Abstract
There exists an interplay between vaccine coverage, disease prevalence and the vaccinating behaviour of individuals. Moreover, because of herd immunity, there is also a strategic interaction between individuals when they are deciding whether or not to vaccinate, because the probability that an individual becomes infected depends upon how many other individuals are vaccinated. To understand this potentially complex interplay, a game dynamic model is developed in which individuals adopt strategies according to an imitation dynamic (a learning process), and base vaccination decisions on disease prevalence and perceived risks of vaccines and disease. The model predicts that oscillations in vaccine uptake are more likely in populations where individuals imitate others more readily or where vaccinating behaviour is more sensitive to changes in disease prevalence. Oscillations are also more likely when the perceived risk of vaccines is high. The model reproduces salient features of the time evolution of vaccine uptake and disease prevalence during the whole-cell pertussis vaccine scare in England and Wales during the 1970s. This suggests that using game theoretical models to predict, and even manage, the population dynamics of vaccinating behaviour may be feasible.
Keywords: game theory, replicator equations, imitation dynamic, childhood diseases, vaccine scares, vaccination policy
1. Introduction
Vaccination has benefited public health enormously. Vaccination campaigns have significantly reduced morbidity and mortality from many infectious diseases, and have come close to eradicating polio (Bonanni 1998; CDC 2004). However, to date, only smallpox has been eliminated through vaccination (Fenner et al. 1998; Andre 2003). Part of the resiliency of vaccine-preventable diseases is due to a paradox inherent to infectious disease epidemiology. As more individuals become vaccinated, the remaining unvaccinated individuals are increasingly unlikely to become infected, because of herd immunity (Anderson & May 1991; Brisson & Edmunds 2003). For a population with sufficiently high vaccine coverage, a disease can be eradicated without vaccinating everyone. Therefore, as coverage increases, there is a greater individual incentive not to vaccinate, since non-vaccinators can gain the benefits of herd immunity without the perceived risk of vaccine complications. Evidence suggests that many individuals are aware of the potential to exploit herd immunity in this way (Asch et al. 1994; Health Canada 2004). This paradox makes eradication difficult under a voluntary vaccination policy (Cullen & West 1979; Fine & Clarkson 1986; Geoffard & Philipson 1997; Bauch et al. 2003; Bauch & Earn 2004), and represents a clash between the optimal vaccinating behaviour for the individual and the level of vaccine coverage that is best for the population as a whole (Fine & Clarkson 1986; Bauch et al. 2003).
Additionally, the perceived risks of vaccines and diseases evolve over time. There have been numerous episodes in which resistance to vaccination has been widespread, sometimes causing major declines in vaccine uptake, the rate at which individuals are vaccinated (Durbach 2000; Albert et al. 2001; Poland & Jacobsen 2001; Plotkin 2002; Biroscak et al. 2003). These episodes have often been associated with concerns about vaccine safety or underestimation of the risks of disease (Asch et al. 1994; Roberts et al. 1995; Lashuay et al. 2000; Evans et al. 2001; Schmitt 2002; Bellaby 2003; Smailbegovic et al. 2003). Even a modest increase in perceived vaccine risk, when combined with a minimal incentive to vaccinate when prevalence is low, can cause a sharp decline in vaccine uptake. Presently, for instance, the common belief that the measles–mumps–rubella (MMR) vaccine causes autism and irritable bowel syndrome has caused a serious decline in MMR vaccine uptake in Britain (Jansen et al. 2003), despite the lack of scientific evidence for a connection (Nicoll et al. 1998; Afzal et al. 2000; Dales et al. 2001; Madsen et al. 2002). Likewise, in the 1970s, safety concerns about the whole-cell pertussis vaccine led to declines in uptake in many countries (Gangarosa et al. 1998; Baker 2003). In England and Wales, a particularly steep decline resulted in a series of large pertussis outbreaks (figure 1a).
Figure 1.
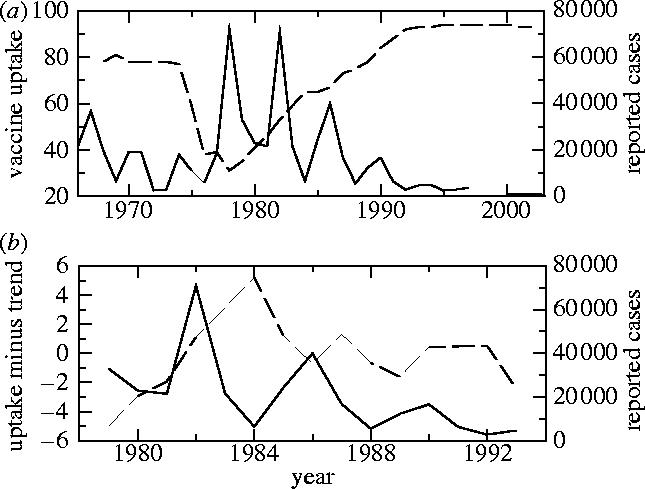
(a) Pertussis vaccine uptake (dashed) and case notifications (solid) in England and Wales from 1967 to 2003 and (b) pertussis vaccine uptake minus a linear least-squares fit (dashed) and case notifications (black) in England and Wales from 1979 to 1993. The vaccine uptake datum at year t is the proportion of children born in year t−2 who completed the recommended course of pertussis immunizations by year t, and the case notification datum at year t is simply the total number of cases reported in that year. Data are from the WHO (2004) and Miller & Gay (1997).
