Abstract
Regeneration of the visual chromophore, 11-cis-retinal, is a critical step in restoring photoreceptors to their dark-adapted conditions. This regeneration process, called the retinoid cycle, takes place in the photoreceptor outer segments and the retinal pigment epithelium (RPE). Disabling mutations in nearly all of the retinoid cycle genes are linked to human conditions that cause congenital or progressive defects in vision. Several mouse models with disrupted genes related to this cycle contain abnormal fatty acid retinyl ester levels in the RPE. To investigate the mechanisms of retinyl ester accumulation, we generated single or double knockout mice lacking retinoid cycle genes. All-trans-retinyl esters accumulated in mice lacking RPE65, but they are reduced in double knockout mice also lacking opsin, suggesting a connection between visual pigment regeneration and the retinoid cycle. Only Rdh5-deficient mice accumulate cis-retinyl esters, regardless of the simultaneous disruption of RPE65, opsin, and prRDH. 13-cis-Retinoids are produced at higher levels when the flow of retinoid through the cycle was increased, and these esters are stored in specific structures called retinosomes. Most importantly, retinylamine, a specific and effective inhibitor of the 11-cis-retinol formation, also inhibits the production of 13-cis-retinyl esters. The data presented here support the idea that 13-cis-retinyl esters are formed through an aberrant enzymatic isomerization process.
Vitamin A transformations in the eye are essential for vision. Absorption of light by the vitamin A-derived chromophore of visual pigments, 11-cis-retinal, leads to its photoisomerization to all-trans-retinal and the initiation of the signal transduction cascade (1–4). Through a chain of reactions, all-trans-retinal is recycled enzymatically back to 11-cis-retinal (5–8). These retinoid cycle reactions (Figure 1) take place in the outer segments of photoreceptor cells and in the adjacent retinal pigment epithelium (RPE).1 Characterization of the knockout mice lacking genes involved in the retinoid cycle provides important insight into the flow of retinoids in the eye.
Figure 1.

Chemistry of the retinoid cycle reactions in the vertebrate retina. The retinoid cycle reactions were reviewed recently (6). In the rod outer segments (ROS), light causes the isomerization of the rhodopsin chromophore, 11-cis-retinylidene, to all-trans-retinylidene, which is hydrolyzed and released from opsin. All-trans-retinal is then reduced in a reaction catalyzed by all-trans-retinal-specific RDH(s) including prRDH. All-trans-retinol diffuses to retinal pigment epithelium (RPE) where it is esterified by LRAT to fatty acid all-trans-retinyl esters. All-trans-retinyl esters or its derivative is isomerized to 11-cis-retinol in a reaction that involves an abundant RPE protein, termed RPE65. 11-cis-Retinol is then oxidized by 11-cis-RDH (RDH5, RDH11) and other dehydrogenases to 11-cis-retinal, completing the cycle. 11-cis-Retinal diffuses across the extracellular space, is taken up by the ROS, and recombines with opsin to regenerate rhodopsin. In aberrant reactions, all-trans-retinyl esters or its derivative is isomerized to 13-cis-retinol. 13-cis-Retinol can also be esterified by LRAT to form 13-cis-retinyl esters, stored in retinosomes.
11-cis-Retinol dehydrogenase (RDH), also known as RDH5, catalyzes the final oxidation reaction of 11-cis-retinol in the RPE (9, 10). Although RDH5 is responsible for the majority of 11-cis-RDH activity, RDH11 and other RDHs also have a measurable role in regenerating the visual pigment by complementing RDH5 in RPE cells (11). Disruption of the gene encoding RDH5 causes fundus albipunctatus in humans (12, 13). Fundus albipunctatus is an autosomal recessive form of congenital stationary night blindness characterized by the appearance of numerous small white dots located in the RPE, a delayed course of dark adaptation, and occasionally progressive cone dystrophy (14). Deletion of 11-cis-RDHs in mice, Rdh5−/− and Rdh11−/−, causes delayed dark adaptation and delayed 11-cis-retinal regeneration (15–18).
Lipid droplet-like organelles known as retinosomes were characterized as specific sites of all-trans-retinyl ester accumulation in the RPE (19, 20). All-trans-retinyl esters accumulate in the RPE of wild-type mice as they are trapped from circulation and also during the recycling of all-trans chromophore from visual pigments (Figure 1). The vast majority of retinyl esters in wild-type mice maintain an all-trans configuration. 11-cis-Retinal appears to be produced on demand when high numbers of visual pigments are uncoupled from their chromophore (21). Deletion of specific genes of the retinoid cycle, however, leads to severe abnormalities in ester levels. For example, the levels of all-trans-retinyl esters are highly elevated in Rpe65−/− mice (22), while in Rdh5−/− mice, 13-cis-retinyl ester levels are surprisingly high (15). RPE65 has been proposed to be the isomerase responsible for transformation of all-trans-retinyl esters to 11-cis-retinol (23–26) (Figure 1). However, in a purified form, it has been reported that RPE65 does not possess the isomerase activity (27).
In this study we examine the formation, accumulation, and utilization of retinyl esters in the retinoid cycle, employing knockout mice lacking genes that encode proteins of the phototransduction and retinoid cycle. Our results point to relaxed stereospecificity of the isomerization complex as the cause of abnormal all-trans- to 13-cis-retinoid isomerization. 13-cis-Retinyl esters are stored in retinosomes. We show also a connection/communication between rod photoreceptors and RPE in the eye.
EXPERIMENTAL PROCEDURES
Animals
All animal experiments employed procedures approved by the Case Western Reserve University, and initially by University of Washington, Animal Care Committee, and conformed to the recommendations of the American Veterinary Medical Association Panel on Euthanasia and the recommendations of the Association of Research for Vision and Ophthalmology. Animals were maintained in complete darkness or on a 12 h light/12 h dark cycle, and all manipulations were done under dim red light employing a Kodak No. 1 safelight filter (transmittance >560 nm). Typically, 2–3-month-old mice were used in all of the experiments. Rdh11-deficient mice were generated by Dr. P. Nelson (Fred Hutchinson Cancer Research Center, Seattle, WA), and their genotyping and characterization were described previously (11). Rdh5-deficient mice were previously generated and characterized by Drs. C. Driessen and J. J. Janssen (15). Genotypes of Rdh5−/− mice were determined by PCR (15). Opsin−/− mice were obtained from Dr. J. Lem (Tufts University, Boston, MA). These mice were genotyped as described previously (28). Rpe65-deficient mice were obtained from Dr. M. Redmond (NEI, National Institutes of Health) and genotyped as described previously (22). Prrdh-and Lrat-deficient mice were generated and characterized in our laboratory in collaboration with Dr. W. Baehr (University of Utah) as described previously (29, 30). Double knockout mice were generated by cross-breeding single knockout mice to genetic homogeneity. All mice were outbred by standard procedures into the pigmented C57BL/ 6J strain (Jackson, Bar Harbor, ME).
