Abstract
Linkage maps are lacking for many highly influential model organisms in evolutionary research, including all passerine birds. Consequently, their full potential as research models is severely hampered. Here, we provide a partial linkage map and give novel estimates of sex-specific recombination rates in a passerine bird, the great reed warbler (Acrocephalus arundinaceus). Linkage analysis of genotypic data at 51 autosomal microsatellites and seven markers on the Z-chromosome (one of the sex chromosomes) from an extended pedigree resulted in 12 linkage groups with 2–8 loci. A striking feature of the map was the pronounced sex-dimorphism: males had a substantially lower recombination rate than females, which resulted in a suppressed autosomal map in males (sum of linkage groups: 110.2 cM) compared to females (237.2 cM; female/male map ratio: 2.15). The sex-specific recombination rates will facilitate the building of a denser linkage map and cast light on hypotheses about sex-specific recombination rates.
Keywords: genetic map, linkage, recombination, heterochiasmy, bird, microsatellite
1. Introduction
Passerine birds are highly influential model organisms in evolutionary research. Work on this group has provided significant insights in, for example, the evolution of mating systems (Komdeur 1992), sex-ratios (Badyaev et al. 2002), inbreeding and inbreeding depression (Keller et al. 1994), life-history trade-offs (Gustafsson & Sutherland 1988), heritability and quantitative genetics (Merilä et al. 2001), natural (Richman & Price 1992) and sexual selection (Norris 1993), hybridization (Veen et al. 2001) and speciation (Irwin et al. 2001). However, the passerines' full potential as general research models is severely hampered by the fact that their genomes are not yet characterized. Recently, Derjusheva et al. (2004) used fluorescent in situ hybridization to reveal high chromosome conservation between chicken (Gallus gallus), pigeon (Columba livia) and two passerine birds, chaffinch (Fringilla coelebs) and redwing (Turdus iliacus). This suggests that the physical map of chicken (International Chicken Genome Sequencing Consortium 2004) can be used as a framework for genome organization in other bird species (Ellegren 2005). However, a physical map only reflects how genes are organized on chromosomes, whereas the actual association between genes is set by antagonistic counterplay involving selection, mutation and drift on the one hand, and recombination on the other. Recombination maps are highly desirable in evolutionary genetic research, but are not currently available for passerines. To date, in passerines, even first-generation recombination linkage maps based on moderate numbers of molecular markers, and basic evaluations of recombination rates and map sizes, are lacking.
In the present study, we provide a partial linkage map and give novel estimates of sex-dimorphic map distances in a passerine bird, the great reed warbler (Acrocephalus arundinaceus). We used this species because a two-decade long study of the breeding ecology of a Swedish great reed warbler population provided us with a unique extended pedigree in which true genetic mothers and fathers were fully resolved for all offspring and for which DNA samples were available (Hasselquist 1998; Arlt et al. 2004; Hansson et al. 2004a). Moreover, previous molecular work on the great reed warbler offered us a first set of microsatellite loci with which to study segregation (Hansson et al. 2000b, 2004a,c). In addition, we saw the potential to detect many more informative markers in the focal species by using already published microsatellite primers from the closely related Seychelles warbler (Acrocephalus sechellensis; Richardson et al. 2000) and from among the many microsatellites developed in many other passerines (e.g. Petren 1998; Saladin et al. 2003) and from primers derived from chicken sequence data (Sætre et al. 2003). Finally, we chose to study the great reed warbler because of its previous importance in various fields of molecular-based avian research, including extra-pair paternity and realized fitness, inbreeding and inbreeding depression, offspring sex-ratios and parasite–host interactions (Bensch et al. 1994; Hasselquist et al. 1996; Westerdahl et al. 2000, 2004; Hansson et al. 2004c).
In several previous mapping studies, it has been observed that the size of the linkage map differs when based on maternal and paternal meioses, respectively (e.g. Dietrich et al. 1996; Sakamoto et al. 2000; Kong et al. 2002). This is due to sex-specific recombination rates, a phenomenon termed ‘heterochiasmy’. Despite the fact that heterochiasmy was documented early in the last century (Haldane 1922; Huxley 1928), there is no consensus as to which of the several proposed hypotheses may explain its occurrence (reviewed in Lenormand 2003). In the present study, we investigate heterochiasmy when building the map by using sex-specific linkage analyses, and then discuss our findings in relation to the proposed hypotheses.
2. Material and methods
(a) Study species and pedigree
The great reed warbler (A. arundinaceus) is a large-sized reed-warbler of the family Sylviidae (Helbig & Seibold 1999). The species is a long-distance migrant that winters in Africa and breeds in reed lakes in Eurasia (Bensch 1996; Hansson et al. 2003). Great reed warblers are facultatively socially polygynous and males may form pair bonds with up to five females in a season (Catchpole et al. 1985; Hasselquist 1998). The karyotype is not yet described, but two other passerines, chaffinch and redwing, have similar numbers of macro- and microchromosomes (2n=80; Derjusheva et al. 2004) as chicken (2n=78; Masabanda et al. 2004) and other Galliformes (Japanese quail, Coturnix japonica: 2n=78, Kayang et al. 2004; turkey, Meleagris gallopavo: 2n=80, Burt et al. 2003). In birds, males are the homogametic (ZZ) and females the heterogametic sex (ZW).
In the present study, we use pedigree data and DNA from a great reed warbler population at Lake Kvismaren, southern central Sweden (59°10′N, 15°25′E), where a detailed ecological study of the whole population has been ongoing since 1983 (Bensch 1996; Hasselquist 1998; Hansson et al. 2000a). In this population, the territories of great reed warblers have been visited on a daily basis throughout the breeding season. Almost all breeding birds and unpaired territorial males have been captured in mist nets, marked with individual-specific combinations of aluminium and colour plastic rings, and blood-sampled (ca 25 μl blood were obtained from the tarsus vein). This ringing scheme allowed us to track the whole breeding population and collect data on the reproductive success of individual birds. We visited nests approximately every third day to measure clutch size, hatching success and number of fledglings. Nestlings were ringed, measured, weighed and blood-sampled nine days after hatching.
The pedigree consists of 151 broods from the years 1987 to 1998. In all of these broods, we have investigated the occurrence of extra-pair young and assigned true maternity and paternity to all extra-pair and legitimate young. This was done by using either minisatellite DNA-fingerprinting or microsatellite genotyping (Hasselquist et al. 1996; Arlt et al. 2004; Hansson et al. 2004a). In the present study, only five offspring from two broods were extra-pair young and in these cases the true genetic father was included in the pedigree. Great reed warblers are highly philopatric (Hansson et al. 2002) and several offspring entered the pedigree as parents in subsequent years. Moreover, several adults bred in more than one year and/or with more than one partner per year. In this study, we genotyped a subset of individuals for a large number of loci (table 1). In total, 812 unique individuals were included in the mapping pedigree. Eighty-three different males and 96 different females were included as parents (χ21=1.24, p>0.1). There was an even sex-ratio among the 693 nestlings (359 males and 334 females, i.e. 51.8% males; χ21=0.9, p>0.1).
Table 1.