Just as reduced vaccine uptake eventually leads to higher disease prevalence, higher prevalence can also increase vaccine uptake. Outbreaks may cause more individuals to seek vaccination, or may cause a redoubling of vaccination programme efforts. In England and Wales, the period of heightened pertussis prevalence in the late 1970s and early 1980s was accompanied by an increase in vaccine uptake (figure 1a). Some of this increase was probably attributable to a decrease in the perceived vaccine risk. However, vaccine uptake during this time tends to rise and fall with the pattern of naturally recurrent outbreaks, indicating that prevalence indeed plays a role (figure 1b). A similar pattern has been observed in other places, for example, in Australia, where a doubling of annual incidence due to a large pertussis outbreak in 1997 was followed by a jump in vaccine uptake from a constant 86% between 1994 and 1998 (for the third dose of diphtheria, tetanus, and pertussis vaccine) to 92% by 2000 (WHO 2004).
Vaccine coverage under a voluntary policy is the collective result of individual decisions to vaccinate or not to vaccinate. These decisions may depend upon a number of factors, such as trust of medical authorities, perceived risks of vaccines and diseases, and societal norms regarding individual rights. However, these decisions also depend upon a strategic interaction among individuals. Because of herd immunity, the probability that an unvaccinated individual is infected depends upon how many others have decided to vaccinate. Previous approaches do not explicitly model the process by which individual decision-making gives rise to the observed prevalence and vaccine uptake, but rather rely upon phenomenological approaches, or describe the differences between criteria for individual versus group optima (Fine & Clarkson 1986; Geoffard & Philipson 1997). To capture this process explicitly, one must apply game theory, which formalizes strategic interactions in a group where individuals attempt to maximize their pay-offs (von Neumann & Morgenstern 1944; Maynard-Smith 1982; Hofbauer & Sigmund 1998). Because vaccine uptake is ultimately determined by individual behaviour, a game theoretical approach to determining the relationship between vaccine uptake and disease prevalence represents an approach from first principles.
Previous game theoretical analyses of vaccinating behaviour rely upon classical game theory, assuming a static game where individuals always act with perfect information on their probability of becoming infected (Bauch et al. 2003; Bauch & Earn 2004). In reality, vaccination games unfold through time, and individuals cannot precisely determine their probability of becoming infected. Moreover, humans adopt new strategies through learning, by imitating others who appear to have adopted more successful strategies. Here, we address these issues by developing a dynamics for the vaccination game, where an imitation dynamic (a learning process between individuals) governs the time evolution of the frequencies of strategies in the population (Helbing 1992; Bjoernerstedt & Weibull 1996; Hofbauer & Sigmund 1998). This is similar to the replicator dynamics (equations) of evolutionary game theory (Weibull 1995; Hofbauer & Sigmund 1998). Furthermore, it is not assumed that individuals have perfect knowledge of their probability of infection; instead, they rely upon a ‘rule of thumb’ to estimate this probability. This is similar to the concept of bounded rationality in psychological game theory (Colman 2003).
2. Model
Here, the focus is on childhood diseases with lifelong, or long-term, natural immunity (e.g. measles, mumps, rubella and pertussis), and for which vaccination is scheduled at a young age. The players of the game are parents, who decide whether to vaccinate their child at birth, and adopt either a vaccinator or a non-vaccinator strategy. The perceived payoff fv for vaccinators is simply
| 2.1 |
where rv is the perceived probability of significant morbidity from the vaccine (and perfect vaccine efficacy is assumed). The perceived payoff for non-vaccinators depends upon the perceived probability ri of suffering significant morbidity upon infection, and the perceived probability of eventually becoming infected, which is assumed to increase linearly with the current disease prevalence I=I(t), the proportion infected at time t. With this rule of thumb, the perceived payoff fv for non-vaccinators is
| 2.2 |
The parameter m quantifies the sensitivity of vaccinating behaviour to changes in prevalence. We note that parents of the current generation play not only against one another, but also against previous generations of behaviourally identical parents, since the current prevalence (and thus payoff) is partly determined by the history of vaccine uptake in the population. Hence, the non-vaccinator payoff depends implicitly upon the strategy history and relevant behavioural and epidemiological parameters, although in practice it may not be possible to derive a simple, closed-form expression in terms of those quantities.