Immunoblotting
The immunoblotting was carried out according to standard protocols using Immobilon-P to adsorb proteins [poly(vinylidene difluoride); Millipore Corp.]. Monoclonal anti-rhodopsin antibody was provided by Dr. R. Molday, rabbit polyclonal anti-RPE65 was a gift from Dr. J. Saari, and monoclonal anti-LRAT antibody was generated in our laboratory (29). Alkaline phosphatase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG (Promega) was used as a secondary antibody. Protein bands were visualized with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium color development substrate (Promega).
Retinoids: Analyses, Synthesis, and Treatments
All of the experimental procedures related to the analysis of dissected mouse eyes, derivatization, and separation of retinoids have been described previously (31). Retinoic acids and their derivatives were measured as reported previously (32). Typically, two mouse eyes were used per assay, and the assays were repeated three to six times. Retinylamine (Ret-NH2) was synthesized by the method described previously (33). Oral gavage was carried out as described previously (31, 34).
Isomerization Assay
Isomerization assay and preparation of proteins were carried out as described in prior publications (35, 36).
Intraocular Injection
Mice were anesthetized by intraperitoneal injection using 20 μL/g body weight of 6 mg/mL ketamine and 0.44 mg/mL xylazine diluted with 10 mM sodium phosphate, pH 7.2, containing 100 mM NaCl. Anesthetized mice were injected with 1 μL of DMSO solution containing all-trans-retinol in the vitreous cavity of the eye. The injection was performed with a 30 gauge Hamilton microneedle syringe through the sclera at a point 1 mm from the limbus to avoid puncture through the lens.
Two-Photon Vitamin A Imaging
Two-photon excitation microscopy was performed using a confocal/two-photon laser scanning microscope (LSM 510 MP-NLO; Carl Zeiss, Inc., Thornwood, NY) with LSM510 software, version 3.0 (19, 20). Briefly, 76 MHz, 100 fs pulses of 730 nm light from a mode-locked Ti:Sapphire laser (Mira-900; Coherent, Mountain View, CA) were focused on the sample by a Plan-Neofluar 40×/1.3 NA objective lens (Carl Zeiss). The intensity of the laser was measured at the back aperture of the objective lens and kept at ×3 mW. Autofluorescence from the sample (390–545 nm) was collected by the objective, separated from the excitation light by a dichroic mirror, filtered to remove scattered excitation light, and directed to a photomultiplier tube detector. The objective lens was heated to 37 °C by an air stream incubator. A temperature-controlled microscopic stage was installed on the microscope to maintain the reaction at 37 °C. Fluorescent intensities, expressed in pixel values, were calculated by offline analysis of the collected raw images (SCION image; Scion Co., Frederick, MD). Fluorescent intensity was measured for the tangential sections of the RPE cells and averaged per pixel for randomly chosen areas (mean ± SD, n = 3) enclosing 200 × 200 pixels (~32 × 32 μm2).
Mice were light adapted under room light for 1 h, and isolated eyecups were located at the center of a glass-bottomed 35 mm dish and perfused with the oxygenized (95% O2, 5% CO2) Ames medium (Sigma) at 37 °C. In case of a slight movement of the eye, the same area of the retina was traced by using the unique texture of the RPE cell layer formed by randomly arranged single and double nucleated RPE cells.
Electron Microscopy
For transmission electron microscopy, mouse eyecups were analyzed as described previously (30).
RESULTS
To investigate the mechanisms of retinyl ester accumulation, we used single or double knockout mice with disrupted genes of the retinoid cycle (Figure 1). The roles of these gene products are described in Table 1.
Table 1.
Single Gene Knockout Mice Used in the Experiments Described in This Report
| genotype | role of the gene | phenotype | refa |
|---|---|---|---|
| opsin−/− | photoreceptor of rod cells, G protein-coupled receptor | lack of ROS formation, lack of rod-mediated vision | 49 |
| Rpe65−/− | abundant RPE protein involved in the isomerization process of all-trans-retinyl esters to 11-cis-retinol (isomerase) | lack of 11-cis-retinal production and residual visual functions, accumulation of retinyl esters in large inclusion droplets | 22 |
| Rdh5−/− | cis-retinol dehydrogenase | delayed dark adaptation, accumulation of 13-cis- and 11-cis-retinyl esters | 15 |
| Lrat−/− | lecithin:retinol acyltransferase, a major enzyme involved in retinal esterification in many tissues | lack of 11-cis-retinal production and residual visual functions, lack of significant levels of retinyl esters in the eye and liver | 29 |
| Rdh11−/− | dual specificity (cis/trans) retinol dehydrogenase | delayed dark adaptation | 11 |
| Prrdh−/− | photoreceptor-specific all-trans-retinol dehydrogenase | delayed dark adaptation | 30 |
Reference to original report on mouse genotype.
Lack of Rdh5 Results in cis-Retinyl Ester Accumulation
cis-Retinyl esters, mostly in the form of 13-cis-retinyl esters, accumulated to significant levels only in mice lacking the functional Rdh5 gene (Figure 2) (Table 2). The highest levels of 13-cis-retinyl esters were observed in the eyes of Rdh5−/−Rdh11−/− mice. Interestingly, high levels of cis-retinyl esters were observed not only in opsin−/−Rdh5−/− mice (Table 2) but also in heterozygote opsin+/−Rdh5−/− mice (Figure 3). Disruption of the Rpe65 and Prrdh genes did not considerably affect the level of cis-retinyl esters. The ablation of Rdh5 and Rdh11 genes did not appreciably affect the expression levels of LRAT or RPE65 (Figure 4).
Figure 2.
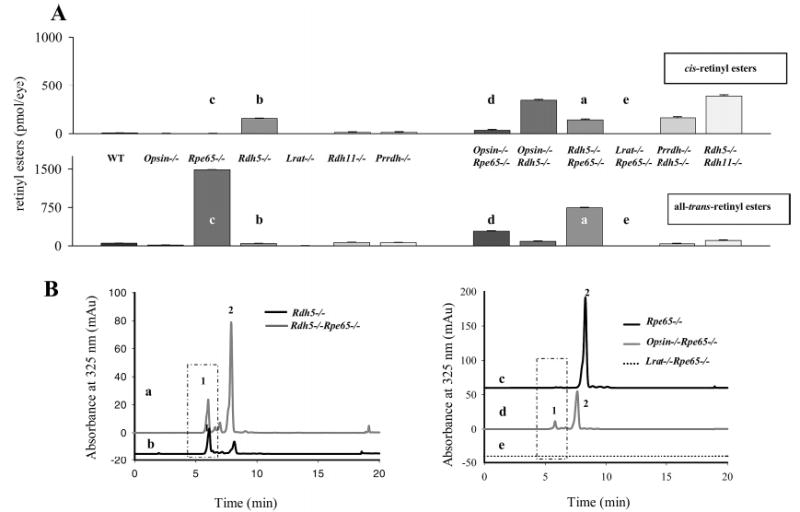
Retinyl esters in the eyes of mice of different genetic backgrounds. (A) All-trans- and cis-retinyl esters in mice of different genetic backgrounds. Retinoids were extracted from 6-week-old eyes and separated on normal-phase HPLC as described in Experimental Procedures. (B) Chromatographic separation of all-trans- and cis-retinyl esters from (a) Rpe65−/−, (b) opsin−/−Rdh65−/−, (c) Lrat−/−Rpe65−/−, (d) Rdh5−/−Rpe65−/−, and (e) Rdh5−/− mice. Retinoids were extracted from 6-week-old eyes and separated on normal-phase HPLC. The box represents cis-retinyl esters (>90% 13-cis-retinyl esters) also indicated as peak 1. Peak 2 represents all-trans-retinyl esters. The mice were reared under dim red light.
