Abstract
A growing body of literature points to a large-scale research approach as essential for understanding population and community ecology. Many of our advances regarding the spatial ecology of predators and prey can be attributed to research with insect parasitoids and their hosts. In this review, we focus on the progress that has been made in the study of the movement and population dynamics of hosts and their parasitoids in heterogeneous landscapes, and how this research approach may be beneficial to pest management programs. To date, few studies have quantified prey and predator rates and ranges of dispersal and population dynamics at the patch level—the minimum of information needed to characterize population structure. From host–parasitoid studies with sufficient data, it is clear that the spatial scale of dispersal can differ significantly between a prey and its predators, local prey extinctions can be attributed to predators and predator extinction risk at the patch level often exceeds that of the prey. It is also evident that populations can be organized as a single, highly connected (patchy) population or as semi-independent extinction-prone local populations that collectively form a persistent metapopulation. A prey and its predators can also differ in population structure. At the landscape level, agricultural studies indicate that predator effects on its prey often spill over between the crop and surrounding area (matrix) and can depend strongly on landscape structure (e.g. the proportion of suitable habitat) at scales extending well beyond the crop margins. In light of existing empirical data, predator–prey models are typically spatially unrealistic, lacking important details on boundary responses and movement behaviour within and among patches. The tools exist for conducting empirical and theoretical research at the landscape level and we hope that this review calls attention to fertile areas for future exploration.
Keywords: dispersal behaviour, extinction risk, landscape, metapopulation, spillover effects
1. Introduction
The classic paper by Pimentel et al. (1963) demonstrated that the persistence times of the braconid parasitoid, Nasonia vitripennis, and its housefly host, Musca domestica, were substantially longer in complex interconnected laboratory cages than in single cages. Similar results were found by Huffaker (1958) for herbivorous and predatory mites among semi-isolated oranges on a laboratory bench. One implication of these studies was that the addition of ‘space–time structure’ to the environment promotes predator–prey coexistence. Building on these classic works, theoretical and empirical evidence over several decades has strongly suggested that spatial considerations such as the size, spatial arrangement, quality and connectivity of habitats and landscape composition can impact animal foraging behaviour, population dynamics, interactions within and among trophic levels and community structure (Kareiva 1987; Ricklefs & Schluter 1993; Gering et al. 2003; Tscharntke & Brandl 2004). In fact, larger-scale processes can potentially dominate local-scale processes (e.g. Steffan-Dewenter et al. 2002; Thies et al. 2003; Cronin 2004; Cronin & Haynes 2004). From a conservation perspective, the primary threat to global biodiversity—the loss and fragmentation of suitable habitat (Debinski & Holt 2000; Fahrig 2003)—is a phenomenon that is often best understood at landscape-level scales (e.g. Bascompte & Rodriguez 2001; Tscharntke et al. 2002; Aune et al. 2005). Clearly, a large-scale approach to studying population and community ecology is essential (Polis et al. 1997; Tscharntke 2000; Tscharntke & Brandl 2004).
Many of our advances regarding predator–prey spatial ecology can be attributed to research with hosts and their parasitoids (Godfray 1994; Hawkins 1994; Hassell 2000). Historically, modelling efforts have far surpassed the contributions of empirical research to this field. However, recent empirical work with hosts and parasitoids represents some of the best large-scale research on predator–prey interactions. We focus this review on the movement and dynamics of populations in heterogeneous landscapes, emphasizing research and progress with natural systems. We leave discussion of the spatial distribution of parasitism among host patches and its dynamical effects to the thorough review by Hassell (2000). Community-level issues (e.g. diversity, structure, succession) are also beyond the scope of this review. Predator–prey models that include a spatial aspect are also reviewed and new modelling approaches, particularly those that have been motivated by empiricism, are discussed. Finally, we discuss how the field of landscape ecology is helping to shape pest management practices and identify fruitful new research directions for the theoretical and field ecologist.