Information on markers.
| locus | EMBL accession number | NGI | NIM | TAa | F-primer (5′–3′) | R-primer (5′–3′) | LG | reference |
|---|---|---|---|---|---|---|---|---|
| Aar1 | AF234985 | 764 | 953 | 62 | TGAGGAGCAGCTGGGAAG | GCAGCAGGAGCTCAGGAG | Z | Hansson et al. (2000b) |
| Aar2 | AF234986 | 796 | 109 | 60 | TGTCTGCAGCAGCTCTGG | GACCTGAAGCGTTTCCGTAA | — | Hansson et al. (2000b) |
| Aar3 | AF234987 | 772 | 868 | 54 | GCATCTGGTCTCCGATTGTT | TTTGGGTTACATCTGAGTGTGC | 7 | Hansson et al. (2000b) |
| Aar4 | AF234988 | 791 | 684 | 53 | GATGACTAAGGTCTCTGGTGTG | GTTTGTGCATCAATTAGTCATG | 6 | Hansson et al. (2000b) |
| (Mcyμ4) | (U82388) | (ATAAGATGACTAAGGTCTCTGGTG) | (TAGCAATTGTCTATCATGGTTTG) | Double et al. (1997) | ||||
| Aar5 | no sequence | 792 | 1126 | 56 | GAGCTCTGTATGTGCGTG | TCTGAGTGGACTCAGGAGT | — | Hansson et al. (2000b) |
| (G7) | (DQ115906) | (GAGCTCTGTATGTGCGTGCG) | (CTGAGTGGACTCAGGAGTGC) | I. Nishiumi (unpublished data) | ||||
| Aar8 | AF234991 | 758 | 700 | 56 | TAGTGATGCCCTGCTAGGTA | AAGTGCTCCTTAATATTTGGCA | 5 | Hansson et al. (2000b) |
| (Escμ6) | (X77082) | (CATAGTGATGCCCTGCTAGG) | (GCAAGTGCTCCTTAATATTTGG) | Hanotte et al. (1994) | ||||
| Ase7 | AJ287390 | 769 | 732 | 60 | AATCAACTTCAAATGCTCACAG | ACTACATGACTCCAGGCTCAG | 1 | Richardson et al. (2000) |
| Ase8rd | no sequence | 746 | 689 | 55 | TACCTCTCCTTGGCTGAG | CCAGCCCTAGCTGTTTCA | 5 | This study |
| (Ase8) | (AJ287391) | (TACCTCTCCTTGGCTGAGCA) | (CCAGCCCTAGCTGTTTCACC) | Richardson et al. (2000) | ||||
| Ase9 | AJ287392 | 779 | 924 | 60 | GACTGAAGTCCTTTCTGGCTTC | CACCAGGAATACAAGTCCATTG | 1 | Richardson et al. (2000) |
| Ase10 | AJ287393 | 769 | 701 | TD | CATTGGGGTACTATGGAAAGACC | TCCTGAGTGGAAGGAACATAGG | 3 | Richardson et al. (2000) |
| Ase11 | AJ287394 | 785 | 1196 | 60 | TCCCCAAATCTCTCAATTCC | AGTTCTAAGCCTGCCTGTGC | — | Richardson et al. (2000) |
| Ase12 | AJ287395 | 755 | 366 | 60 | TCAAGGAAACACAACTACAGCC | TTTCCTCACAGCCTTGACTG | 11 | Richardson et al. (2000) |
| Ase15 | AJ287398 | 796 | 218 | 55 | CTGAACCCAAACATAAGCACAC | GCTCCAAACACGCCAGAG | 7 | D. S. Richardson et al. (unpublished data), see Hansson et al. (2004c) and http://www.shef.ac.uk/misc/groups/molecol/deborah-dawson-birdmarkers.html |
| Ase18 | AJ276375 | 793 | 359 | 60 | ATCCAGTCTTCGCAAAAGCC | TGCCCCAGAGGGAAGAAG | 1 | Richardson et al. (2000) |
| Ase21 | AJ276378 | 745 | 759 | 58 | TTAGAACCATTTGATAGTTGCCAC | ATGGGTTTCTTGGGGAAGAG | 6 | Richardson et al. (2000) |
| Ase27 | AJ276384 | 419 | 165 | 60 | TTAACATTGCATGCTCCTGC | AGTCAAGGTACAGGCTAGATAGCC | — | Richardson et al. (2000) |
| Ase32 | AJ276635 | 661 | 747 | 58 | AATGAGCAATACCATGACAGC | GATCTTTCAGTCAGGAACAAGC | 3 | Richardson et al. (2000) |
| Ase34 | AJ276636 | 774 | 649 | 60 | GTTATTCTTTTGGCCCTCAGC | GGAGACACCACACCAATGC | 8 | Richardson et al. (2000) |
| Ase38rd | no sequence | 750 | 682 | 55 | ATCCGAGAACCCAATCAC | GCAGCATTACAGTCTCAAAGA | 5 | This study |
| (Ase38) | (AJ276640) | (ATCCGAGAACCCAATCACTT) | (GCAGCATTACAGTCTCAAAGAAC) | Richardson et al. (2000) | ||||
| Ase42 | AJ276644 | 777 | 1180 | 60 | CATGGGTAGGTTGGGATGTC | AGGTGAGGGTATGCAAACATG | 9 | Richardson et al. (2000) |
| Ase44 | AJ276646 | 772 | 762 | 60 | TTCCCGTAATTATGACCTCTCTTG | ACCAGAACTTGTTGTCTGGGAG | 3 | Richardson et al. (2000) |
| Ase48 | AJ276777 | 482 | 241 | 58 | TTTATTTCCTGGACTGGAACAATC | GAACATTGGGCTACTGGGC | 6 | Richardson et al. (2000) |
| Ase50 | AJ276779 | 803 | 780 | 60 | CTGTGGAATGCTGTCTGGC | ATGGACTCCCGTCTAACTTGC | Z | Richardson et al. (2000) |
| Ase51 | AJ276780 | 747 | 925 | 60 | AATTCCCCTAGACAGGCAGC | TCACTGGAGAGCCAAATTCC | 1 | Richardson et al. (2000) |
| Ase53rd | no sequence | 481 | 186 | 53 | ATGGAGAATTCTGGGTGC | CCCAATAATGAGGTAACACC | 4 | This study |
| (Ase53) | (AJ276782) | (ATGGAGAATTCTGGGTGCTG) | (CCCAATAATGAGGTAACACCAA) | Richardson et al. (2000) | ||||
| Ase55 | AJ276784 | 729 | 292 | 60 | GTGTGGACTCTGGTGGCTC | TCCCAAAGCACTCAAACTAGG | 1 | Richardson et al. (2000) |
| Ase56 | AJ276785 | 746 | 343 | 60 | TTCACTGAGAAGTGAGAATGTG | GTCCTTGATTGATTACAGGCT | 5 | Richardson et al. (2000) |
| Ase57rd | no sequence | 776 | 1090 | 50 | GCAAGTGCAGATGTTTCC | CCAAAGCAGGACAATGC | — | This study |
| (Ase57) | (AJ276786) | (GCAAGTGCAGATGTTTCCCT) | (CCAAAGCAGGACAATGCTG) | Richardson et al. (2000) | ||||
| Ase58 | AJ276787 | 772 | 1046 | 60 | ATTCCAGGGATTGGGCAG | CTCAAAGCGAAATTGAGCAGT | 8 | Richardson et al. (2000) |
| Ase60 | AJ276789 | 775 | 1180 | 55 | CATGAAAAGGAACTCTCCAGC | TTCCATCTCTGTTCTACTGCG | 1 | Richardson et al. (2000) |
| Ase61 | AJ276790 | 459 | 191 | 54 | AGGATTTTTAATGGGATATACACATCTG | AGCCACATTTTAGCCCACAG | 7 | Richardson et al. (2000) |
| Ase63 | AJ276792 | 717 | 513 | 60 | TTTGGGGTTTAGGAATAGCAGA | GGCTTCAGCCTGAGAAAGTC | 5 | Richardson et al. (2000) |
| Ase64 | AJ276793 | 766 | 1152 | TD | CCACCTTTCATACTGGGGAG | TTCAGCCAGTCAGTGTAGCC | 10 | Richardson et al. (2000) |
| BRM12rdAb | DQ073912 | 784b | 1019b | 55 | CAACACACTGGCAAAACCAG | TGCACACCTGACAAAAGAGTG | Z | This study |
| (BRM12) | (X91638) | (CCCTATCTCATCATTGTTCC) | (CACAGAAGGAGCCCATTTGT) | Sætre et al. (2003) and G.-P. Sætre et al. (unpublished data) | ||||
| BRM12rdBb | DQ073912 | 783b | 722b | 52 | ATGCGTTTTCCTTTCTCCAA | AAAAATAACAACACAACTCCTCAA | Z | This study |
| (BRM12) | (X91638) | (CCCTATCTCATCATTGTTCC) | (CACAGAAGGAGCCCATTTGT) | Sætre et al. (2003) and G.-P. Sætre et al. (unpublished data) | ||||