Table 2.
Retinoid Levels in Genetically Varied Mice Lacking Proteins of the Retinoid Cyclea
| genotype | 13-cis-/11-cis- retinyl esters (pmol/eye) | all-trans- retinyl esters (pmol/eye) | 11-cis-retinal (pmol/eye) | 11-cis-retinol (pmol/eye) | all-trans-retinal (pmol/eye) | all-trans-retinol (pmol/eye) |
|---|---|---|---|---|---|---|
| single knockout | ||||||
| WT | 6.6 ± 0.8 | 52.6 ± 4 | 575 ± 30 | 18 ± 4 | 45 ± 6.5 | 4.6 ± 0.6 |
| opsin−/− | 2.0 ± 0.5 | 15.8 ± 4.6 | 13.7 ± 1.2 | 7.1 ± 1.9 | trace | 4.8 ± 1.3 |
| Rpe65−/− | trace | 1488 ± 65 | none | none | 3 ± 0.1 | 7.4 ± 0.7 |
| Rdh5−/− | 155 ± 40 | 47 ± 9.8 | 508 ± 24 | 9.2 ± 3.5 | 22.3 ± 2.8 | 4.8 ± 1.3 |
| Lrat−/− | trace | trace | trace | trace | trace | 7.9 ± 1.3 |
| Rdh11−/− | 12 ± 1.4 | 67 ± 13.4 | 510 ± 24 | 13.1 ± 1.1 | 17 ± 0.1 | 0.8 ± 1.5 |
| Prrdh−/− | 12 ± 0.8 | 62 ± 4.2 | 514 ± 30 | 20 ± 7 | 40.9 ± 9.4 | 7.9 ± 1.0 |
| double knockout | ||||||
| opsin−/−Rpe65−/− | 34 ± 7.5 | 288 ± 107 | none | trace | trace | 89 ± 3.2 |
| opsin−/−Rdh5−/− | 343 ± 91 | 88.8 ± 29 | 10.1 ± 3.9 | trace | trace | trace |
| Rdh5−/−Rpe65−/− | 140 ± 24 | 744 ± 98 | none | trace | trace | 96 ± 21 |
| Lrat−/−Rpe65−/− | trace | trace | none | trace | trace | 8 ± 2.1.4 |
| Prrdh−/−Rdh5−/− | 162 ± 17 | 39 ± 7.9 | 536 ± 39 | 19.7 ± 2.5 | 9.2 ± 6.5 | 3.5 ± 1.0 |
| Rdh5−/−Rdh11−/− | 186 ± 34 | 104 ± 6 | 480 ± 18 | trace | 29.5 ± 2.0 | trace |
Mice were genotyped, and retinoid analysis was performed as described in Experimental Procedures. cis-Retinyl esters were integrated as 13-cis-retinyl esters (>90% cis-retinyl esters).
Figure 3.

Chromatographic separation of nonpolar retinoids from opsin−/−Rdh5−/− mice. Retinoids were extracted from 6-week-old eyes and separated on normal-phase HPLC. The box represents cis-retinyl esters (>90% 13-cis-retinyl esters). A representative chromatogram is shown, and the average data from three mice are indicated with standard deviation above the ester peaks (mean ± SD).
Figure 4.

Immunoblotting of eyecup extracts from wild-type and Rdh5−/−Rdh11−/− mice probed with anti-rhodopsin (1D4), anti-RPE65, or anti-LRAT antibodies. The eyecup extracts were prepared from mice by homogenizing with the sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer. The proteins (30 μg) were analyzed by 12.5% SDS–PAGE. The eyecup extract from Rdh5−/−Rdh11−/− mice showed no significant difference in the levels of rhodopsin, RPE65, or LRAT compared with wild-type mice.
All-trans-retinyl esters accumulated to significant levels only in mice lacking RPE65 (Figure 2A) (Table 2). Additional disruption of the opsin gene significantly lowered the level of all-trans-retinyl esters in Rpe65-deficient animals and increased levels of cis esters in Rdh5-deficient animals. In opsin−/− animals, 11-cis-retinal was present at a low level (Figure 2A) (Table 2), of which a majority most likely bound to CRALBP, as the cone pigments are sparse in mice (37). Simultaneous disruption of Rdh5 and Rpe65 genes led to lower levels of all-trans-retinyl esters than did single knockouts, while these and other retinoids were not detected in Lrat−/−Rpe65−/− mice.
Employing electron microscopy, we analyzed the retinas for morphological changes in double knockout Lrat−/−Rpe65−/− mice and compared them with Rpe65−/− mice (19, 22). The outer segments of Lrat−/−Rpe65−/− mice were shortened, loaded with opsin instead of rhodopsin as indicated by retinoid analysis, and displayed an underdeveloped synaptic inner plexiform layer (IPL) (Figure 5A–E), which was likely due to overstimulation by free opsin in the rod photoreceptor cells. No lipid droplets were observed in Lrat−/−Rpe65−/− mice. Similarly, the lack of opsin in opsin−/−Rpe65−/− mice reduced the formation of these ester droplets (Figure 5F–H), while they remained prominent, although reduced in size, in Rdh5−/−Rpe65−/− mice, which had fewer total esters than Rpe65−/− mice (Figure 5I–K). Reducing levels of retinyl esters by rescuing visual pigments with 9-cis-retinal also led to the shrinkage of these droplets in Rpe65−/− mice (34).
Figure 5.