2. Literature review
(a) The structure and dynamics of fragmented populations
Spatially explicit field studies of host–parasitoid ecology and dynamics have come to the forefront as metapopulation theory has matured. A metapopulation is an assemblage of spatially discrete local populations that are linked together by migration (Levins 1970), a structure thought common to many species (Hanski 1999). One of the most influential and prevalent spatially explicit metapopulation models is the incidence function model (Hanski 1994). This model assumes a finite number of discrete and suitable habitat patches that can vary in size and degree of isolation (Hanski 1999). Furthermore, all patches are assumed equal in quality and are embedded in an inhospitable matrix (akin to an island archipelago). In general, this model framework is likely to be most useful if the following conditions are met (Hanski 1997): (i) all local populations have a substantial risk of extinction; (ii) habitat patches are not so isolated that recolonization is impossible; and (iii) local populations have asynchronous dynamics. The latter condition minimizes the likelihood that all local populations simultaneously go extinct and that the metapopulation persists. These spatially explicit models are appealing because they predict persistence of the ensemble of patches despite evanescent local dynamics (see also Hanski et al. 1996a). The model offers useful predictions regarding the effective metapopulation size (minimum number of patches or amount of suitable habitat necessary for long-term persistence), the contribution of each patch to regional persistence and how changes to real landscapes might affect metapopulation dynamics for a single species (Hanski et al. 1996b; Hanski & Ovaskainen 2000; Ovaskainen & Hanski 2003, 2004). Spatially realistic models have since been modified to incorporate variation in habitat quality, environmental and demographic stochasticity, spatially and temporally varying environments and within-patch population dynamics (e.g. Gyllenberg et al. 1997; Heino et al. 1997; Ovaskainen 2002; Ovaskainen & Hanski 2004).
Despite the wealth of single species, spatially explicit population models—the development of models involving predator–prey/host–parasitoid interactions in space—remains rather limited. Classic host–parasitoid models included variation in parasitoid densities across host patches, but also assumed complete remixing of the host and parasitoid population in each generation (e.g. Bailey et al. 1962; Hassell & May 1974; May 1978; Hassell et al. 1991), making them best suited to describing dynamics on a local scale. Later models linked collections of local populations through a dispersal pool or to their nearest neighbours in a two-dimensional lattice (e.g. Reeve 1988; Comins et al. 1992; Wilson & Hassell 1997; Childs et al. 2004). Under a variety of conditions, persistence of these systems can occur even when the underlying local dynamics are unstable (see Briggs & Hoopes 2004).
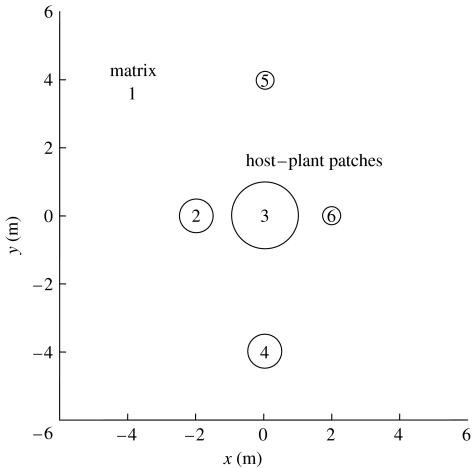
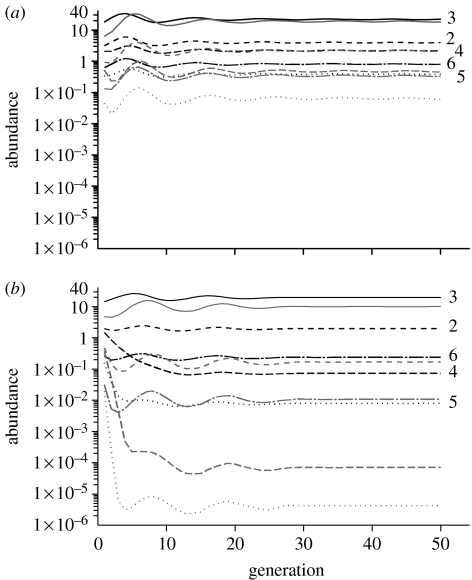
A drawback of these models is the simplistic way in which space and dispersal are described. It is difficult to see how they could be applied to natural systems, where habitat patches differ in size, spatial arrangement and matrix type. Reaction–diffusion versions of predator–prey models can provide a more realistic description of dispersal behaviour in space, but generally treat space as a continuum (Okubo et al. 2001). An exciting recent development has been the incorporation of boundary behaviour for habitat patches within the diffusion framework (Cantrell & Cosner 2003; Ovaskainen & Cornell 2003; Ovaskainen 2004). Combined with recent improvements in software for reaction–diffusion models, it is possible to construct host–parasitoid models across a collection of discrete habitat patches. We are currently developing such models for a planthopper (Prokelisia crocea) and its egg parasitoid (Anagrus columbi) residing in a landscape consisting of host–plant patches embedded in a heterogeneous matrix (Reeve et al. in