For the imitation dynamic, it is assumed that individuals randomly sample other members of the population at some constant rate. If the strategy of the sampled member provides a higher payoff, then his or her strategy is adopted with a probability proportional to the expected gain in payoff (Helbing 1992; Bjoernerstedt & Weibull 1996; Hofbauer & Sigmund 1998). The payoff gain for switching to a vaccinator strategy is ΔE≡fv−fn(I). Consider first the case where ΔE>0, such that non-vaccinators may become vaccinators but not vice versa, and let x denote the relative frequency of vaccinators. If individuals sample at rate σ, then a non-vaccinator samples vaccinators at rate σx. Then, a non-vaccinator switches to the vaccinator strategy with probability ρΔE, where ρ is the proportionality constant. Accordingly, the time evolution of x when ΔE>0 is governed by
| 2.3 |
where (1−x) is the frequency of non-vaccinators. By comparison, if ΔE<0, then vaccinators may become non-vaccinators and the equation of motion for x is
| 2.4 |
If we take k≡σρ to represent the combined imitation rate at which individuals sample others and switch strategies, then equations (2.3) and (2.4) simplify to
| 2.5 |
It can be shown that equation (2.5) is formally identical to the replicator equations for an evolutionary population game (Hofbauer & Sigmund 1998), except that the payoffs here are time varying.
The prevalence I(t) can be determined from an SIR (susceptible–infectious–recovered) compartmental epidemic model with births and deaths (Anderson & May 1991). Compartmental models divide the population into mutually exclusive categories based on their epidemiological status, and define ordinary differential equations that govern the flow between compartments. Here, we make the standard assumption that the rate at which new infected individuals arise is proportional to the product of the density of susceptible and infected individuals. With the inclusion of the imitation dynamic, such that a proportion x of children are vaccinated at birth, the vaccination game with imitation dynamics is described by the system of ordinary differential equations
| 2.6 |
| 2.7 |
| 2.8 |
| 2.9 |
Here, S is the proportion susceptible, I is the proportion infected (or equivalently, the prevalence), R is the proportion immune, either naturally or through vaccination, μ is the mean birth (and death) rate per capita, β is the mean transmission rate and 1/γ is the mean duration of infectiousness. Because a proportion x of children are vaccinated at birth, the frequency of vaccinator strategists at time t is equal to the vaccine uptake at time t. Although parents may switch from vaccinator to non-vaccinator, or vice versa, at any time (in accordance with the rules of the imitation dynamic), the decision whether or not to actually vaccinate can be made only once, at birth, and so it is not possible for vaccinated individuals to become unvaccinated later in life (or even for unvaccinated individuals to later become vaccinated).
The differential equation for R can be eliminated since S+I+R=1. Furthermore, it is possible to write the differential equation for x in terms of just two parameters, κ≡krv and ω≡mri/rv. Hence
| 2.10 |
| 2.11 |
| 2.12 |
Hereafter, β is expressed in terms of the basic reproductive number 0 (the average number of secondary infections produced by an infected individual in an otherwise susceptible population) using the relation (Anderson & May 1991). This system of equations admits a disease-free, pure non-vaccinator equilibrium and a disease-free, pure vaccinator equilibrium . There is also an endemic, pure non-vaccinator equilibrium
| 2.13 |
which exists when 0>1 (and where ). Finally, there is an endemic equilibrium which corresponds to a mixed state of vaccinators and non-vaccinators
| 2.14 |
and which exists when 0>1 and ω>ω0.
3. Analysis
The disease-free, pure vaccinator equilibrium 2 is always unstable owing to the incentive not to vaccinate when coverage is high (Bauch et al. 2003; Bauch & Earn 2004). When 0<1, the disease-free, pure non-vaccinator equilibrium 1 is globally stable. This occurs because when 0<1 (such that each infected individual on average yields less than one secondary infection), the disease eventually disappears from the population, hence there is no incentive to vaccinate. When 0>1, such that sustained disease transmission (and an endemic equilibrium) is possible, 1 also destabilizes and the system exhibits three dynamical regimes in the κ–ω parameter plane (figures 2–4). In region I (0<ω<ω0), the endemic, pure non-vaccinator equilibrium 3 is the only locally stable equilibrium, hence no one chooses to vaccinate and the prevalence is constant and non-zero. By comparison, in region II, the endemic, mixed equilibrium 4 is the only locally stable equilibrium, so both the frequency of vaccinators and the prevalence of disease are constant and non-zero. In region III, 4 destabilizes through a Hopf bifurcation, leaving no stable equilibria. In this region, the frequency of vaccinators and the prevalence oscillate in a stable limit cycle about 4. Stability analysis of the non-trivial equilibria, and continuation of limit cycles in region III, were performed numerically using Content 1.5 (Kuznetsov 1999).
Figure 2.