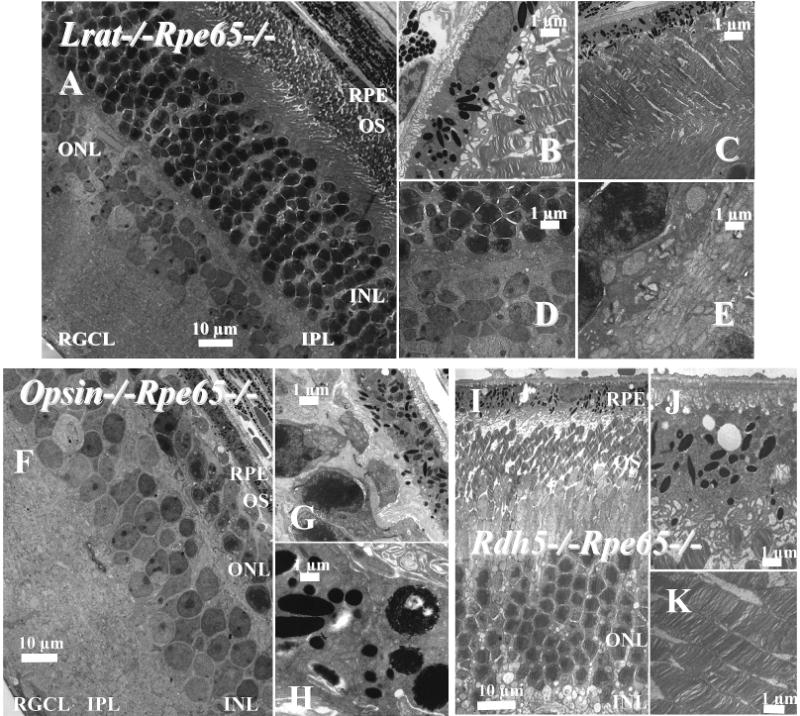
Montage of cross sections of the retinas of 2-month-old Lrat−/−Rpe65−/− mice analyzed by transmission electron microscopy. Panel A shows the cross section of the RPE and the photoreceptor cells. Panels B and C show higher magnification sections of the RPE and ROS (B, C) and the IPL (D, E). (F–H) Montage of cross sections of the retinas of 2-month-old opsin−/−Rpe65−/− mice analyzed by transmission electron microscopy. Panel F shows the cross section of the RPE and the photoreceptor cells. Panels G and H show higher magnification sections of the RPE and ROS, repectively. (I–K) Montage of cross sections of the retinas of 2-month-old Rdh5−/−Rpe65−/−mice analyzed by transmission electron microscopy. Panel I shows the cross section of the RPE and photoreceptor cells. Panels J and K show a higher magnification of the RPE and ROS. The sections were prepared as described in Experimental Procedures. The scale bar represents 1 or 10 μm as indicated.
13-cis-Retinoids Are Produced at Higher Levels in Light/ Dark-Reared Mice
13-cis-Retinoids were produced at higher levels in light/dark-reared Rdh5−/−Rdh11−/− mice than in mice never exposed to light (Figure 6A), while light exposure did not lead to accumulation of these esters in wild-type mice. Exposure of dark-adapted Rdh5−/−Rdh11−/− mice to intense illumination enhanced production of 13-cis-retinyl esters. After exposure to light, transiently elevated all-trans-retinyl esters decayed to normal dark-adapted levels concurrently with the regeneration of rhodopsin, while 13-cis-retinylesters once formed remained stable for at least 1 week (data not shown). As investigated by two-photon microscopy (19), 13-cis-retinyl esters likely accumulated in the retinosomes (Figure 6B), because these structures became inflated and displayed higher RPE fluorescence compared with those of control wild-type and Rdh5+/− Rdh11+/− mice (Figure 6C).
Figure 6.
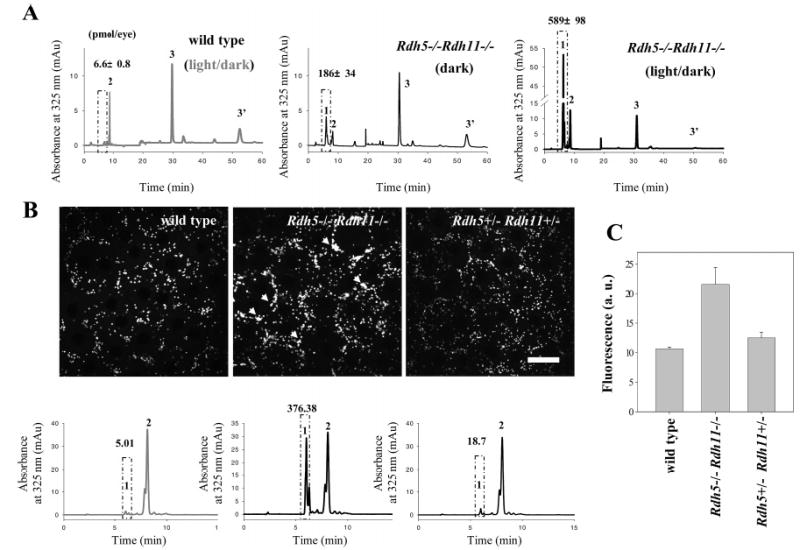
Light-dependent formation and storage of retinyl esters in eyes from Rdh5−/−Rdh11−/− mice. (A) Effect of dark or light rearing on the accumulation of 13-cis-retinyl esters in eyes from Rdh5−/−Rdh11−/− mice and wild-type controls. The dark-reared mice were exposed to no other light than dim red illumination. Peaks 1, 2, and 3/3′ represent cis-retinyl esters, all-trans-retinyl esters, and 11-cis-retinal oximes, respectively. The box represents cis-retinyl esters (>90% 13-cis-retinyl esters). A representative chromatogram is shown, and the average data from three mice are indicated with standard deviation above the ester peaks (mean ± SD). (B) Imaging of retinyl esters by two-photon microscopy (top row) and quantification of retinyl esters by HPLC (bottom row). Left column: Wild-type mice contained all-trans-retinyl esters in retinosomes (RESTs). Middle column: Rdh5−/−Rdh11−/− mice stored all-trans- and 13-cis-retinyl esters in retinosomes (white arrow). Retinosome fluorescence in Rdh5−/−Rdh11−/− mice is more intense than in wild-type mice (arrowheads). Right column: Distribution and quantity of all-trans-retinyl esters in eyes from Rdh5+/−Rdh+/− mice were similar to those of wild type. The box represents cis-retinyl esters (>90% 13-cis-retinyl esters). A representative chromatogram is shown, and the average data from three mice are indicated with standard deviation above the ester peaks (mean ± SD). (C) Quantification of fluorescence intensity measured by two-photon microscopy. Fluorescence intensity is higher in Rdh5−/−Rdh11−/− compared to wild-type and Rdh5+/−Rdh+/−mice (n = 3). The mean ± SD was indicated.
13-cis-Retinol Is Formed through an Enzymatic Isomerization Process
Biochemical data described below supported the idea that 13-cis-retinyl esters are formed through an isomerization process. When all-trans-retinol was injected into the eyes of Rdh5−/−Rdh11−/− mice, the amount of 13-cis-retinyl esters formed in darkness increased from 83.1 ± 9.3 pmol (in control mice treated only with DMSO) to 220.4 ± 33.2 pmol in 30 min and to 498.5 ± 49 pmol in 2 days (Figure 7). Thus, 13-cis-retinyl esters are formed independently of light. Second, when Rdh5−/−Rdh11−/−mice were gavaged with a potent inhibitor of 11-cis-retinal production, all-trans-Ret-NH2 (38), and then exposed 24 h later to intense light for 3 min at 500 cd·m−2, only a modest increase in 13-cis-retinyl ester production was observed, whereas untreated control mice showed a severalfold increase in 13-cis-retinyl esters. No formation of these cis esters was observed in wild-type treated or untreated mice (Figure 8A). Finally, we examined the effects of Ret-NH2 on 13-cis-retinyl ester production after intraocular injection of all-trans-retinol. The inhibitor completely stopped isomerization to 13-cis-retinyl esters in Rdh5−/−Rdh11−/− mice (Figure 8B).