preparation), in which movement rates and boundary behaviour vary with the composition of the matrix (Haynes 2004). We briefly illustrate this approach on a hypothetical landscape (figure 1) using the software package Femlab 3.1 (Comsol AB, Burlington, MA, 2005). The solution process consists of drawing the landscape and then specifying diffusion, oviposition, mortality and parasitoid attack rates within each domain, as well as boundary behaviour on the patch–matrix edge. Numerical solutions are shown in figure 2 for two matrix types, mudflat and brome (a grass), using parameter values estimated from observations of P. crocea movement (Reeve et al. in preparation). Observations have yet to be made for A. columbi, so for simplicity we assume its dispersal behaviour is similar to P. crocea. The solutions illustrate the importance of matrix type, patch size and edge behaviour on host and parasitoid abundance. Densities were consistently higher for a cordgrass–mudflat (figure 2a) versus cordgrass–brome landscape (figure 2b), because boundary behaviour on the cordgrass–mudflat landscape retains dispersing insects within cordgrass patches. Large cordgrass patches also had higher densities than small ones, as would be expected, and patches closer to the large central patch also had higher densities.
Figure 1.
Hypothetical landscape consisting of five circular host–plant patches surrounded by a matrix of grass (smooth brome) or mudflat. The landscape is divided into six domains (matrix=1, patches=2–6). The diffusion models for host and parasitoid movement incorporated boundary behaviour in the form of a biased random walk, where individuals on the patch–matrix boundary move toward the patch with probability k1, while 1−k1 is the probability for the matrix (Ovaskainen 2004). The outer boundary for the landscape was assumed to be absorbing.
Figure 2.
Abundance of host eggs and juvenile parasitoids (parasitized eggs) for 50 generations across the landscape depicted in figure 1. Numbered lines in black refer to host eggs in host–plant patches 2–6, while grey lines in the same style are juvenile parasitoids. The model was initialized by adding a small number of adult hosts and parasitoids to the patches. Host and parasitoid are then assumed to move and interact for a period of time, generating a distribution of host eggs and juvenile parasitoids in space. This distribution is then used to initialize the adult host and parasitoid distribution in the next generation, after adjusting for mortality in these stages. (a) Patch–mudflat landscape with diffusion rate Dpatch=0.12 m2 d−1, Dmudflat=5.8 m2 d−1 and k1=0.95; (b) patch-brome landscape with Dpatch=Dbrome=0.12 m2 d−1 and k1=0.5. Note that k1 is much higher for the patch–mudflat landscape, implying that dispersing individuals are more likely to be retained in patches surrounded by mudflat versus brome.
For parasitoids and their hosts, their small body sizes, high rates of population increase and specific resource requirements are thought to predispose them to metapopulation dynamics (Murphy et al. 1990; Bonsall et al. 2002; Bonsall & Hastings 2004). To date, there are few field studies that have characterized in detail the movement and spatial (meta)population structure of a host and its parasitoid among spatially discrete habitat patches (table 1). Perhaps, the best-studied example involves the Glanville fritillary (Melitaea cinxia) and its specialist braconid parasitoid Cotesia melitaearum. Both M. cinxia and C. melitaearum exhibit classic extinction–colonization metapopulation dynamics among dry meadow patches of the Åland islands in southwestern Finland (Lei & Hanski 1997; van Nouhuys & Hanski 2002). As an interesting contrast, van Nouhuys & Hanski (2002) found that the population structure of Hyposoter horticola (Ichneumonidae), another parasitoid of M. cinxia, is best described as a patchy population. Hyposoter horticola is much more dispersive than its host and as a consequence most host patches are occupied. Even at the scale of 3500 km2, there was no evidence of genetic population structure for H. horticola (Kankare et al. 2005).