| BRM15rd | DQ073913 | 788 | 1143 | 55 | AGCACCTTTGAACAGTGGTT | AAATCCCTAAGTACCAGTGCAAA | Z | This study |
| (BRM15) | (X91638) | (AGCACCTTTGAACAGTGGTT) | (TACTTTATGGAGACGACGGA) | Sætre et al. (2003) and G.-P. Sætre et al. (unpublished data) | ||||
| Cdi38 | AB089175 | 765 | 895 | 54 | ACATCTTCGGCACGGCT | GAGCTGGAAGTGGTGGA | 2 | Otsuka et al. (2003) |
| CHDZ20rd | DQ073914 | 782 | 803 | 52 | GCCCCAAAGTAAACTTGGAA | TGATTAGCAGGAGAGCCATTT | Z | This study |
| (CHDZ20) | (AF004397) | (GAAGAGAGCTGAAACTCGG) | (TCATCTTCATCCATATTGG) | Sætre et al. (2003) and G.-P. Sætre et al. (unpublished data) | ||||
| Cuμ02 | AF122890 | 262 | 211 | 60 | CCTTGGATTGCTTCCAAATG | CCAATTTCCTGCAGACTCTTTC | 4 | Gibbs et al. (1999) |
| Cuμ04 | AF122891 | 744 | 216 | 55 | AATTGCATAAATGTGATCCAC | AAATGAAATGTGGTAGAATTCC | 6 | Gibbs et al. (1999) |
| Cuμ28 | AF122894 | 755 | 329 | 60 | GAGGCACAGAAATGTGAATT | TAAGTAGAAGGACTTGATGGCT | 12 | Gibbs et al. (1999) |
| G61 | no sequence | 797 | 1172 | 56 | GAGCAGAAGCTACAGAAATC | GCAAAGTCTGATTGTAAGCAG | Z | Nishiumi et al. (1996) |
| Gf08 | AF081932 | 744 | 417 | 62 | TGGGAGAGCAAGGTGGGAACAG | TGGAGTGGTGATTAAACCAGCAGG | — | Petren (1998) |
| Gf15 | AF081939 | 702 | 460 | 62 | CTCCACCTCCCACTAACTGCTACC | CAACACCTGGAGTGGAAGTGCC | 3 | Petren (1998) |
| HrU5 | X84090 | 641 | 116 | 45 | TCAACAAGTGTCATTAGGTTC | AACTTAGATAAGGAAGGTATAT | 5 | Primmer et al. (1995) |
| LOX1 | Y16820 | 719 | 639 | 54 | ATGATGGTAAGTCTAATGAAAGC | CCACACACATTCACTCTATTG | 3 | Piertney et al. (1998) |
| MSLP2 | AB031374 | 747 | 297 | 50 | TAACTACAGCCAGTTAGAAG | TGAAGTTACTGGTAGCCTTTG | 6 | Ishibashi et al. (2000) |
| PAT MP 2–43 | no sequence | 749 | 321 | 60 | ACAGGTAGTCAGAAATGGAAAG | GTATCCAGAGTCTTTGCTGATG | 3 | Otter et al. (1998) |
| Pdoμ4 | X93505 | 332 | 297 | 54 | CGATAAGCTTGGATCAGGACTAC | CTTGGGAAGAGAATGAGTCAGGA | 3 | Neumann & Wetton (1996) |
| Pdoμ6 | Y15125 | 220 | 99 | 59 | CTGATCATGTGTAGATGTAAGACTGC | CAGATCCTTAAGCAGGAAGTTAGG | 5 | Griffith et al. (1999) |
| PmaTGAn42 | AY260540 | 747 | 1127 | 57 | ACTTCCACATGCCAGTTTCC | TGTTAAGGCAGAGAGGTGGG | 3 | Saladin et al. (2003) |
| Ppi2 | AJ272375 | 789 | 1122 | 53 | CACAGACCATTCGAAGCAGA | GCTCCGATGGTGAATGAAGT | 10 | Martinez et al. (1999) |
| Sjr4 | no sequence | 479 | 642 | 54 | TCCAGGCTGTGCTTGCACTTG | TGCCAGACCACCAACTAAATC | — | D. B. McDonald and W. K. Potts (unpublished data) |
| VLDLR9rd | DQ073915 | 783 | 1015 | 52 | TCTGTTACCAAGTGTGGAATGG | TCGGTTGGTGAAAATCAGAC | Z | This study |
| TGTTACCAAGTGTGGAATGC | ||||||||
| (VLDLR9) | (X80207) | (AAGTGTGAATGTAGCCGTGG) | (TCGGTTGGTGAAAATCAGAC) | Sætre et al. (2003) and G.-P. Sætre et al. (unpublished data) | ||||
| WBSW7 | AF130434 | 751 | 266 | 54 | TCTGGAGTTCTGGGACCTGT | CTCACTCAACAGCAGGACCA | 6 | McRae & Amos (1999) |
| ZL18 | AF076668 | 721 | 810 | 58 | CTGCAGAGGAGCTCAGGTAAC | CTCGGTGCTGCCAGAACTCAG | — | Degnan et al. (1999) |
| ZL44 | AJ517996 | 698 | 460 | 53 | CTGTCCCTGCCCTCTCATC | ACCATGGCAGAGGCACCAA | 9 | Frentiu et al. (2003) |
| ZL45 | AJ517997 | 756 | 74 | 58 | CCGGAGCACCACGCACAGC | GTTTGGGTCCAAGCGCCTCGAG | 9 | Frentiu et al. (2003) |
| ZL54 | AJ518005 | 681 | 486 | 58 | CACGACTTCTCAAGCAGAC | GAGCCTTGCACAAACGGAC | 2 | Frentiu et al. (2003) |
All loci are microsatellites with the exceptions of three indels (BRM12, BRM15 and CHDZ20) and one SNP (VLDLR9; see §2b). Number of genotyped individuals (NGI), number of informative meioses (NIM), annealing temperature used in PCR (TA), primer sequences, linkage group (LG) and reference are given for each locus. In cases where re-designed primers were used, the original locus name and primer sequences are given in parenthesis.
TD, Touch down PCR with TA continuously falling from 60° to 50°.
BRM12rdA and BRM12rdB score two indels at the same locus and were evaluated simultaneously; NGI=786, NIM=1053.
(b) Molecular markers and genotyping
We isolated DNA from blood samples with phenol/chloroform-isoamylalcohol extraction (Bensch et al. 1994).
We have previously screened 3–8 unrelated great reed warblers for polymorphism at 130 passerine microsatellite markers, including five which were specifically developed from great reed warbler microsatellite clones (Hansson et al. 2000b, 2004b,c; Richardson et al. 2000), and specifically for this study we screened an additional 88 microsatellites. This effort resulted in 51 polymorphic autosomal microsatellites and three polymorphic microsatellites on the Z-chromosome (table 1). Some of these markers had segregating non-amplifying alleles (‘null alleles’); we tried to eliminate these problematic alleles by re-designing the primers (see table 1). This was done by excluding two bases in the 3′-end of the original primers because we suspected that the null alleles resulted from mismatches between template and primers at these sites. To increase the number of markers on the Z-chromosome, we tested 20 primer pairs derived from sequence data of Z-linked chicken genes (Sætre et al. 2003). We sequenced the PCR-products of four unrelated great reed warblers at loci that yielded a single product of expected length (DYEnamics sequence kit, QIAGEN). This survey yielded three loci with length polymorphism (BRM12, BRM15, CHDZ20; table 1) and one locus with single-nucleotide polymorphism (‘SNP’; locus VLDLR9; table 1). In total, we ended up with 51 autosomal and seven Z-linked markers (table 1).