Figure 7.
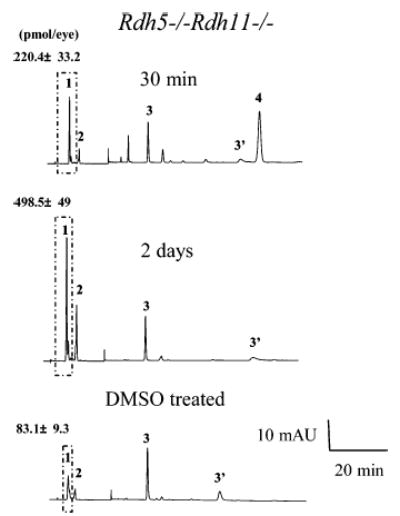
Enzymatic production of 13-cis-retinyl esters by Rdh5−/−Rdh11−/− mice. Mice were dark adapted for 48 h, followed by intravenous injection of 40 nmol of all-trans-retinol in 1 μL of DMSO with a 30 gauge needle under anesthesia. Retinoid analysis was performed both 30 min and 2 days after injection. All experiments were carried out in the dark. A representative chromatogram is shown, and the average data from three mice are indicated with standard deviation above the ester peaks (mean ± SD). Retinoids were extracted from 6-week-old eyes and separated on normal-phase HPLC. The box represents cis-retinyl esters (>90% 13-cis-retinyl esters). Peaks 1, 2, 3/3′, and 4 represent cis-retinyl esters, all-trans-retinyl esters, 11-cis-retinal oximes, and all-trans-retinol, respectively.
Figure 8.
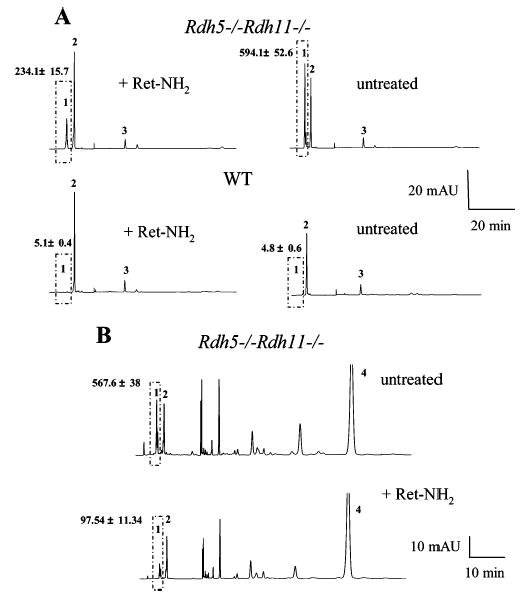
Effects of Ret-NH2 on 13-cis-retinyl ester production. (A) Effects of Ret-NH2 on 13-cis-retinyl ester production induced by light. Mice were dark adapted for 48 h, gavaged with 1 mg of Ret-NH2, and then exposed to intense light for 3 min at 500 cd·m−2, 24 h postgavage. A representative chromatogram is shown, and the average data from three mice are indicated with standard deviation above the ester peaks (mean ± SD). The box represents cis-retinyl esters (>90% 13-cis-retinyl esters). (B) Effects of Ret-NH2 on 13-cis-retinyl ester production from injected all-trans-retinol. Mice were dark adapted for 48 h and gavaged with 1 mg of Ret-NH2. After 24 h, 400 nmol of all-trans-retinol in 1 μL of DMSO was intravenously injected. A representative chromatogram is shown, and the average data from three mice are indicated with standard deviation above the ester peaks (mean ± SD). The box represents cis-retinyl esters (>90% 13-cis-retinyl esters). Peaks 1, 2, 3/3′, and 4 represent cis-retinyl esters, all-trans-retinyl esters, 11-cis-retinal oximes, and all-trans-retinol, respectively.
To prove that the inhibitor blocks this aberrant isomerization, we demonstrated that Ret-NH2 stops production of 13-cis-retinol in vitro in the presence of bovine serum albumin (BSA) (Figure 9). These experiments are an extension of the observations made by McBee and coworkers establishing that isomerization depends on the specificity of the acceptor protein used in the isomerization reaction (36). Production of both 11-cis- and 13-cis-retinol in the presence of Ret-NH2 was inhibited in the presence of appropriate retinoid-binding proteins (Figure 9). Injection of 13-cis-retinol into the eyes of Rdh5−/−Rdh11−/− and wild-type mice did not lead to differences in production of retinoic acid or its oxidation products (data not shown), indicating that the accumulation of 13-cis-retinyl esters in these knockout mice is not the result of a defective clearing process in the oxidation pathway (32).
Figure 9.
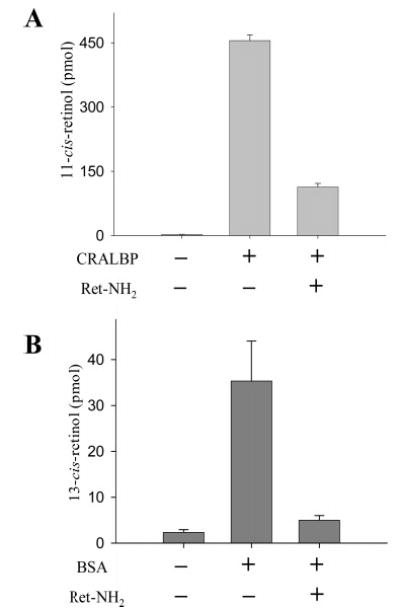
Influence of retinoid-binding proteins and Ret-NH2 on the formation of 11-cis-retinol and 13-cis-retinol. The isomerization reaction was performed in 10 mM BTP buffer, pH 7.5, containing 1 mM ATP and 10% BSA. UV-treated bovine RPE microsomes were used as a source of enzyme (150 μg of protein). For inhibition assays, all-trans-Ret-NH2 was used at a concentration of 3 mM. The reaction was initiated by adding all-trans-retinol in DMF and a solution of CRALBP or BSA to the final concentration of 10 mM or 10%, respectively. The reaction mixture was incubated in 37 °C for 1 h.
DISCUSSION
The retinoid cycle is a two-cell biochemical pathway that requires the concerted action of several enzymes. It has been recently reported that RPE65 possesses retinol isomerase activity (23–26), while RDH5 is one of the enzymes catalyzing the last step of the retinoid cycle, oxidation of 11-cis-retinol to 11-cis-retinal (11, 12, 17, 39). In mice, disruption of the Rdh5 gene results in the accumulation of cis-retinyl esters (15). Interestingly, 13-cis-retinyl esters are observed in Rdh5−/−Rpe65−/− mice, which we believe are formed enzymatically through a molecular mechanism similar to that for 11-cis-retinol (36). To gain insight into the mechanism of 13-cis-retinyl ester formation, we produced animals with simultaneous disruption of Rdh5 and other genes of the retinoid cycle as well as opsin. Disruption of the Rdh5 gene concomitantly with the Rpe65 gene results in a different phenotype than the disruption of the Rpe65 gene alone. For example, Rpe65−/− mice accumulate all-trans-retinyl esters; however, Rdh5−/−Rpe65−/− double knockouts have far fewer all-trans-retinyl esters than the Rpe65−/− mice, but these mice still accumulated 13-cis-retinyl esters. This suggests that the process of hydrolysis–isomerization–oxidation of all-trans-retinyl esters to 11-cis-retinal is aberrant in a unique way when one or more components of the retinoid cycle genes are eliminated. Hence, the retinoid analysis of mice with a combination of different retinoid cycle genes provides the opportunities to uncover interconnections between these gene products and understanding in more detail their involvements in the cycle.