Table 1.
Field studies on hosts and parasitoid spatial population structure.
| host and parasitoid(s) | dispersal rangea | local extinction risk | parasitoid can cause local host extinction? | type of population dynamics | sources |
|---|---|---|---|---|---|
| fruitfly (Uurophora cardui) and Eurytoma robusta | H>P | unknown | yes | classic metapopulation | Eber & Brandl (1994, 1996, 1997), Eber (2001), Johannesen & Seitz (2003) |
| fruitfly (Tephritis bardanae) and Habrocytus albipennis and Bracon minutator | H>P | unknown | no | patchy population | Dempster et al. (1995a,b) |
| California red scale (Aonidiella aurantii) and Aphytis melinus and Ecarsia perniciosi | H<P | none | no | locally and regionally stable | Murdoch et al. (1996) |
| Glanville fritillary (Melitaea cinxia) and Cotesia melitaearum | H>P | H<P | yes | classic metapopulation | Lei & Hanski (1997), van Nouhuys & Hanski (1999, 2002), van Nouhuys & Tay (2001) |
| cinnabar moth (Tyria jacobaea) and Cotesia popularis | H>P | unknown | no | host: classic metapopulation; parasitoid: unknown | van der Meijden & van der Veen-van Wijk (1997) |
| Glanville fritillary and Hyposoter horticola | H<P | host driven | no | host: classic metapopulation; parasitoid: patchy population | van Nouhuys & Hanski (2002), van Nouhuys & Ehrnsten (2004), Kankare et al. (2005) |
| aphid (Metopeurum fuscoviride) and Lysiphlebus hirticornis | H≈P | host driven | yes | classic metapopulation | Weisser (2000) |
| planthopper (Prokelisia crocea) and Anagrus columbi | H>P | H<P | no | mainland-island metapopulation | Cronin (2003a,b, 2004), Cronin & Haynes (2004) |
For inclusion in the table, host–plant patches must be discrete, and information about the relative dispersal of the host and parasitoid(s) and local extinction–colonization dynamics must be provided.
Is host (H) dispersal range less than, greater than or approximately equal to the dispersal range of the parasitoid (P)? Information is based on mark–recapture or gene flow studies.
Cronin (2003a,b, 2004) provides another example of extinction–colonization dynamics with P. crocea and A. columbi in the tall-grass prairies of North America. Host–plant patches (prairie cordgrass) have a heavily skewed size distribution such that a few large patches (greater than 4 ha) are intermixed with many small patches (less than 10 m2). Local planthopper and parasitoid extinctions decrease in likelihood with increasing patch size and no instance of extinction has ever been recorded in the largest patches (Cronin 2004). The population structures of P. crocea and A. columbi were similar, exhibiting mainland (large patches)-island dynamics (see also Hanski 1986; Harrison 1991; Berendonk & Bonsall 2002).
Most of the studies in table 1 infer parasitoid local dynamics from the distribution of parasitized hosts (Eber & Brandl 1994; Dempster et al. 1995a,b; Eber & Brandl 1996, 1997; van der Meijden & van der Veen-van Wijk 1997). Typically, little information is available on the extinction dynamics of the parasitoid independent of host extinctions. The major bottleneck in our understanding of host–parasitoid interactions among spatially discrete patches remains limited information on host, and especially parasitoid dispersal. Secondarily, we lack even rudimentary information on the local dynamics of patches within a metapopulation context. These limitations are common to predator–prey and parasite–host systems as well (but see Walde 1994; Antonovics 2004; Keeling et al. 2004).
Despite the limited number of case studies, the examples in table 1 serve to highlight several important issues with regard to predator–prey spatial ecology. First, the population structures of these systems are quite variable, ranging from classic metapopulations, to mainland-island metapopulations, to patchy populations with stable or unstable local dynamics. These results support the view that a classic metapopulation is but one point along a continuum of possible spatial population structures (Harrison & Taylor 1997; Thomas & Kunin 1999).
