.1476-5381.1985.tb08860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodríguez-Moreno A, Sistiaga A, Lerma J, Sánchez-Prieto J. Neuron. 1998;21:1477–1486. doi: 10.1016/s0896-6273(00)80665-0. [DOI] [PubMed] [Google Scholar]
- 21.Paternain A V, Rodriguez-Moreno A, Villarroel A, Lerma J. Neuropharmacology. 1998;37:1249–1259. doi: 10.1016/s0028-3908(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 22.Kullmann D M, Asztely F. Trends Neurosci. 1998;21:8–14. doi: 10.1016/s0166-2236(97)01150-8. [DOI] [PubMed] [Google Scholar]
- 23.Dzubay J A, Jahr C E. J Neurosci. 1999;19:5265–5274. doi: 10.1523/JNEUROSCI.19-13-05265.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min M-Y, Melyan Z, Kullmann D M. Proc Natl Acad Sci USA. 1999;96:9932–9937. doi: 10.1073/pnas.96.17.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melyan Z, Kullmann D M. Soc Neurosci Abstr. 1999;25:1796. [Google Scholar]
- 26.Frerking M, Petersen C C H, Nicoll R A. Proc Natl Acad Sci USA. 1999;96:12917–12922. doi: 10.1073/pnas.96.22.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunha R A, Constantino M D, Ribeiro J A. Eur J Pharmacol. 1997;323:167–172. doi: 10.1016/s0014-2999(97)00043-5. [DOI] [PubMed] [Google Scholar]
- 28.Perkinton M S, Sihra T S. Neuroscience. 1999;90:1281–1292. doi: 10.1016/s0306-4522(98)00573-9. [DOI] [PubMed] [Google Scholar]