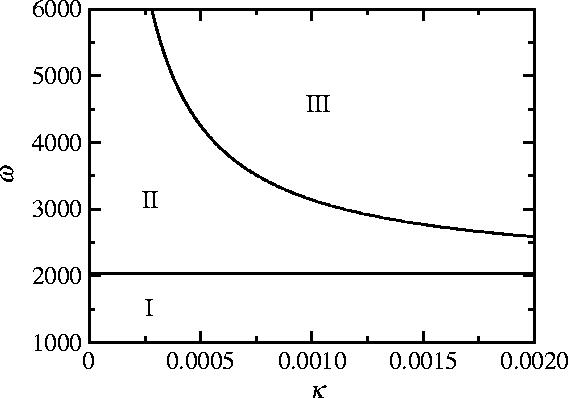
The κ–ω parameter plane for the vaccination game with imitation dynamics, equations (2.10)–(2.12). See text for description of regions I, II and III. Along the Hopf curve dividing regions II and III, κ(ω−ω0) is a constant. The line ω=ω0 divides regions I and II. Other parameters are 0=10, 1/γ=10 days, 1/μ=50 years.
Figure 3.
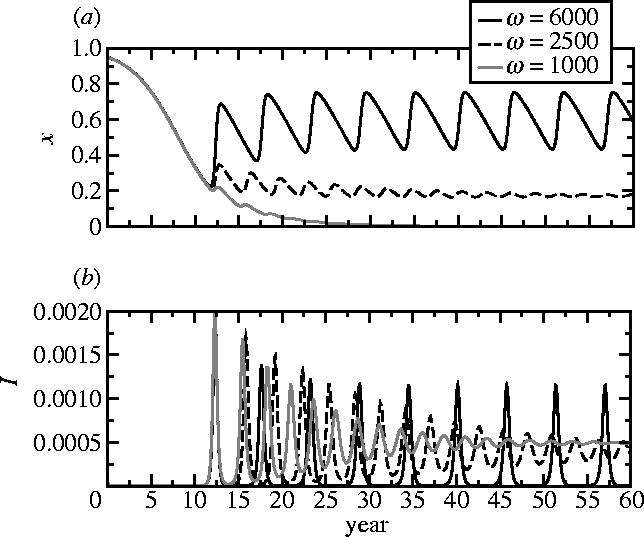
Representative time-series of x, (a) the frequency of vaccinators, and (b) I, the disease prevalence, from regions I, II and III of the κ–ω parameter plane, for three values of ω. Other parameters are 1/μ=50 years, 1/γ=10 days, 0=10, κ=0.001. Initial conditions are S=0.05, I=0.0001, x=0.95. Given that immigration of infected individuals becomes important in the inter-epidemic troughs, a term +α is included for these time-series in equation (2.10), and a term −α in equation (2.12), where α=10−8 day−1.
Figure 4.
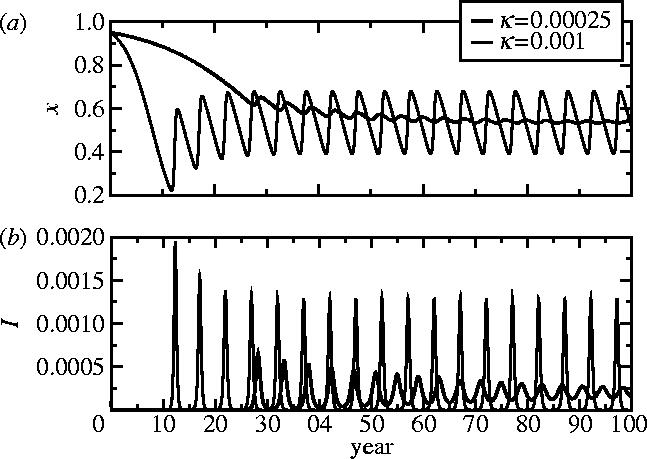
Representative time-series of x, (a) the frequency of vaccinators, and (b) I, the disease prevalence, from regions II and III of the κ–ω parameter plane, for two values of κ. Other parameters are 1/μ=50 years, 1/γ=10 days, 0=10, ω=5000, α=10−8 day−1 (see figure 3 caption). Initial conditions are S=0.05, I=0.0001, x=0.95.
In most cases, both static vaccination games (Bauch et al. 2003; Bauch & Earn 2004; G. Heal & H. Kunreuther 2005, unpublished work), and the vaccination game with imitation dynamics predict a single stable equilibrium for any given choice of parameter values. However, in region III of figure 2, the vaccination game with imitation dynamics diverges from static vaccination games, predicting no stable equilibria at all, but rather stable limit cycles. Once strategy frequencies are allowed to evolve through time according to an imitation dynamic, the occurrence of stable limit cycles becomes not only possible but also probable, given the natural propensity of epidemiological systems to oscillate (Bauch & Earn 2003).