Second, a prey and its predators often differ significantly in the scales at which they disperse or respond to spatial subdivision (e.g. Roland & Taylor 1997; Althoff & Thompson 1999; Cronin et al. 2000; Ryall & Fahrig 2005). This is particularly evident in the case of the Glanville fritillary and H. horticola whereby the dispersal discrepancy leads to very different population structures—a classic metapopulation for the host and a patchy population for the highly mobile parasitoid. Differences in dispersal between interacting species can be important to their regional population dynamics. In a number of prey–predator models, stability is achieved in instances where the prey is more dispersive than the predator, i.e. the host stays one step ahead of its natural enemy (e.g. Comins et al. 1992; McCauley et al. 1996; Holt 1997; Hassell 2000). However, unequal dispersal rates are neither necessary nor sufficient for interaction persistence (Nee et al. 1997; Briggs & Hoopes 2004). Once again, it is clear that we need information on the ranges and rates of dispersal of interacting species if we wish to understand the mechanisms underlying their persistence.
Third, host extinctions are caused by their parasitoids in a number of instances (Lei & Hanski 1997; Weisser 2000; van Nouhuys & Tay 2001). For example, Weisser (2000) found that the aphidiid parasitoid Lysiphlebus hirticornis caused 100% parasitism of 12% of the local aphid populations residing in clonal patches of common tansy. These results are significant because they suggest that the host–parasitoid interaction within a patch is inherently unstable—an important feature of predator–prey metapopulation theory (Harrison & Taylor 1997; Weisser 2000). Predator- or parasite-induced extinctions of local prey populations are more difficult to document, but they do exist (e.g. Walde 1994; Antonovics 2004; Cronin et al. 2004).
Fourth, for those systems in which extinction probabilities have been quantified, the parasitoid is more extinction prone than its host (van Nouhuys & Tay 2001; van Nouhuys & Hanski 2002; Cronin 2004). These studies support the theoretical expectation that higher trophic levels are at greater risk of extinction than lower trophic levels (Pimm & Lawton 1977; Pimm 1991; Holt 1996). For P. crocea and A. columbi, the most likely explanation for this result was that the parasitoid's extinction risk was dependent on three trophic levels (densities of plants, hosts and conspecifics), whereas the host's extinction risk was dependent only on aspects of the landscape (patch size and matrix composition) (Cronin 2004). Also, A. columbi experienced a more fragmented landscape than its host because on average only 77% of the host–plant patches were occupied at a given time (Cronin 2004). Several studies have shown that predators, parasitoids and parasites are more sensitive to habitat fragmentation than their hosts (e.g. Kruess & Tscharntke 1994, 2000; Komonen et al. 2000; Thies et al. 2003; Antonovics 2004).
The chasm between theoretical and empirical research on fragmented populations of interacting species is at its widest with regard to the establishment of a causal link between habitat connectivity, fragmentation and population dynamics (e.g. Donahue et al. 2003; Bowne & Bowers 2004; Cronin & Haynes 2004). Laboratory microcosm experiments with consumer–resource systems have generally supported theoretical predictions (e.g. Huffaker 1958; Pimentel et al. 1963; Holyoak & Lawler 1996a,b, Ellner et al. 2001; Bonsall et al. 2002; Donahue et al. 2003; Bonsall & Hastings 2004). In contrast, most field studies report on surveys of the distribution of organisms over space and time, and hence cannot ascribe cause and effect relationships with much confidence. Experimental manipulations of patch structure, fragmentation, habitat loss, connectivity and its resulting effect on local or regional dynamics of a prey and its predators are also quite scarce (but see Kruess & Tscharntke 1994, 2000; Braschler et al. 2003; Cronin & Haynes 2004). No study has examined the impact of fragmentation per se (the breaking up of suitable habitat, independent of habitat loss; Fahrig 2003) on parasitoid abundance or parasitism rates. In the study by Cronin & Haynes (2004), in which replicate patch networks were created that differed in degree of functional connectivity, increased connectivity resulted in increased local and regional extinctions of the planthopper and its parasitoid. In this particular case, high connectivity caused patches to function like sieves (see Thomas & Kunin 1999), losing individuals faster than they could be gained and essentially countering the rescue effect (Brown & Kodric-Brown 1977; Hanski 1999). These unanticipated results were only revealed through experimentation.