Accumulation of 13-cis-Retinyl Esters in the RPE in Rdh5−/− Mice
Accumulation of 13-cis-retinyl esters is a result of isomerization of all-trans-retinoids to 13-cis-retinol and esterification of the product by LRAT to 13-cis-retinyl esters (Figures 1 and 2). Even Rdh5−/−Rdh11−/− mice kept in normal room light showed very high levels of 13-cis-retinyl esters, but after exposure to high-intensity light these mice further accumulated 13-cis-retinyl esters. These esters were not observed in wild-type mice. The retinosomes of the RPE are the storage site of 13-cis-retinyl and all-trans-retinyl esters. The storage amount was enlarged to 400–600 pmol/eye, and accumulated 13-cis-retinyl esters remained for more than 1 week in the RPE.
What can account for this mechanism? For one, the lack of RDH5 may perturb the highly compartmentalized retinoid cycle (see, for example, ref 20), resulting in the production of cis-retinol according to the thermodynamics of the isomerization reaction and distribution of the hydration product of the putative carbocation intermediate (40). This aberrant isomer is produced not only from all-trans-retinal liberated from opsin during a high demand for 11-cis-retinal but also in Rdh5−/−Rpe65−/− mice lacking rhodopsin. The product of this isomerization is not utilized but is stored in the retinosomes. Perhaps this is due to the lack of 13-cis-retinol to 13-cis-retinal oxidation activity in Rdh5−/− mice (16) and further oxidation reactions producing inactive oxidized retinoid, which could eliminate this aldehyde.
Alternatively, the isomerization complex lacking RDH5 may have relaxed specificity, producing both 11-cis- and 13-cis-retinoids. The fact that there is a substantial 13-cis-retinyl ester formation in the Rdh5−/−Rpe65−/− mice supports the notion that RPE65 is not required for 13-cis-retinoid formation, but these animals have only trace amounts of 11-cis-retinoids, consistent with RPE65 being essential for the 11-cis-retinal production. Hence, RPE65 is not responsible for enzymatic conversion of all-trans-retinoids to their corresponding 13-cis isomers but essential for 11-cis-retinal production (22).
In wild-type mice, light can convert a small amount of all-trans-retinoids to 13-cis-retinoids, and potentially RPE65 is capable of interconverting all-trans-, 11-cis-, and 13-cis-retinol, but in normal conditions the reaction is driven by RDH5 and the retinoid-binding proteins selectively removing the 11-cis product. However, our Rdh5−/− mice maintained under dim red light to avoid photoisomerization still accumulated 13-cis-retinyl esters, diminishing the probability of this mechanism. Moreover, Rdh5−/−Rpe65−/− mice lacking visible light absorbing rhodopsin still produce 13-cis-retinoids. Alternatively, not light, but thermal isomerization of retinoids could be the responsible factor. To test this possibility, we employed a very potent inhibitor of isomerization, Ret-NH2, which does not bind to most of the RPE65 pool present in the RPE (38, 41). This inhibitor prevents the isomerization of all-trans- to both the 11-cis-and the 13-cis-retinol configuration, providing evidence that this reaction is enzymatic. This conclusion is also supported by the earlier chemical evidence of a similar mechanism of enzymatic isomerization of trans isomers to 11-cis- and 13-cis-retinol through the alkyl cleavage (36). Observed differences between Rpe65-null (no production of 11-cis-retinal) and Ret-NH2-administrated Rdh5−/−Rdh11−/− mice (in-hibition of 11-cis- or 13-cis-retinoid production) thus suggest the existence of an enzyme which requires the other protein components such as RPE65 and RDH5 for the production of 13-cis isomer or an activity which is suppressed by the presence of RPE65 and RDH5 in wild-type mice. This observation is puzzling when it is considered that RPE65 has been recently proposed to be an isomerohydrolase, the enzyme responsible for isomerization and hydrolysis of all-trans-retinyl esters to 11-cis-retinol (23–26). It should be noted that purified RPE65 has no isomerase activity (27). Further studies are required to understand the protein complex responsible for the retinoid isomerization in the RPE cells.
This aberrant isomerization process to 13-cis-retinoids occurs to a significant degree when RDH5 is eliminated, and it is further enhanced when opsin is deleted (Figures 2 and 3). High levels of cis-retinyl esters were observed not only in opsin−/−Rdh5−/− mice but also in heterozygote op-sin+/−Rdh5−/− mice (Figure 3), suggesting that the lack of 11-cis-retinal, the final product of the retinoid cycle, or the lack of the full complement of rhodopsin in rod outer segments of opsin+/− mice (42) enhances this aberrant isomerization. Opsin, or the physical interaction of the rod outer segment structure with the RPE processes, can influence the retinoid cycle through an uncovered yet signaling pathway. This hypothesis is supported by accumulation of all-trans-retinyl esters in mice of different genetic backgrounds and production of 11-cis-retinal on demand.
Accumulation of All-trans-Retinyl Esters in the RPE
If esterification and hydrolysis depended only on mass action, a steady level of retinyl esters would be present in the eye. However, this is not what is observed in knockout mice deprived of key proteins of the retinoid cycle. In addition, excessive all-trans-retinyl esters normally accumulate in the eyes of wild-type mice with age (34).
Redmond et al. first observed that Rpe65−/− mice do not possess an 11-cis-retinal chromophore and also tend to overaccumulate retinyl esters in lipid droplets (22). The esters accumulated with age (34) by means of the budding of naturally occurring retinyl ester storage particles, termed retinosomes (19, 20). Two hypotheses can be postulated that are not exclusive of each other. First, the RPE may recognize a signal from photoreceptor cells by an undefined chemical sensory means that opsin is unliganded and more 11-cis-retinal needs to be produced. In such a scenario, the RPE would first accumulate retinyl esters to provide a substrate for further transformations leading to the production of 11-cis-retinal. A genetic defect or a lack of RPE65 would stall the isomerization process, and consequently the chromophore would not be produced, while the esters would continue to accumulate. Recent identification of RPE65 as the isomerase is consistent with this view (23–26). When the opsin gene is deleted from Rpe65−/− mice, a 3–4-fold decrease in the ester content is observed (Figure 2), which also supports this hypothesis. This effect of opsin on the level of all-trans-retinyl ester levels revealed a very important connection between phototransduction and the retinoid cycle. This hypothesis is also supported by the observation that the retinyl ester accumulation defect can be bypassed via oral gavage of 9-cis-retinal very early in life, slowing the accumulation of esters (34). It appears that unliganded opsin sends a signal to RPE, which responds to increased absorption of all-trans-retinol from circulation.