It is helpful to interpret these results in terms of the original epidemiological and behavioural parameters that appear in equations (2.6)–(2.9). Populations where vaccinating behaviour is more responsive to changes in disease prevalence (larger m) will have a higher frequency of vaccinators, and for sufficiently large m they will exhibit oscillations in vaccine uptake and prevalence. In populations where individuals imitate more quickly (larger k), oscillations in prevalence and the frequency of vaccinators are also more probable (as long as ω>ω0), although the average vaccine uptake and disease prevalence remain unchanged. As k or ω increase, the amplitude of oscillations also increases. Populations converge to asymptotic states more rapidly for larger k (figure 4). When I is very small, the time evolution of the frequency of vaccinators is entirely determined by the value of k (figures 3 and 4). Furthermore, because κ(ω−ω0) is constant along the Hopf curve dividing regions II and III, a higher perceived vaccine risk rv can move the population into region III, again producing oscillations (although the change in rv must be relatively large for this to happen). Finally, increasing 0 shifts the Hopf curve to the right while moving the ω=ω0 line downward, diminishing the region of pure non-vaccinating behaviour.
The impact of a vaccine scare can be studied by considering a population initially near the endemic, mixed equilibrium 4 with a high uptake and low incidence, in which the perceived vaccine risk rv increases instantaneously at a specified time and returns later to pre-scare levels. Parameters and initial conditions are chosen to reflect pertussis epidemiology and vaccine uptake in England and Wales before the 1970s vaccine scare. The perceived risk rv is increased eightfold at t=5 years in order to produce a drop in uptake similar to that observed in England and Wales between 1974 and 1978 (figures 1 and 5). When rv is restored to pre-scare levels at t=10 years, vaccine uptake returns to pre-scare levels much more slowly (and we note that a more gradual restoration of perceived vaccine risk to pre-scare levels would only exaggerate this effect). This pattern is observed in the data from England and Wales (figure 1a) and is characteristic of the model. This pattern is also predicted by simpler static vaccination games (Bauch & Earn 2004). We emphasize that the decline and restoration of vaccine uptake observed in England and Wales during the pertussis vaccine scare is not interpreted here as the result of endogenous oscillations of the vaccination game (as observed in figures 3 and 4) but rather as the result of an exogenous change in the perceived vaccine risk rv.
Figure 5.
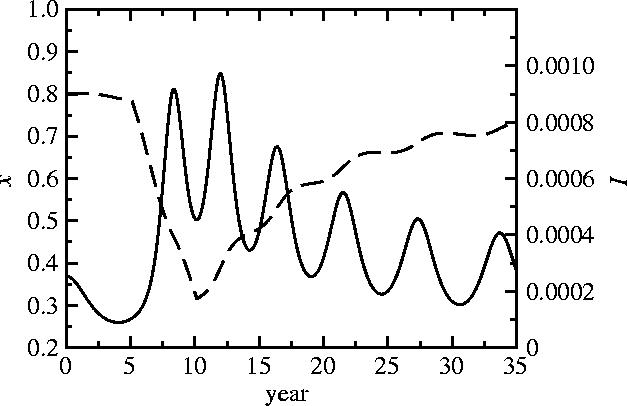
A simulated pertussis vaccine scare, with time-series of frequency of vaccinators x (dashed line; equivalent to vaccine uptake in figure 1a) and disease prevalence I (solid line; equivalent to case reports in figure 1a). Parameters are 0=15, 1/γ=22 days, 1/μ=50 years, κ=0.002, ω=5000; these parameter values yield a mean vaccine uptake and period of oscillation similar to that observed in England and Wales before the whole-cell pertussis vaccine scare of the 1970s (figure 1; Bauch & Earn 2003). The population is near the equilibrium 4 at t=0 years. At t=5 years, the perceived vaccine risk rv is increased eightfold in order to produce a decline similar to that observed in England and Wales from 1973 to 1978. At t=10 years, the pre-scare value of rv is restored.
4. Discussion
A vaccination game with imitation dynamics has been introduced that reproduces salient features observed in time-series of pertussis vaccine uptake and case notifications in England and Wales during the 1970s. These features include apparent oscillations in vaccine uptake in response to the naturally recurrent pattern of disease outbreaks, and a relatively slow recovery of vaccine uptake after a vaccine scare. The model also predicts that oscillations in vaccine uptake are more probable when individuals imitate others more readily (higher k), or exhibit a stronger response to increases in prevalence (higher m). Larger values of either k or m will increase the amplitude of oscillations. A higher perceived vaccine risk, rv, will also tend to induce such oscillations. This model applies to any childhood disease for which a highly efficacious vaccine is available.
Other factors in addition to those considered here may also influence vaccinating behaviour. For instance, although the disease-free, pure vaccinator equilibrium 2 is never stable in the vaccination game with imitation dynamics, in real populations, a high vaccine uptake is often observed to persist over long periods of time. This stability may be attributable to a high level of trust for medical authorities, to mandatory vaccination policies or to altruism. Individuals may also vaccinate despite low prevalence in order to reduce risks from potential future outbreaks, or on the basis of historical prevalence, although available evidence seems to suggest that short-term thinking dominates vaccinating behaviour in at least several countries (e.g. figure 1b).