(b) Landscape-level studies
Unlike the dichotomous view of landscapes inherent in classic metapopulation theory (i.e. discrete habitat patches of identical quality embedded in a uniformly inhospitable matrix), real landscapes are composed of patches that may have indistinct boundaries, their geometry and occurrence may be transient and the matrix may be quite heterogeneous (Wiens 1997; With 2004). Moreover, patch quality can vary with respect to edaphic and topographic conditions (Dias 1996; Haynes & Cronin 2004; With 2004). The consideration of these aspects of the landscape and their effect on ecological processes encapsulates the burgeoning field of landscape ecology (Turner 1989). Theoretical and empirical landscape studies focus on how the spatial arrangement and composition of landscape elements (i.e. landscape context) influence within-patch dynamics, boundary or edge responses, spillover among adjacent elements, functional connectivity and distribution of organisms (Tscharntke 2000; Tscharntke & Brandl 2004; With 2004). As we outline below, landscape ecology and biological pest management have become intertwined fields of study (Roland 2000; Tscharntke 2000; Thies et al. 2005).
Incorporation of the mosaic structure of real landscapes into metapopulation models has been viewed as a main promise of landscape ecology (Wiens 1997). One difficulty from a modelling perspective is that patch-based connectivity measures, such as nearest neighbour distance (Moilanen & Hanski 2001), may be incorrect for real landscapes. Connectivity is therefore often assessed in a functional way (i.e. dependent upon the movement behaviour of the species in question) with individual-based simulation models. The models must include individual behaviour at habitat boundaries and movement patterns through the different landscape elements (e.g. With & Crist 1995; Ovaskainen & Hanski 2004; Revilla et al. 2004). As we have mentioned previously, however, there is a dearth of spatially realistic predator–prey models that also incorporate realistic dispersal behaviour of animals within and between landscape elements.
The idea that the composition of the matrix affects animal movement and connectivity among patches has been well established (e.g. Crist et al. 1992; Ricketts 2001; Revilla et al. 2004), but very few studies on this subject have been conducted with parasitoids, predators or parasites (but see Morrison 1996; Elliott et al. 2002; Cronin 2003a, Cronin & Haynes 2004; Grez et al. 2005). In the study by Cronin & Haynes (2004), both P. crocea and A. columbi had higher emigration rates, moved longer distances and had higher colonization rates when dispersing through a grass as compared to a bare-ground matrix. By affecting functional connectivity, the matrix influenced the mean and variance in densities and extinction risk of local host and parasitoid populations. Also, aside from patterns of the distribution of predation/parasitism or natural enemies across ecotones or boundaries (e.g. Tscharntke et al. 2002; Cronin 2003a, Ries & Fagan 2003), we have very little data on how patch boundaries affect enemy and prey movement behaviour (but see Cronin 2003a for a rare parasitoid example).
Many of our advances in the arena of predator–prey landscape ecology have derived from studies of agricultural pests and their parasitoids (Roland 2000; Tscharntke 2000; Thies et al. 2005). We will emphasize two areas of study, spillover effects between adjacent landscape elements (i.e. spatial subsidies; Polis et al. 1997) and landscape context effects on host–parasitoid interactions. Spillover effects between adjacent landscape elements should be common when natural enemies and their prey are not restricted to a single habitat type and use different landscape elements for feeding, oviposition or hibernation (Landis et al. 2000; Gurr et al. 2003). The strength of pest–enemy interactions may depend on the juxtaposition of these different elements (Tscharntke & Brandl 2004). For example, spillover from non-crop habitat often results in higher parasitism rates near the crop edge than interior (e.g. Baggen & Gurr 1998; Thies & Tscharntke 1999; Tylianakis et al. 2004). The reverse has also been shown—spillover from crop to non-crop habitat can magnify the impact of parasitoids on non-crop insects resident in adjacent natural habitats (Barratt et al. 1997). Whether the adjacent matrix functions as a source (net exporter) or sink (net importer) for natural enemies probably depends on the nature of the matrix (i.e. its composition, size and age). Consequently, effective top-down control of plant pests may be contingent upon the composition of adjacent matrix habitats (Landis et al. 2000; Gurr et al. 2003; Snyder et al. in press). Although various pest management programs actively modify adjacent non-crop habitat to facilitate natural enemy production and spillover onto agricultural crops, we generally know too little about spillover effects to make predictions or management recommendations for many crop systems (Landis et al. 2000).