Alleles were amplified in standard PCRs following Hansson et al. (2000b) and Richardson et al. (2000). The PCR-mix contained 4 pmol of each primer, 1×NH4-buffer, 15 nmol MgCl2, 2 nmol dNTP, 0.5 U BIOTAQ DNA-polymerase (Bioline) and 1–5 ng template in 10 μl reaction volume. PCR-condition was as follows: 94 °C for 2 min, then 35 cycles at 94 °C for 30 s/TA for 30 s/72 °C for 30 s, followed by 72 °C for 10 min; where TA is the locus-specific annealing temperature (table 1). The SNP (locus VLDLR9) was scored by using two allele-specific forward primers (2 pmol of each primer in PCR) and one reverse primer (4 pmol in PCR), where the 3′-ends of the forward primers were made to match each of the SNP-alleles and the 5′-ends differed in length by two bases (table 1), which resulted in a designed length polymorphism (Hansson & Kawabe 2005). One primer in each primer combination was labelled with fluorescent dye and the PCR-products were separated and the alleles detected in an ABI 3730 capillary sequencer (Applied Biosystems). When possible, several loci per individual were run simultaneously in the sequencer, and each run included a size ladder (ABI GeneScan LIZ500; Applied Biosystems). Genotypes were read in Genemapper v. 3.0 (Applied Biosystems).
We genotyped 50.4±8.7 s.d. loci per individual, and 706.1±136.1 s.d. individuals per locus (table 1). The number of informative meioses (which increases with number of genotyped individuals and with increasing genetic variability) ranged from 75 at locus ZL45 to 1196 at locus Ase11 (table 1).
(c) Linkage analyses
The segregation of alleles in the pedigree was evaluated (detection of genotype and scoring errors, and null alleles) by eye and by running the data in PEDCHECK v. 1.1 (http://hpcio.cit.nih.gov/lserver/PEDCHECK.html; O'Connell & Weeks 1998). All scoring errors were corrected and new PCRs were run in some cases. As stated above, we re-designed some primers to avoid null alleles. At some loci, null alleles could not be completely eliminated, and we only included segregation that could be easily interpreted in these cases.
The linkage map was constructed in CRIMAP v. 2.4 (http://biobase.dk/Embnetut/Crimap/analyse1.html; Lander & Green 1987). This program calculates two-point recombination fractions, provides logarithmic odds (LOD) scores for recombination estimates, and tests marker order. In line with previous mapping studies (e.g. Kayang et al. 2004; Samollow et al. 2004), we assigned markers to linkage groups at a threshold of LOD>3.0. Autosomal and Z-linked loci were evaluated separately. For the autosomal loci, we first calculated the recombination fractions between all pairs of markers with the TWOPOINT option in CRIMAP, and then determined the most parsimonious ordering of significantly linked loci (i.e. groups of pair-wise markers with LOD>3.0) with the options FLIPSN and FIXED. The analyses revealed pronounced heterochiasmy, and all our results are from sex-specific analyses. One of the loci on the Z-chromosome (G61) was not linked at LOD>3.0 to any other locus, but when we had determined the most parsimonious ordering of the other six Z-linked loci, we could use the combined dataset and the FIXED option in CRIMAP to determine the relative position of this locus. All map distances are given in Kosambi centiMorgans (cM).
3. Results
Forty-three of the 51 autosomal loci (84.3%) scored LOD>3.0 to at least one other locus. There were 86 pairs with LOD>3.0 and among these pairs LOD scores ranged from 4.3 (Ase18 versus Ase55) to 201.6 (Ase64 versus Ppi2). The 43 linked autosomal loci were placed on the map (figure 1a). The number of informative meioses was not associated with probability that a marker either scored LOD>3.0 or LOD≤3.0 with another marker (logistic regression: χ21=0.02, p=0.89), which suggests that most apparently unlinked markers were indeed located on unique chromosomes or chromosome arms.
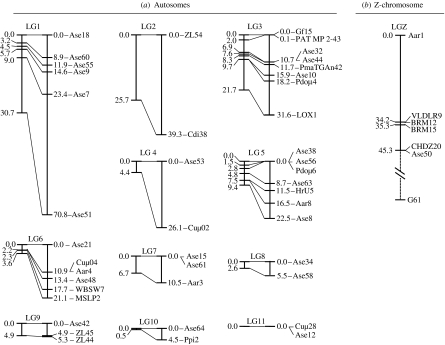
Figure 1.
(a) Male- and female-specific autosomal recombination linkage map of great reed warbler (based on 43 microsatellite markers). Male maps are to the left in each linkage group. (b) Male-specific Z-chromosome linkage group (based on three microsatellite and four chicken-derived markers; see table 1). Note that the location of G61 is preliminary (see §3). Locus positions are given by their distance in cM.
There was pronounced heterochiasmy. Between pairs of loci with LOD>3.0, the recombination rate in females (0.101±0.099 s.d.) was almost twice that of males (0.052±0.066 s.d.). These loci constituted 11 autosomal linkage groups with 2–8 loci, with an average size of 10.0 (±10.8 s.d.) cM in males and 21.6 (±20.6 s.d.) cM in females (paired t-test: t=3.2, d.f.=10, p=0.009). In total, the autosomal linkage map was much smaller in males (110.2 cM) compared to females (237.2 cM; figure 1a), with a female-to-male map ratio of 2.15.
Among the Z-linked markers, six out of seven (i.e. all loci excluding G61) scored LOD>3.0 to the other loci (ranging between LOD 3.6 for Aar1 versus BRM15 and 67.1 for BRM12 versus BRM15). This linkage group spanned 45.3 cM (figure 1b). When the combined information from the other six loci was used, there was significant support for locating G61 together with Ase50 and CHDZ20 rather than together with Aar1 at the other end of the chromosome (ΔLOD=3.57; figure 1b).
4. Discussion
In the present study, we have constructed a partial linkage map in a passerine bird, the great reed warbler. Our analyses revealed a pronounced heterochiasmy in the species: males had substantially lower meiotic recombination rate than females. This resulted in a reduced male autosomal linkage map compared to that of the females, and a female-to-male map length ratio of 2.15.
Heterochiasmy has previously been documented in several animals and plants, and the phenomenon was first described by Haldane (1922) and Huxley (1928). They hypothesized that in species where the sexes differed much in recombination rate, it is usually the heterogametic sex that has suppressed recombination (the ‘Haldane–Huxley rule’). In support of this rule, reduced recombination rate in the heterogametic sex has been found in several mammals, with a female-to-male map ratio of 1.56–1.65 in human (Broman et al. 1998; Kong et al. 2002), 1.36–1.41 in dog (Canis familiaris; Mellersh et al. 1997; Neff et al. 1999), 1.30–1.55 in pig (Sus scrofa; Archibald et al. 1995) and 1.25 in mouse (Mus musculus; Dietrich et al. 1996). In fish, heterochiasmy seems to be particularly strong, where, for example, rainbow trout (Oncorhynchus mykiss) has a female-to-male map ratio of 3.25 (Sakamoto et al. 2000).