The type of epidemic model used can also influence model behaviour. Factors such as stochasticity (Bartlett 1956; Rohani et al. 2002; Bauch & Earn 2003), seasonality (Schenzle 1984; Earn et al. 2000; Bauch & Earn 2003), the distribution of latent and infectious periods (Brauer 1990; Hethcote & van den Driessche 2000; Lloyd 2001) and spatial structure (Grenfell et al. 2001) can all influence epidemic dynamics. Because vaccination schedules typically distribute doses over several years, age-structured models (Keeling & Grenfell 1997) would be suitable for some policy-specific questions, particularly because parents may delay vaccination rather then avoiding it altogether (as appears to happen frequently with the present MMR vaccine scare).
In order to specifically test the assumed imitation dynamic, the empirical case notification time-series can be used instead of a model-generated prevalence time-series. Additionally, risk perception can evolve through time in ways other than what has been assumed here, altering the observed time evolution of vaccine uptake during and after a vaccine scare. Hence, it may be desirable to incorporate perceived risk as a state variable of the imitation dynamics, instead of incorporating it as a model parameter. Finally, evolutionary stable states (Hofbauer & Sigmund 1998) for games with time-varying payoffs should be more thoroughly characterized for application to vaccination games.
The epidemic modelling literature is extensive (Hethcote 2000; Brauer & van den Driessche 2003), and many epidemic models have been tested against empirical data. Likewise, game theory has been applied fruitfully in areas as diverse as economics (von Neumann & Morgenstern 1944), psychology (Colman 2003), evolution (Maynard-Smith 1982) and virology (Turner & Chao 1999). The application of game theory to vaccination policy may likewise prove beneficial to public health. However, the development of game theoretical applications is limited by the availability of suitable data. Although there are long time-series of annual case notifications for many countries, the corresponding time-series for vaccine uptake tend to be incomplete. This is changing, as more countries begin to compile and report vaccine uptake statistics (WHO 2004). In order to further advance understanding of vaccinating behaviour and vaccine scares, representative population-wide surveys detailing both quantitative and qualitative aspects of risk perception and vaccinating behaviour are necessary.
The history of vaccination suggests that high vaccine coverage cannot be taken for granted in countries with a voluntary vaccination policy. There remains a need to understand the interplay between disease prevalence, vaccine coverage and individual vaccinating behaviour. The results obtained in this paper suggest that game theory can capture this interplay, and may help in managing the population dynamics of vaccinating behaviour for the benefit of public health.
Acknowledgments
C.T.B. is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC). C.T.B. thanks Audrey Pereira and the Australian Centre for Immunisation Research for assistance with data collection; David Earn and Madhur Anand for comments on the manuscript; and Alison Galvani and Timothy Reluga for interesting discussions.
References
- Afzal M, Minor P, Schild G. Clinical safety issues of measles, mumps and rubella vaccines. Bull. WHO. 2000;78:199–204. [PMC free article] [PubMed] [Google Scholar]
- Albert M.R, Ostheimer K.G, Breman J.G. The last smallpox epidemic in Boston and the vaccination controversy, 1901–1903. N. Engl. J. Med. 2001;344:375–379. doi: 10.1056/NEJM200102013440511. [DOI] [PubMed] [Google Scholar]
- Anderson R.M, May R.M. Oxford University Press; 1991. Infectious diseases of humans. [Google Scholar]
- Andre F. Vaccinology: past achievements, present roadblocks and future promises. Vaccine. 2003;21:593–595. doi: 10.1016/s0264-410x(02)00702-8. [DOI] [PubMed] [Google Scholar]
- Asch D.A, Baron J, Hershey J.C, Kunreuther H, Meszaros J, Ritov I, Spranca M. Omission bias and pertussis vaccination. Med. Decis. Making. 1994;14:118–123. doi: 10.1177/0272989X9401400204. [DOI] [PubMed] [Google Scholar]
- Baker J. The pertussis controversy in Great Britain, 1974–1986. Vaccine. 2003;21:4003–4010. doi: 10.1016/s0264-410x(03)00302-5. [DOI] [PubMed] [Google Scholar]