Studies of landscape-context effects on predator–prey interactions also come primarily from parasitoids and hosts in agroecosystems (but see Aviron et al. 2005; Schmidt & Tscharntke 2005). The typical study involves a collection of suitable host–plant patches (e.g. a crop field) surrounded by a mixture of crop and non-crop habitat (table 2). The landscape context ranges from the simple (high percentage of crop habitat in the surrounding area) to the complex (a high percentage of non-crop habitat). What was once a laborious task to classify vegetation structure over wide geographical regions has become quite simple thanks to the availability of medium-to-high resolution thermal and multispectral satellite imagery (Lillesand et al. 2003). Satellite images with a resolution of less than 5 m are globally available and can be used to identify the location of dominant vegetation types and even individual plant species (e.g. Mehner et al. 2004; Rocchini et al. 2004; Casady et al. 2005).
Table 2.
Effect of landscape context on host–parasitoid interactions.
| study system | landscape context | statistics | significant results | source |
|---|---|---|---|---|
| armyworm (Pseudaletia unpuncta) and parasitoids in maize | simple (cropland) versus complex (cropland surrounded by hedgerows and woodlots) landscapes | richness, parasitism | parasitism higher in complex landscapes | Marino & Landis (1996) |
| armyworms and parasitoids in maize | simple (cropland) versus complex (cropland intermixed with non-crop) landscapes | richness, parasitism | inconsistent among sites and years | Menalled et al. (1999, 2003) |
| rape pollen beetle (Meligethes aenus) and parasitoids in oilseed rape | presence/absence of oldfield margin strips adjacent to oilseed rape fields | crop damage, parasitism | crop damage lowest and percentage parasitism highest for crop fields near margin strips | Thies & Tscharntke (1999) |
| agromyzid (Melanagromyza aeneoventris) and parasitoids in creeping thistle | percentage of non-crop area | host abundance, parasitism | host abundance, but not percentage parasitism, increase with increasing percentage non-crop area | Kruess (2003) |
| trap-nesting bees and wasps and their parasitoids in orchards | matrix diversity; percentage of semi-natural habitat (orchards/meadows) | richness, parasitism | parasitoid richness increase with matrix diversity | Steffan-Dewenter (2003) |
| rape pollen beetle and parasitoids in oilseed rape | structural complexity (proportion of non-crop habitat) | crop damage, parasitism | plant damage decrease and parasitism increase with increasing complexity | Thies et al. (2003) |
| noctuid moth (Pseudaletia unipuncta) and parasitoids in maize | simple (cropland) versus complex (cropland intermixed with non-crop) landscapes | richness, diversity, parasitism | trend toward increasing richness in simple landscapes | Costamagna et al. (2004) |
| tansy leaf beetle (Galeruca tanaceti) and egg parasitoid (Oomyzus galerucivorus) in grasslands | percentage of area covered by non-host shrubs and percentage of area covered by suitable habitat | host incidence, parasitism | incidence and parasitism decrease with increasing coverage by shrubs and increase with coverage of suitable habitat | Meiners & Obermaier (2004), E. Obermaier, A. Heißwolf, H. J. Poethke, B. Randkofer and T. Meiners (unpublished data) |
| cereal aphids and parasitoids in winter wheat fields | structural complexity of landscape (percentage of arable land) | host abundance, parasitism | host density and parasitism increase with structural complexity | Roschewitz et al. (2005), Thies et al. (2005) |
In most studies with parasitoids of agricultural pests, parasitoid species richness and/or parasitism in the crop habitat increased with increasing landscape complexity (but see Menalled et al. 1999; Kruess 2003; Meiners & Obermaier 2004; table 2). For example, Thies et al. (2003) found that parasitism of the rape pollen beetle (Meligethes aenus) generally increased with the proportion of non-crop area within 6 km of the focal plants. In six out of eight studies, landscape context contributed significantly to variation in parasitoid richness or percentage parasitism. There is also evidence from these studies that herbivore and parasitoid responses to landscape context change with scale and differ from one another (e.g. Kruess 2003; Thies et al. 2003, 2005). In the study by E. Obermaier, A. Heißwolf, H. J. Poethke, B. Randkofer and T. Meiners (unpublished data), tansy leaf beetle (Galeruca tanaceti) oviposition was positively correlated with the percentage of suitable habitat within 200 m, whereas parasitism by Oomyzus galerucivorus was only positively correlated with the percentage of suitable habitat within 500 m. These scale-dependent differences between hosts and their parasitoids probably link back to their differences in dispersal ability (Thies et al. 2005). The above studies suggest that the biological control of plant pests is influenced by landscape-scale processes and that pest management programs may benefit considerably from thinking beyond the boundaries of a single agricultural field.