In chicken, Japanese quail and turkey, which to our knowledge are the only other bird species with available maps, the sexes have almost identical map distances (Groenen et al. 2000; Burt et al. 2003; Kayang et al. 2004). Thus, the highly sex-dimorphic linkage map in great reed warblers contrasts strongly with that found in chicken and other Galliformes. Moreover, in birds females are the heterogametic sex and therefore the sex-bias observed in great reed warblers—that females have higher recombination rate than males—opposes the Haldane–Huxley rule. Stauss et al. (2003) studied recombination between three allozyme loci in a single linkage group in two other passerines, great tit (Parus major) and blue tit (P. caeruleus). Although, genome-wide recombination rates should not be inferred from a single linkage group (Lynn et al. 2000; Sakamoto et al. 2000), their data indicate that heterochiasmy occurs in other passerines: the female-to-male recombination ratio was 1.91 in great tits and 0.44–0.56 in blue tits (Stauss et al. 2003). Thus, the great tit may be another exception to the Haldane–Huxley rule among birds. Other firmly established exceptions are found in marsupials, insects and plants (Lenormand 2003; Samollow et al. 2004). In the short-tailed opossum (Monodelphis domestica), for instance, males are the heterogametic sex but females have suppressed recombination (female/male map ratio: 0.50; Samollow et al. 2004).
Consequently, there is no clear-cut association between heterogamety and heterochiasmy, and other (not necessarily exclusive) hypotheses have to be explored to explain the phenomenon of sex-dimorphic map distances. Several other hypotheses exist (reviewed in Lenormand 2003; Samollow et al. 2004). For example, Samollow et al. (2004) suggested that systematic differences between placental mammals and marsupials in the X-chromosome inactivation process may explain the opposing female-to-male map ratios of these groups of animals. In marsupials (females have suppressed recombination), it is always the paternally inherited X-chromosome that is inactivated in females, whereas in placentals some genes on the paternal and some genes on the maternal X-chromosome are inactivated. If this scenario were to explain the pattern found in the present study (suppressed recombination in males), the maternally inherited Z-chromosome should be inactivated in male offspring. However, work on chicken, spotted turtle-dove (Streptopelia chinensis) and house sparrow (Passer domesticus) suggests that neither of the Z-chromosomes are inactivated (Baverstock et al. 1982; Kuroda et al. 2001; but see McQueen et al. 2001), and although further research on other species is necessary to confirm that this is a general pattern in birds, the current data in birds fail to support this hypothesis for heterochiasmy.
Another group of hypotheses for heterochiasmy is based on selection. It is well known that recombination may evolve to optimize the allelic associations between loci as an epistatic response to natural selection—a process that will shape the sex-average rate of recombination (reviewed in Otto & Lenormand 2002; Rice 2002). In line with this theory, selection may also cause the sexes to diverge in recombination rate. Trivers (1988) proposed that sexual selection may cause heterochiasmy. He suggested that genes and combinations of genes that pass through the sex with highest variance in reproductive success would be superior on average and thus a reduced rate of recombination will be selected for in this sex. Lenormand (2003) suggested that heterochiasmy can result when epistasis varies with sex during the haploid phase or between chromosomes inherited from the father and the mother during the diploid phase (‘sex-of-origin effect’). This scenario requires that genes are expressed during the haploid phase, which is common in plants but uncommon in animals (Christians et al. 1999; Xu et al. 1999), or a mechanism such as imprinting that produces the sex-of-origin effect in diploids, but imprinting probably only affects a very small proportion of the genome (ca 0.1% of the genes are imprinted in mammals; Burns et al. 2001). In polygynous species such as the great reed warbler, males are likely to have higher variance in reproduction success than females. Thus, Trivers' (1988) ideas about heterochiasmy and sexual selection may fit our observation. However, Lenormand's (2003) suggestion may also be correct; more recombination in females could result from the fact that the haploid phase is shorter in females than in males and that there is little scope for selection to reduce recombination in females.
It has also been suggested that heterochiasmy may occur for mechanistic reasons due to sex-differences in the internal or external environment. For instance, recombination rates may be affected by the metabolic rate or ambient temperature (Bernstein et al. 1988; Plomion & O'Malley 1996). However, it is difficult with this hypothesis to explain heterochiasmy in hermaphrodites where both male and female meiosis may occur simultaneously, and in plants where timing of meiosis is similar in the two sexes. Still, the hypothesis may apply in species with such sex-differences, but relevant data and predictions are lacking for passerines. Clearly, more data on, for example, timing of meiosis in males and females, haploid gene-expression and imprinting are needed to understand the occurrence of heterochiasmy in great reed warblers and other organisms.
To our surprise a very high proportion of the autosomal markers tested (84.3%) were significantly linked to at least one other marker. This could partly stem from the fact that we genotyped many individuals and therefore achieved high statistical power. Our result could also be explained in terms of non-random distribution of microsatellites in the genome. If our markers were predominantly located in areas with low recombination rate, they would be more probably associated with other markers in this type of linkage analysis. In chicken, there is an even distribution of microsatellites over macrochromosomes and intermediate-sized chromosomes, whereas the microchromosomes, large parts of the sex chromosomes, and most centromeres and telomeres have very low densities of microsatellites (Primmer et al. 1997). Microchromosomes constitute only a moderate part of the genome and the general scarcity of microsatellites on these chromosomes is not sufficient to explain why we ended up with a very high proportion of linked markers. Neither can very low densities of microsatellites near centromeres explain our finding. The recombination rate is reduced around centromeres (Hulten 1974; Lynn et al. 2000), so a low density of markers in this region would have reduced the chances of finding linked markers. Instead, comparative data in great reed warblers and chicken suggest another explanation as to why many of our markers were significantly linked, namely that the recombination rate is relatively low in great reed warblers. Based on data for orthologous loci on seven chromosomes (Dawson et al. 2006), we have provisionally estimated that the recombination rates in male and female great reed warblers were only ca 17% and 32% that of the chicken, respectively (B. Hansson, D. A. Dawson, M. Åkesson, T. Burke, J. M. Pemberton and J. Slate, unpublished data).
We have used markers that we know are polymorphic in many other passerines. Several of the microsatellites developed in the Seychelles warbler are polymorphic in other Acrocephalus spp. (Richardson et al. 2000), and many other markers are frequently used in parental analyses of a vide variety of passerines (e.g. Ppi2, Aar4/Mcyμ4, Aar8/Escμ6; Hanotte et al. 1994; Double et al. 1997; Martinez et al. 1999; see also http://www.shef.ac.uk/misc/groups/molecol/deborah-dawson-birdmarkers.html). These markers can be used as framework loci to link future passerine maps and enable comparative work on passerine genomes. The pronounced heterochiasmy has practical implications for experimental design: it will facilitate the building of a high-resolution map in great reed warblers—work that we have already initiated by genotyping individuals in the pedigree with the AFLP-technique (M. Åkesson, unpublished data). Potentially, our and other future passerine linkage maps will cast light on important issues in evolutionary research (Ellegren 2005), including hypotheses of heterozygosity-fitness correlations (Hansson et al. 2004c), causes of heterochiasmy (Lenormand 2003), distribution of quantitative trait loci (Flint & Mott 2001; Slate et al. 2002) and avian genome organization (Hurst et al. 2004).
Acknowledgments
We thank all field workers at Lake Kvismaren, especially S. Bensch, D. Hasselquist, and B. Nielsen for their long-term efforts. Also, we are grateful to T. Burke, D. Richardson, G.-P. Sætre and T. Borge for sharing previously unpublished primers; to D. A. Dawson for primer and microsatellite sequence information, data for orthologous loci in great reed warblers and chicken and the bird marker database at http://www.shef.ac.uk/misc/groups/molecol/deborah-dawson-birdmarkers.html; to T. Thomasson for efficient genotyping; to D. Beraldi for support with PEDCHECK and CRIMAP runs; and to S. Bensch and D. Hasselquist for initiating the great reed warbler project and supporting all steps of this work. The manuscript was greatly improved by the comments of two anonymous reviewers. The work was supported by a post-doctoral grant from the Swedish Research Council and STINT (to BH), by a Marie-Curie post-doctoral fellowship (to BH/JMP), the National Environmental Research Council (to L. E. B. Kruuk), Lunds Djurskyddsfond (to BH), Berggrens Stiftelse (to BH), Schwartz Stiftelse (to BH), and Kvismare Bird Observatory (report no. 138).