- Bartlett M.S. Deterministic and stochastic models for recurrent epidemics. Proc. Third Berkeley Symp. Math. Stat. Probab. 1956;4:81–108. [Google Scholar]
- Bauch C.T, Earn D.J.D. Vaccination and the theory of games. Proc. Natl Acad. Sci. USA. 2004;101:13 391–13 394. doi: 10.1073/pnas.0403823101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauch C.T, Earn D.J.D. Transients and attractors in epidemics. Proc. R. Soc. B. 2003;270:1573–1578. doi: 10.1098/rspb.2003.2410. 10.1098/rspb.2003.2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauch C.T, Galvani A.P, Earn D.J.D. Group interest versus self interest in smallpox vaccination policy. Proc. Natl Acad. Sci. USA. 2003;100:10 564–10 567. doi: 10.1073/pnas.1731324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaby P. Communication and miscommunication of risk: understanding UK parents' attitudes to combined MMR vaccination. Br. Med. J. 2003;327:725–728. doi: 10.1136/bmj.327.7417.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biroscak B, Fiore A, Fasano N, Fineis P, Collins M, Stoltman G. Impact of the thimerosal controversy on hepatitis B vaccine coverage of infants born to women of unknown hepatitis B surface antigen status in Michigan. Pediatrics. 2003;111:e645–e649. doi: 10.1542/peds.111.6.e645. [DOI] [PubMed] [Google Scholar]
- Bjoernerstedt J, Weibull J. Nash equilibrium and evolution by imitation. In: Arrow K, Colombatto E, editors. The rational foundations of economic behaviour. MacMillan; New York: 1996. pp. 155–171. [Google Scholar]
- Bonanni P. Demographic impact of vaccination: a review. Vaccine. 1998;17(Suppl. 3):S120–S125. doi: 10.1016/s0264-410x(99)00306-0. [DOI] [PubMed] [Google Scholar]
- Brauer F. Models for the spread of universally fatal diseases. J. Math. Biol. 1990;28:451–462. doi: 10.1007/BF00178328. [DOI] [PubMed] [Google Scholar]
- Brauer F, van den Driessche P. Some directions for mathematical epidemiology. Fields Inst. Commun. 2003;36:95–112. [Google Scholar]
- Brisson M, Edmunds W. Economic evaluation of vaccination programs: the impact of herd-immunity. Med. Decis. Making. 2003;23:76–82. doi: 10.1177/0272989X02239651. [DOI] [PubMed] [Google Scholar]
- CDC. Progress toward poliomyelitis eradication—Nigeria, January 2003–March 2004. MMWR Wkly. 2004;53:343–346. [PubMed] [Google Scholar]
- Colman A. Cooperation, psychological game theory, and limitations of rationality in social interaction. Behav. Brain Sci. 2003;26:139–153. doi: 10.1017/s0140525x03000050. [DOI] [PubMed] [Google Scholar]
- Cullen J, West P. Martin Robertson; Oxford: 1979. The economics of health. An introduction. [Google Scholar]
- Dales L, Hammer S, Smith N. Time trends in autism and in MMR immunization coverage in California. J. Am. Med. Assoc. 2001;285:1183–1185. doi: 10.1001/jama.285.9.1183. [DOI] [PubMed] [Google Scholar]
- Durbach N. They might as well brand us: working-class resistance to compulsory vaccination in Victorian England. Soc. Hist. Med. 2000;13(1):45–62. doi: 10.1093/shm/13.1.45. [DOI] [PubMed] [Google Scholar]
- Earn D.J.D, Rohani P, Bolker B.M, Grenfell B.T. A simple model for complex dynamical transitions in epidemics. Science. 2000;287:667–670. doi: 10.1126/science.287.5453.667. [DOI] [PubMed] [Google Scholar]
- Evans M, Stoddart H, Condon L, Freeman E, Grizzell M, Mullen R. Parents' perspectives on the MMR immunisation: a focus group study. Br. J. Gen. Pract. 2001;51:904–910. [PMC free article] [PubMed] [Google Scholar]
- Fenner F, Henderson D, Arita I, Jezek Z, Ladnyi I. World Health Organization; Geneva: 1998. Smallpox and its eradication. [Google Scholar]
- Fine P, Clarkson J. Individual versus public priorities in the determination of optimal vaccination policies. Am. J. Epidemiol. 1986;124:1012–1020. doi: 10.1093/oxfordjournals.aje.a114471. [DOI] [PubMed] [Google Scholar]
- Gangarosa E.J, Galazka A.M, Wolfe C.R, Phillips L.M, Gangarosa R.E, Miller E, Chen R.T. Impact of anti-vaccine movements on pertussis control: the untold story. Lancet. 1998;351:356–361. doi: 10.1016/s0140-6736(97)04334-1. [DOI] [PubMed] [Google Scholar]
- Geoffard P, Philipson T. Disease eradication: private versus public vaccination. Am. Econ. Rev. 1997;87:222–230. [Google Scholar]
- Grenfell B, Bjornstad O, Kappey J. Travelling waves and spatial hierarchies in measles epidemics. Nature. 2001;414:716–723. doi: 10.1038/414716a. [DOI] [PubMed] [Google Scholar]
- Health Canada. 2004. Division of immunization and respiratory diseases, FAQ.http://www.hc-sc.gc.ca/pphb-dgspsp/dird-dimr/vs-sv [Google Scholar]