A second possibility is that RPE65 is also involved in the mobilization of stored retinyl esters (43). The accumulation of retinyl esters is clearly dependent on LRAT activity (Figure 2), and these esters accumulate in Rpe65−/− mice. RPE65 was also proposed to be a retinyl ester-binding protein (27, 44, 45), hinting of its possible role in this process. Most insightful was the observation made by Pepperberg et al. that, in normal RPE, there is a substantial exchange of all-trans-retinol with the blood circulation, whereas in Rpe65−/−mice, despite the presence of abnormally high molar levels of RPE retinyl esters, the outward movement is inhibited (43).
There are other circumstances in which retinyl esters accumulate in knockout mice, as in Rgr−/− (46), Cralbp−/−(47), and Crbp−/− mice (48) after bleach. However, this accumulation is due to the dynamic process during the retinoid cycle, a different process than discussed in this study. Here, we demonstrated that 13-cis-retinyl esters are formed through an aberrant enzymatic isomerization process involving an enzyme that is inhibited by Ret-NH2 as is the isomerase that normally produces 11-cis-retinoids.
Acknowledgments
We thank Daniel Possin for electron microscopic analysis, Amy Look for technical help during the course of this study, and Rebecca Birdsong for help with manuscript preparation.
Footnotes
This research was supported by NIH Grant EY09339.
Abbreviations: CRALBP, cellular retinaldehyde-binding protein; CRBP, cellular retinoid-binding protein; LRAT, lecithin:retinol acyl-transferase; RDH, retinol dehydrogenase; RPE, retinal pigment epithelium; retinosomes, retinyl ester storage particles; Ret-NH2, retinylamine; RPE65, an RPE-specific 65 kDa protein.
References
- 1.Filipek S, Stenkamp RE, Teller DC, Palczewski K. G protein-coupled receptor rhodopsin: A prospectus. Annu Rev Physiol. 2003;65:851–879. doi: 10.1146/annurev.physiol.65.092101.142611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridge KD, Abdulaev NG, Sousa M, Palczewski K. Phototransduction: crystal clear. Trends Biochem Sci. 2003;28:479–487. doi: 10.1016/S0968-0004(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 3.Okada T, Ernst OP, Palczewski K, Hofmann KP. Activation of rhodopsin: new insights from structural and biochemical studies. Trends Biochem Sci. 2001;26:318–324. doi: 10.1016/s0968-0004(01)01799-6. [DOI] [PubMed] [Google Scholar]
- 4.Palczewski, K. (2006) G Protein-coupled receptor rhodopsin, Annu. Rev. Biochem. (in press). [DOI] [PMC free article] [PubMed]
- 5.Rando, R. R. (1991) Membrane phospholipids as an energy source in the operation of the visual cycle, Biochemistry 30, 595–602. [DOI] [PubMed]
- 6.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retinal Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 7.Thompson DA, Gal A. Vitamin A metabolism in the retinal pigment epithelium: genes, mutations, and diseases. Prog Retinal Eye Res. 2003;22:683–703. doi: 10.1016/s1350-9462(03)00051-x. [DOI] [PubMed] [Google Scholar]
- 8.Gottesman, M. E., Quadro, L., and Blaner, W. S. (2001) Studies of vitamin A metabolism in mouse model systems, BioEssays 23, 409–419. [DOI] [PubMed]
- 9.Simon, A., Lagercrantz, J., Bajalica-Lagercrantz, S., and Eriksson, U. (1996) Primary structure of human 11-cis retinol dehydrogenase and organization and chromosomal localization of the corresponding gene, Genomics 36, 424–430. [DOI] [PubMed]
- 10.Simon A, Hellman U, Wernstedt C, Eriksson U. The retinal pigment epithelial-specific 11-cis retinol dehydrogenase belongs to the family of short chain alcohol dehydrogenases. J Biol Chem. 1995;270:1107–1112. [PubMed] [Google Scholar]
- 11.Kim TS, Maeda A, Maeda T, Heinlein C, Kedishvili N, Palczewski K, Nelson PS. Delayed dark adaptation in 11-cis-retinol dehydrogenase deficient mice: A role of RDH11 in visual processes in vivo. J Biol Chem. 2005;280:8694–8704. doi: 10.1074/jbc.M413172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto H, Simon A, Eriksson U, Harris E, Berson EL, Dryja TP. Mutations in the gene encoding 11-cis retinol dehydrogenase cause delayed dark adaptation and fundus albipunctatus. Nat Genet. 1999;22:188–191. doi: 10.1038/9707. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura M, Hotta Y, Tanikawa A, Terasaki H, Miyake Y. A high association with cone dystrophy in fundus albipunctatus caused by mutations of the RDH5 gene. Invest Ophthalmol Visual Sci. 2000;41:3925–3932. [PubMed] [Google Scholar]
- 14.Marmor, M. F., Haeseleer, F., and Palczewski, K. (2003) Albi-punctate retinopathy with cone dysfunction and no abnormality in the RDH5 or RLBP1 genes, Retina 23, 543–546. [DOI] [PubMed]
- 15.Driessen CA, Winkens HJ, Hoffmann K, Kuhlmann LD, Janssen BP, Van Vugt AH, Van Hooser JP, Wieringa BE, Deutman AF, Palczewski K, Ruether K, Janssen JJ. Disruption of the 11-cis-retinol dehydrogenase gene leads to accumulation of cis-retinols and cis-retinyl esters. Mol Cell Biol. 2000;20:4275–4287. doi: 10.1128/mcb.20.12.4275-4287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang GF, McBee JK, Alekseev AM, Haeseleer F, Palczewski K. Stereoisomeric specificity of the retinoid cycle in the vertebrate retina. J Biol Chem. 2000;275:28128–28138. doi: 10.1074/jbc.M004488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang GF, Van Hooser JP, Kuksa V, McBee JK, He YG, Janssen JJ, Driessen CA, Palczewski K. Characterization of a dehydrogenase activity responsible for oxidation of 11-cis-retinol in the retinal pigment epithelium of mice with a disrupted RDH5 gene. A model for the human hereditary disease fundus albipunctatus. J Biol Chem. 2001;276:32456–32465. doi: 10.1074/jbc.M104949200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang E, Lai K, Packer AI, Paik J, Blaner WS, de Morais Vieira M, Gouras P, Wolgemuth DJ. Targeted disruption of the mouse cis-retinol dehydrogenase gene: visual and nonvisual functions. J Lipid Res. 2002;43:590–597. [PubMed] [Google Scholar]
- 19.Imanishi Y, Batten ML, Piston DW, Baehr W, Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J Cell Biol. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imanishi Y, Gerke V, Palczewski K. Retinosomes: new insights into intracellular managing of hydrophobic substances in lipid bodies. J Cell Biol. 2004;166:447–453. doi: 10.1083/jcb.200405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saari JC, Garwin GG, Van Hooser JP, Palczewski K. Reduction of all-trans-retinal limits regeneration of visual pigment in mice. Vision Res. 1998;38:1325–1333. doi: 10.1016/s0042-6989(97)00198-3. [DOI] [PubMed] [Google Scholar]