References
- Archibald A.L, et al. The pigmap consortium linkage map of the pig (Sus scrofa) Mamm. Genome. 1995;6:157–175. doi: 10.1007/BF00293008. doi:10.1007/BF00293008 [DOI] [PubMed] [Google Scholar]
- Arlt D, Hansson B, Bensch S, von Schantz T, Hasselquist D. Breeding synchrony does not affect extra-pair paternity in great reed warblers. Behaviour. 2004;141:863–880. doi:10.1163/1568539042265699 [Google Scholar]
- Badyaev A.V, Hill G.E, Beck M.L, Dervan A.A, Duckworth R.A, McGraw K.J, Nolan P.M, Whittingham L.A. Sex-biased hatching order and adaptive population divergence in a passerine bird. Science. 2002;295:316–318. doi: 10.1126/science.1066651. doi:10.1126/science.1066651 [DOI] [PubMed] [Google Scholar]
- Baverstock P.R, Adams M, Polkinghorne R.W, Gelder M. A sex-linked enzyme in birds—Z-chromosome conservation but no dosage compensation. Nature. 1982;296:763–766. doi: 10.1038/296763a0. doi:10.1038/296763a0 [DOI] [PubMed] [Google Scholar]
- Bensch S. Female mating status and reproductive success in the great reed warbler: is there a potential cost of polygyny that requires compensation? J. Anim. Ecol. 1996;65:283–296. [Google Scholar]
- Bensch S, Hasselquist D, von Schantz T. Genetic similarity between parents predicts hatching failure: nonincestuous inbreeding in the great reed warbler. Evolution. 1994;48:317–326. doi: 10.1111/j.1558-5646.1994.tb01314.x. [DOI] [PubMed] [Google Scholar]
- Bernstein H, Hopf F, Michod R. Is meiotic recombination and adaptation for repairing DNA, producing genetic variability, or both? In: Michod R, Levin B, editors. The evolution of sex. Sinauer; Sunderland, MA: 1988. pp. 139–160. [Google Scholar]
- Broman K.W, Murray J.C, Sheffield V.C, White R.L, Weber J.L. Comprehensive human genetic maps: Individual and sex-specific variation in recombination. Am. J. Hum. Genet. 1998;63:861–869. doi: 10.1086/302011. doi:10.1086/302011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J.L, Jackson D.A, Hassan A.B. A view through the clouds of imprinting. FASEB J. 2001;15:1694–1703. doi: 10.1096/fj.010085rev. doi:10.1096/fj.010085rev [DOI] [PubMed] [Google Scholar]
- Burt D.W, Morrice D.R, Sewalem A, Smith J, Paton I.R, Smith E.J, Bentley J, Hocking P.M. Preliminary linkage map of the Turkey (Meleagris gallopavo) based on microsatellite markers. Anim. Genet. 2003;34:399–409. doi: 10.1046/j.1365-2052.2003.01033.x. doi:10.1046/j.1365-2052.2003.01033.x [DOI] [PubMed] [Google Scholar]
- Catchpole C.K, Leisler B, Winkler H. Polygyny in the great reed warbler, Acrocephalus arundinaceus: a possible case of deception. Behav. Ecol. Sociobiol. 1985;16:285–291. doi:10.1007/BF00310992 [Google Scholar]
- Christians E, Boiani M, Garagna S, Dessy C, Redi C.A, Renard J.P, Zuccotti M. Gene expression and chromatin organization during mouse oocyte growth. Dev. Biol. 1999;207:76–85. doi: 10.1006/dbio.1998.9157. doi:10.1006/dbio.1998.9157 [DOI] [PubMed] [Google Scholar]
- Dawson D.A, Burke T, Hansson B, Pandhal J, Hale M.C, Hinten G.H, Slate J. A predicted microsatellite map of the passerine genome based on chicken–passerine sequence similarity. Mol. Ecol. 2006 doi: 10.1111/j.1365-294X.2006.02803.x. (In press.) [DOI] [PubMed] [Google Scholar]
- Degnan S.M, Robertson B.C, Clegg S.B, Moritz C.C. Microsatellite primers for studies of gene flow and mating systems in white-eyes (Zosterops) Mol. Ecol. 1999;8:159–160. doi: 10.1046/j.1365-294x.1999.00799.x. doi:10.1046/j.1365-294X.1999.00799.x [DOI] [PubMed] [Google Scholar]
- Derjusheva S, Kurganova A, Habermann F, Gaginskaya E. High chromosome conservation detected by comparative chromosome painting in chicken, pigeon and passerine birds. Chromosome Res. 2004;12:715–723. doi: 10.1023/B:CHRO.0000045779.50641.00. doi:10.1023/B:CHRO.0000045779.50641.00 [DOI] [PubMed] [Google Scholar]
- Dietrich W.F, et al. A comprehensive genetic map of the mouse genome. Nature. 1996;380:149–152. doi: 10.1038/380149a0. doi:10.1038/380149a0 [DOI] [PubMed] [Google Scholar]
- Double M.C, Dawson D, Burke T, Cockburn A. Finding the fathers in the least faithful bird: a microsatellite-based genotyping system for the superb fairy-wren Malurus cyaneus. Mol. Ecol. 1997;6:691–693. doi:10.1046/j.1365-294X.1997.00228.x [Google Scholar]
- Ellegren H. The avian genome uncovered. Trends Ecol. Evol. 2005;20:180–186. doi: 10.1016/j.tree.2005.01.015. doi:10.1016/j.tree.2005.01.015 [DOI] [PubMed] [Google Scholar]
- Flint J, Mott R. Finding the molecular basis of quantitative traits: successes and pitfalls. Nat. Rev. Genet. 2001;2:437–445. doi: 10.1038/35076585. doi:10.1038/35076585 [DOI] [PubMed] [Google Scholar]
- Frentiu F.D, Lange C.L, Burke T, Owens I.P.F. Isolation of microsatellite loci in the Capricorn silvereye, Zosterops lateralis chlorocephalus (Aves: Zosteropidae) Mol. Ecol. Notes. 2003;3:462–464. doi:10.1046/j.1471-8286.2003.00484.x [Google Scholar]
- Gibbs H.L, Tabak L.M, Hobson K. Characterization of microsatellite DNA loci for a neotropical migrant songbird, the Swainson's thrush (Catharus ustulatus) Mol. Ecol. 1999;8:1551–1552. doi: 10.1046/j.1365-294x.1999.00673.x. doi:10.1046/j.1365-294x.1999.00673.x [DOI] [PubMed] [Google Scholar]
- Griffith S.C, Stewart I.R.K, Dawson D.A, Owens I.P.F, Burke T. Contrasting levels of extra-pair paternity in mainland and island populations of the house sparrow (Passer domesticus): is there an ‘island effect’? Biol. J. Linn. Soc. 1999;68:303–316. doi:10.1006/bijl.1999.0343 [Google Scholar]
- Groenen M.A.M, et al. A consensus linkage map of the chicken genome. Genome Res. 2000;10:137–147. doi: 10.1101/gr.10.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson L, Sutherland W.J. The cost of reproduction in the collared flycatcher Ficedula albicollis. Nature. 1988;335:813–815. doi:10.1038/335813a0 [Google Scholar]
- Haldane J.B.S. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 1922;12:101–109. [Google Scholar]
- Hanotte O, Zanon C, Greig C, Dixon A, Burke T. Isolation and characterization of microsatellite loci in a passerine bird: the reed bunting Emberiza schoeniclus. Mol. Ecol. 1994;3:529–530. doi: 10.1111/j.1365-294x.1994.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Hansson B, Kawabe A. A simple method to score single nucleotide polymorphisms based on allele-specific PCR and primer-induced fragment-length variation. Mol. Ecol. Notes. 2005;5:692–696. doi:10.1111/j.1471-8286.2005.01033.x [Google Scholar]
- Hansson B, Bensch S, Hasselquist D. Patterns of nest predation contribute to polygyny in the great reed warbler. Ecology. 2000a;81:319–328. [Google Scholar]