- Helbing D. A mathematical model for behavioral changes by pair interactions and its relation to game theory. In: Haag G, Mueller U, Troitzsch K, editors. Economic evolution and demographic change. Formal models in social sciences. Springer; Berlin: 1992. pp. 330–348. [Google Scholar]
- Hethcote H.W. The mathematics of infectious diseases. Soc. Ind. Appl. Math. Rev. 2000;42:599–653. [Google Scholar]
- Hethcote H.W, van den Driessche P. Two SIS epidemiologic models with delays. J. Math. Biol. 2000;40:3–26. doi: 10.1007/s002850050003. [DOI] [PubMed] [Google Scholar]
- Hofbauer J, Sigmund K. Cambridge University Press; 1998. Evolutionary games and population dynamics. [Google Scholar]
- Jansen V.A, Stollenwerk N, Jensen H.J, Ramsay M.E, Edmunds W.J, Rhodes C.J. Measles outbreaks in a population with declining vaccine uptake. Science. 2003;301:804. doi: 10.1126/science.1086726. [DOI] [PubMed] [Google Scholar]
- Keeling M.J, Grenfell B.T. Disease extinction and community size: modeling the persistence of measles. Science. 1997;275:65–67. doi: 10.1126/science.275.5296.65. [DOI] [PubMed] [Google Scholar]
- Kuznetsov Y. 1999. Content: a multiplatform environment for continuation and bifurcation analysis of dynamical systems.http://www.enm.bris.ac.uk/staff/hinke/dss/continuation/content.html [Google Scholar]
- Lashuay N, Tjoa T, de Nuncio M.L.Z, Franklin M, Elder J, Jones M. Exposure to immunization media messages among African American parents. Prev. Med. 2000;31:522–528. doi: 10.1006/pmed.2000.0745. [DOI] [PubMed] [Google Scholar]
- Lloyd A.L. Destablization of epidemic models with the inclusion of realistic distributions of infectious periods. Proc. R. Soc. B. 2001;268:985–993. doi: 10.1098/rspb.2001.1599. 10.1098/rspb.2001.1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen K, Hviid H, Vestergaard M. A population-based study of measles, mumps, and rubella vaccination and autism. N. Engl. J. Med. 2002;347:1477–1482. doi: 10.1056/NEJMoa021134. [DOI] [PubMed] [Google Scholar]
- Maynard-Smith J. Cambridge University Press; 1982. Evolution and the theory of games. [Google Scholar]
- Miller E, Gay N.J. Epidemiological determinants of pertussis. Dev. Biol. Stand. 1997;89:15–23. [PubMed] [Google Scholar]
- Nicoll A, Elliman D, Ross E. MMR vaccination and autism 1998. Br. Med. J. 1998;316:715–716. doi: 10.1136/bmj.316.7133.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin S. Lessons learned concerning vaccine safety. Vaccine. 2002;20(Suppl. 1):S16–S19. doi: 10.1016/s0264-410x(01)00303-6. [DOI] [PubMed] [Google Scholar]
- Poland G, Jacobsen R. Understanding those who do not understand: a brief review of the anti-vaccine movement. Vaccine. 2001;19:2440–2445. doi: 10.1016/s0264-410x(00)00469-2. [DOI] [PubMed] [Google Scholar]
- Roberts R.J, Sandifer Q.D, Evans M.R, Nolan-Farrell M.Z, Davis P.M. Reasons for non-uptake of measles, mumps and rubella catch up immunisation in a measles epidemic and side effects of the vaccine. Br. Med. J. 1995;310:1629–1639. doi: 10.1136/bmj.310.6995.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohani P, Keeling M.J, Grenfell B.T. The interplay between determinism and stochasticity in childhood diseases. Am. Nat. 2002;159:469–481. doi: 10.1086/339467. [DOI] [PubMed] [Google Scholar]
- Schenzle D. An age-structure model of pre- and post-vaccination measles transmission. IMA J. Math. Appl. Med. Biol. 1984;1:169–191. doi: 10.1093/imammb/1.2.169. [DOI] [PubMed] [Google Scholar]
- Schmitt H.J. Factors influencing vaccine uptake in Germany. Vaccine. 2002;20(Suppl. 1):S2–S4. doi: 10.1016/s0264-410x(01)00304-8. [DOI] [PubMed] [Google Scholar]
- Smailbegovic M.S, Laing G.J, Bedford H. Why do parents decide against immunization? The effect of health beliefs and health professionals. Child Care Health Dev. 2003;29:303–311. doi: 10.1046/j.1365-2214.2003.00347.x. [DOI] [PubMed] [Google Scholar]
- Turner P.E, Chao L. Prisoner's dilemma in an RNA virus. Nature. 1999;398:441–443. doi: 10.1038/18913. [DOI] [PubMed] [Google Scholar]
- von Neumann J, Morgenstern O. Princeton University Press; 1944. Theory of games and economic behavior. [Google Scholar]
- Weibull J. The MIT Press; Cambridge, MA: 1995. Evolutionary game theory. [Google Scholar]
- WHO. 2004. Immunization, vaccines and biologicals.http://www.who.int/vaccinessurveillance/statsandgraphs.htm [Google Scholar]