- 22.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 23.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci USA. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci USA. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi Y, Moiseyev G, Chen Y, Ma JX. Identification of conserved histidines and glutamic acid as key residues for isomerohydrolase activity of RPE65, an enzyme of the visual cycle in the retinal pigment epithelium. FEBS Lett. 2005;579:5414–5418. doi: 10.1016/j.febslet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Jin, M., Li, S., Moghrabi, W. N., Sun, H., and Travis, G. H. (2005) Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium, Cell 122, 449–459. [DOI] [PMC free article] [PubMed]
- 27.Mata NL, Moghrabi WN, Lee JS, Bui TV, Radu RA, Horwitz J, Travis GH. Rpe65 is a retinyl ester binding protein that presents insoluble substrate to the isomerase in retinal pigment epithelial cells. J Biol Chem. 2004;279:635–643. doi: 10.1074/jbc.M310042200. [DOI] [PubMed] [Google Scholar]
- 28.Lem J, Krasnoperova NV, Calvert PD, Kosaras B, Cameron DA, Nicolo M, Makino CL, Sidman RL. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci USA. 1999;96:736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda A, Maeda T, Imanishi Y, Kuksa V, Alekseev A, Bronson JD, Zhang H, Zhu L, Sun W, Saperstein DA, Rieke F, Baehr W, Palczewski K. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J Biol Chem. 2005;280:18822–18832. doi: 10.1074/jbc.M501757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Hooser JP, Aleman TS, He YG, Cideciyan AV, Kuksa V, Pittler SJ, Stone EM, Jacobson SG, Palczewski K. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc Natl Acad Sci USA. 2000;97:8623–8628. doi: 10.1073/pnas.150236297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moise AR, Kuksa V, Blaner WS, Baehr W, Palczewski K. Metabolism and transactivation activity of 13,14-dihydroretinoic acid. J Biol Chem. 2005;280:27815–27825. doi: 10.1074/jbc.M503520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang T, Snider BB, Oprian DD. Synthesis and characterization of a novel retinylamine analog inhibitor of constitutively active rhodopsin mutants found in patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci USA. 1997;94:13559–13564. doi: 10.1073/pnas.94.25.13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Hooser JP, Liang Y, Maeda T, Kuksa V, Jang GF, He YG, Rieke F, Fong HK, Detwiler PB, Palczewski K. Recovery of visual functions in a mouse model of Leber congenital amaurosis. J Biol Chem. 2002;277:19173–19182. doi: 10.1074/jbc.M112384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stecher H, Gelb MH, Saari JC, Palczewski K. Preferential release of 11-cis-retinol from retinal pigment epithelial cells in the presence of cellular retinaldehyde-binding protein. J Biol Chem. 1999;274:8577–8585. doi: 10.1074/jbc.274.13.8577. [DOI] [PubMed] [Google Scholar]
- 36.McBee, J. K., Kuksa, V., Alvarez, R., de Lera, A. R., Prezhdo, O., Haeseleer, F., Sokal, I., and Palczewski, K. (2000) Isomerization of all-trans-retinol to cis-retinols in bovine retinal pigment epithelial cells: dependence on the specificity of retinoid-binding proteins, Biochemistry 39, 11370–11380. [DOI] [PMC free article] [PubMed]
- 37.Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golczak M, Kuksa V, Maeda T, Moise AR, Palczewski K. Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc Natl Acad Sci USA. 2005;102:8162–8167. doi: 10.1073/pnas.0503318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cideciyan AV, Haeseleer F, Fariss RN, Aleman TS, Jang GF, Verlinde CL, Marmor MF, Jacobson SG, Palczewski K. Rod and cone visual cycle consequences of a null mutation in the 11-cis-retinol dehydrogenase gene in man. Vision Neurosci. 2000;17:667–678. doi: 10.1017/s0952523800175029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuksa V, Imanishi Y, Batten M, Palczewski K, Moise AR. Retinoid cycle in the vertebrate retina: experimental approaches and mechanisms of isomerization. Vision Res. 2003;43:2959–2981. doi: 10.1016/s0042-6989(03)00482-6. [DOI] [PubMed] [Google Scholar]
- 41.Golczak M, Imanishi Y, Kuksa V, Maeda T, Kubota R, Palczewski K. Lecithin:retinol acyltransferase is responsible for amidation of retinylamine, a potent inhibitor of the retinoid cycle. J Biol Chem. 2005;280:42263–42273. doi: 10.1074/jbc.M509351200. [DOI] [PubMed] [Google Scholar]
- 42.Liang Y, Fotiadis D, Maeda T, Maeda A, Modzelewska A, Filipek S, Saperstein DA, Engel A, Palczewski K. Rhodopsin signaling and organization in heterozygote rhodopsin knockout mice. J Biol Chem. 2004;279:48189–48196. doi: 10.1074/jbc.M408362200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qtaishat NM, Redmond TM, Pepperberg DR. Acute radiolabeling of retinoids in eye tissues of normal and rpe65-deficient mice. Invest Ophthalmol Visual Sci. 2003;44:1435–1446. doi: 10.1167/iovs.02-0679. [DOI] [PubMed] [Google Scholar]
- 44.Jahng, W. J., David, C., Nesnas, N., Nakanishi, K., and Rando, R. R. (2003) A cleavable affinity biotinylating agent reveals a retinoid binding role for RPE65, Biochemistry 42, 6159–6168. [DOI] [PMC free article] [PubMed]
- 45.Gollapalli, D. R., Maiti, P., and Rando, R. R. (2004) RPE65 operates in the vertebrate visual cycle by stereospecifically binding all-trans-retinyl esters, Biochemistry 43, 7226. [DOI] [PubMed]
- 46.Maeda T, Van Hooser JP, Driessen CA, Filipek S, Janssen JJ, Palczewski K. Evaluation of the role of the retinal G protein-coupled receptor (RGR) in the vertebrate retina in vivo. J Neurochem. 2003;85:944–956. doi: 10.1046/j.1471-4159.2003.01741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saari, J. C., Nawrot, M., Kennedy, B. N., Garwin, G. G., Hurley, J. B., Huang, J., Possin, D. E., and Crabb, J. W. (2001) Visual cycle impairment in cellular retinaldehyde binding protein (CRALBP) knockout mice results in delayed dark adaptation, Neuron 29, 739–748. [DOI] [PubMed]
- 48.Saari JC, Nawrot M, Garwin GG, Kennedy MJ, Hurley JB, Ghyselinck NB, Chambon P. Analysis of the visual cycle in cellular retinol-binding protein type I (CRBPI) knockout mice. Invest Ophthalmol Visual Sci. 2002;43:1730–1735. [PubMed] [Google Scholar]
- 49.Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, Sieving PA, Sheils DM, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi MR, Humphries P. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]