- Hansson B, Bensch S, Hasselquist D, Lillandt B.-G, Wennerberg L, von Schantz T. Increase of genetic variation over time in a recently founded population of great reed warblers (Acrocephalus arundinaceus) revealed by microsatellites and DNA fingerprinting. Mol. Ecol. 2000;9:1529–1538. doi: 10.1046/j.1365-294x.2000.01028.x. doi:10.1046/j.1365-294x.2000.01028.x [DOI] [PubMed] [Google Scholar]
- Hansson B, Bensch S, Hasselquist D, Nielsen B. Restricted dispersal in a long-distance migrant bird with patchy distribution, the great reed warbler. Oecologia. 2002;130:536–542. doi: 10.1007/s00442-001-0831-2. doi:10.1007/s00442-001-0831-2 [DOI] [PubMed] [Google Scholar]
- Hansson B, Gavrilov E, Gavrilov A. Hybridisation between great reed warblers Acrocephalus arundinaceus and clamorous reed warblers A. stentoreus: morphological and molecular evidence. Avian Sci. 2003;3:145–151. [Google Scholar]
- Hansson B, Bensch S, Hasselquist D. Do female great reed warblers seek extra-pair fertilizations to avoid inbreeding? Proc. R. Soc. B. 2004a;271(Suppl. 5):S290–S292. doi: 10.1098/rsbl.2004.0164. doi:10.1098/rsbl.2004.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson B, Bensch S, Hasselquist D. Lifetime fitness of short- and long-distance dispersing great reed warblers. Evolution. 2004b;58:2546–2557. doi: 10.1111/j.0014-3820.2004.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Hansson B, Westerdahl H, Bensch S, Hasselquist D, Åkesson M. Does linkage disequilibrium generate heterozygosity-fitness correlations in great reed warblers? Evolution. 2004c;58:870–879. doi: 10.1111/j.0014-3820.2004.tb00418.x. [DOI] [PubMed] [Google Scholar]
- Hasselquist D. Polygyny in the great reed warbler: a long-term study of factors contributing to male fitness. Ecology. 1998;79:2376–2390. [Google Scholar]
- Hasselquist D, Bensch S, von Schantz T. Correlation between male song repertoire, extra-pair paternity and offspring survival in the great reed warbler. Nature. 1996;381:229–232. doi:10.1038/381229a0 [Google Scholar]
- Helbig A.J, Seibold I. Molecular phylogeny of the Palearctic-African Acrocephalus and Hippolais warblers (Aves: Sylviidae) Mol. Phylogenet. Evol. 1999;11:246–260. doi: 10.1006/mpev.1998.0571. doi:10.1006/mpev.1998.0571 [DOI] [PubMed] [Google Scholar]
- Hulten M. Chiasma distribution at diakinesis in normal human male. Hereditas. 1974;76:55–78. doi: 10.1111/j.1601-5223.1974.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Hurst L.D, Pal C, Lercher M.J. The evolutionary dynamics of eukaryotic gene order. Nat. Rev. Genet. 2004;5:299–310. doi: 10.1038/nrg1319. doi:10.1038/nrg1319 [DOI] [PubMed] [Google Scholar]
- Huxley J.S. Sexual differences of linkage in Gammarus chevreuxi. J. Genet. 1928;20:145–156. [Google Scholar]
- International Chicken Genome Sequencing Consortium. Sequencing and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. doi:10.1038/nature03154 [DOI] [PubMed] [Google Scholar]
- Irwin D.E, Bensch S, Price T.D. Speciation in a ring. Nature. 2001;409:333–337. doi: 10.1038/35053059. doi:10.1038/35053059 [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Mikami O, Abe S. Isolation and characterization of microsatellite loci in the Japanese marsh warbler Locustella pryeri. Mol. Ecol. 2000;9:373–375. doi: 10.1046/j.1365-294x.2000.00874-5.x. doi:10.1046/j.1365-294x.2000.00874-5.x [DOI] [PubMed] [Google Scholar]
- Kayang B.B, Vignal A, Inoue-Murayama M, Miwa M, Monvoisin J.L, Ito S, Minvielle F. A first-generation microsatellite linkage map of the Japanese quail. Anim. Genet. 2004;35:195–200. doi: 10.1111/j.1365-2052.2004.01135.x. doi:10.1111/j.1365-2052.2004.01135.x [DOI] [PubMed] [Google Scholar]
- Keller L.F, Arcese P, Smith J.N.M, Hochachka W.M, Stearns S.C. Selection against inbred song sparrows during a natural population bottleneck. Nature. 1994;372:356–357. doi: 10.1038/372356a0. doi:10.1038/372356a0 [DOI] [PubMed] [Google Scholar]
- Komdeur J. Importance of habitat saturation and territory quality for the evolution of cooperative breeding in the Seychelles warbler. Nature. 1992;358:493–495. doi:10.1038/358493a0 [Google Scholar]
- Kong A, et al. A high-resolution recombination map of the human genome. Nat. Genet. 2002;31:241–247. doi: 10.1038/ng917. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Arai N, Arita M, Teranishi M, Hori T, Harata M, Mizuno S. Absence of Z-chromosome inactivation for five genes in male chickens. Chromosome Res. 2001;9:457–468. doi: 10.1023/a:1011672227256. doi:10.1023/A:1011672227256 [DOI] [PubMed] [Google Scholar]
- Lander E.S, Green P. Construction of multilocus genetic-linkage maps in humans. Proc. Natl Acad. Sci. USA. 1987;84:2363–2367. doi: 10.1073/pnas.84.8.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenormand T. The evolution of sex dimorphism in recombination. Genetics. 2003;163:811–822. doi: 10.1093/genetics/163.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn A, Kashuk C, Petersen M.B, Bailey J.A, Cox D.R, Antonarakis S.E, Chakravarti A. Patterns of meiotic recombination on the long arm of human chromosome 21. Genome Res. 2000;10:1319–1332. doi: 10.1101/gr.138100. doi:10.1101/gr.138100 [DOI] [PubMed] [Google Scholar]
- McQueen H.A, McBride D, Miele G, Bird A.P, Clinton M. Dosage compensation in birds. Curr. Biol. 2001;11:253–257. doi: 10.1016/s0960-9822(01)00070-7. doi:10.1016/S0960-9822(01)00070-7 [DOI] [PubMed] [Google Scholar]
- McRae S.B, Amos W. Characterization of hypervariable microsatellites in the cooperatively breeding white-browed sparrow weaver Plocepasser mahali. Mol. Ecol. 1999;8:903–904. [PubMed] [Google Scholar]
- Martinez J.G, Soler J.J, Soler M, Møller A.P, Burke T. Comparative population structure and gene flow of a brood parasite, the great spotted cuckoo (Clamator glandarius), and its primary host, the magpie (Pica pica) Evolution. 1999;53:269–278. doi: 10.1111/j.1558-5646.1999.tb05352.x. [DOI] [PubMed] [Google Scholar]
- Masabanda J.S, et al. Molecular cytogenetic definition of the chicken genome: the first complete avian karyotype. Genetics. 2004;166:1367–1373. doi: 10.1534/genetics.166.3.1367. doi:10.1534/genetics.166.3.1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellersh C.S, et al. A linkage map of the canine genome. Genomics. 1997;46:326–336. doi: 10.1006/geno.1997.5098. doi:10.1006/geno.1997.5098 [DOI] [PubMed] [Google Scholar]
- Merilä J, Kruuk L.E.B, Sheldon B.C. Cryptic evolution in a wild bird population. Nature. 2001;412:76–79. doi: 10.1038/35083580. [DOI] [PubMed] [Google Scholar]
- Neff M.W, Broman K.W, Mellersh C.S, Ray K, Acland G.M, Aguirre G.D, Ziegle J.S, Ostrander E.A, Rine J. A second-generation genetic linkage map of the domestic dog, Canis familiaris. Genetics. 1999;151:803–820. doi: 10.1093/genetics/151.2.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann K, Wetton J.H. Highly polymorphic microsatellites in the house sparrow Passer domesticus. Mol. Ecol. 1996;5:307–309. doi:10.1046/j.1365-294X.1996.00095.x [PubMed] [Google Scholar]
- Nishiumi I, Yamagishi S, Maekawa H, Shimoda C. Paternal expenditure is related to brood sex ratio in polygynous great reed warblers. Behav. Ecol. Sociobiol. 1996;39:211–217. doi:10.1007/s002650050283 [Google Scholar]
- Norris K. Heritable variation in a plumage indicator of viability in male great tits Parus major. Nature. 1993;362:537–539. doi:10.1038/362537a0 [Google Scholar]
- O'Connell J.R, Weeks D.E. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am. J. Hum. Genet. 1998;63:259–266. doi: 10.1086/301904. doi:10.1086/301904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka R, Nishiumi I, Wada M. Characterisation of 12 polymorphic microsatellite loci in the Japanese bush warbler Cettia diphone. Mol. Ecol. Notes. 2003;3:44–46. doi:10.1046/j.1471-8286.2003.00345.x [Google Scholar]
- Otter K, Ratcliffe L, Michaud D, Boag P.T. Do female black-capped chickadees prefer high-ranking males as extra-pair partners? Behav. Ecol. Sociobiol. 1998;43:25–36. doi:10.1007/s002650050463 [Google Scholar]
- Otto S.P, Lenormand T. Resolving the paradox of sex and recombination. Nat. Rev. Genet. 2002;3:252–261. doi: 10.1038/nrg761. doi:10.1038/nrg761 [DOI] [PubMed] [Google Scholar]
- Petren K. Microsatellite primers from Geospiza fortis and cross-species amplification in Darwin's finches. Mol. Ecol. 1998;7:1782–1784. doi: 10.1046/j.1365-294x.1998.00518.x. [DOI] [PubMed] [Google Scholar]
- Piertney S.B, Marquiss M, Summers R. Characterization of tetranucleotide microsatellite markers in the Scottish crossbill (Loxia scotica) Mol. Ecol. 1998;7:1261–1263. doi:10.1046/j.1365-294x.1998.00493.x [PubMed] [Google Scholar]
- Plomion C, O'Malley D.M. Recombination rate differences for pollen parents and seed parents in Pinus pinaster. Heredity. 1996;77:341–350. [Google Scholar]
- Primmer C.R, Møller A.P, Ellegren H. Resolving genetic relationship with microsatellite markers: a parentage testing system for the swallow Hirundo rustica. Mol. Ecol. 1995;4:493–498. doi: 10.1111/j.1365-294x.1995.tb00243.x. [DOI] [PubMed] [Google Scholar]
- Primmer C.R, Raudsepp T, Chowdhary B.P, Moller A.P, Ellegren H. Low frequency of microsatellites in the avian genome. Genome Res. 1997;7:471–482. doi: 10.1101/gr.7.5.471. [DOI] [PubMed] [Google Scholar]
- Rice W.R. Experimental tests of the adaptive significance of sexual recombination. Nat. Rev. Genet. 2002;3:241–251. doi: 10.1038/nrg760. doi:10.1038/nrg760 [DOI] [PubMed] [Google Scholar]
- Richardson D.S, Jury F.L, Dawson D.A, Salgueiro P, Komdeur J, Burke T. Fifty Seychelles warbler (Acrocephalus sechellensis) microsatellite loci polymorphic in Sylviidae species and their cross-species amplification in other passerine birds. Mol. Ecol. 2000;9:2226–2231. doi: 10.1046/j.1365-294x.2000.105338.x. doi:10.1046/j.1365-294X.2000.105338.x [DOI] [PubMed] [Google Scholar]
- Richman A.D, Price T. Evolution of ecological differences in the Old World leaf Warblers. Nature. 1992;355:817–821. doi: 10.1038/355817a0. doi:10.1038/355817a0 [DOI] [PubMed] [Google Scholar]
- Sætre G.P, Borge T, Lindroos K, Haavie J, Sheldon B.C, Primmer C, Syvanen A.C. Sex chromosome evolution and speciation in Ficedula flycatchers. Proc. R. Soc. B. 2003;270:53–59. doi: 10.1098/rspb.2002.2204. doi:10.1098/rspb.2002.2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, et al. A microsatellite linkage map of rainbow trout (Oncorhynchus mykiss) characterized by large sex-specific differences in recombination rates. Genetics. 2000;155:1331–1345. doi: 10.1093/genetics/155.3.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin V, Bonfils D, Binz T, Richner H. Isolation and characterization of 16 microsatellite loci in the Great Tit Parus major. Mol. Ecol. Notes. 2003;3:520–522. doi:10.1046/j.1471-8286.2003.00498.x [Google Scholar]
- Samollow P.B, Kammerer C.M, Mahaney S.M, Schneider J.L, Westenberger S.J, VandeBerg J.L, Robinson E.S. First-generation linkage map of the gray, short-tailed opossum, Monodelphis domestica, reveals genome-wide reduction in female recombination rates. Genetics. 2004;166:307–329. doi: 10.1534/genetics.166.1.307. doi:10.1534/genetics.166.1.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slate J, Visscher P.M, MacGregor S, Stevens D, Tate M.L, Pemberton J.M. A genome scan for quantitative trait loci in a wild population of red deer (Cervus elaphus) Genetics. 2002;162:1863–1873. doi: 10.1093/genetics/162.4.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauss M, Tomiuk J, Segelbacher G, Driesel S, Fietz J, Bachmann L, Kompf J. Sex-specific recombination rates in Parus major and P. caeruleus, an exception to Huxley's rule. Hereditas. 2003;139:199–205. doi: 10.1111/j.1601-5223.2003.01698.x. doi:10.1111/j.1601-5223.2003.01698.x [DOI] [PubMed] [Google Scholar]
- Trivers R. Sex differences in rates of recombination and sexual selection. In: Michod R, Levin B, editors. The evolution of sex. Sinauer; Sunderland, MA: 1988. pp. 270–286. [Google Scholar]
- Veen T, Borge T, Griffith S.C, Sætre G.P, Bures S, Gustafsson L, Sheldon B.C. Hybridization and adaptive mate choice in flycatchers. Nature. 2001;411:45–50. doi: 10.1038/35075000. doi:10.1038/35075000 [DOI] [PubMed] [Google Scholar]
- Westerdahl H, Bensch S, Hansson B, Hasselquist D, von Schantz T. Brood sex ratios, female harem status and resources for nestling provisioning in the great reed warbler. Behav. Ecol. Sociobiol. 2000;47:312–318. doi:10.1007/s002650050671 [Google Scholar]
- Westerdahl H, Hansson B, Bensch S, Hasselquist D. Between-year variation of MHC allele frequencies in great reed warblers: selection or drift? J. Evol. Biol. 2004;17:485–492. doi: 10.1111/j.1420-9101.2004.00711.x. doi:10.1111/j.1420-9101.2004.00711.x [DOI] [PubMed] [Google Scholar]
- Xu H.L, Swoboda I, Bhalla P.L, Singh M.B. Male gametic cell-specific gene expression in flowering plants. Proc. Natl Acad. Sci. USA. 1999;96:2554–2558. doi: 10.1073/pnas.96.5.2554. doi:10.1073/pnas.96.5.2554 [DOI] [PMC free article] [PubMed] [Google Scholar]