3. Discussion and future directions
Insect parasitoids have distinct advantages for the study of predator–prey interactions in space because their foraging success and impact on prey are manifest in the parasitism of hosts. Capitalizing on this advantage, ecologists have amassed considerable information on the spatial distribution of predator-induced prey mortality, and provided strong evidence that predators cause local prey extinctions and that prey suppression is impacted by factors that span local, regional and landscape scales. Moreover, it is probably the norm, rather than the exception, that each prey species and its associated enemies disperse, aggregate and respond to landscape structure at different spatial scales. On its own, this implies that predator–prey interactions must be considered across a broad range of spatial scales (see also Dunning et al. 1992; Polis et al. 1997; Tscharntke & Brandl 2004). These data have greatly expanded our understanding of predator–prey spatial ecology and are beginning to affect how agricultural pest management programs are developed (Landis et al. 2000; Tscharntke 2000; Tscharntke & Brandl 2004).
Although we have made significant strides in our understanding of predator–prey spatial ecology, this remains an open and fertile area for research. First, movement studies with parasitoids, predators and parasites need to be conducted in the context of the landscape. In addition to quantifying predator and prey migration rates, there is a critical need for data on movement behaviour at the boundary of suitable patches and other landscape elements and trajectories within different matrix types. This information is essential to understanding the functional connectivity among suitable patches (Ims & Yaccoz 1997; Cronin & Haynes 2004).
Second, we lack experimental studies at the metapopulation or landscape level that address questions regarding the temporal population dynamics of interacting species like hosts and their parasitoids. For example, no studies have examined the effects of habitat fragmentation per se on host–parasitoid interaction persistence at local and regional scales (see Fahrig 2003). Cronin & Haynes (2004, unpublished data) is the only study to our knowledge that created replicate landscapes in the field with the intention of determining the contribution of matrix composition to interaction persistence. In this case, ensembles of host–plant patches embedded in a grass matrix were much more extinction prone, both locally and regionally, than identical arrangements of patches in a bare-ground matrix. Experimental studies such as these are essential if we are to fathom the underlying mechanisms influencing predator–prey population dynamics. Although studies such as this are logistically impractical for widely dispersing species, quasi-experimental studies in agroecosystems remain viable options.
Third, modelling efforts with regard to predator–prey interactions mostly deal in the abstract—little attention is paid to the actual details of movement, the landscape is typically structured as a lattice, and the matrix is neutral in its effects on movement. Clearly, empirical research does not support these modelling simplifications. If the goal is to make qualitative or short-term predictions for threatened or endangered species in which we have limited data, then these simplified models may be our best and only option (Hanski 2002). However, as our own data suggest (Cronin & Haynes 2004), regional persistence may be strongly landscape-context dependent. In lieu of these abstractions, we advocate a more mechanistic, but generalizable modelling approach that includes landscape realism. A significant advantage of this modelling approach is that theoretical landscapes can be made to resemble natural landscapes and hypotheses can be tested for real scenarios.
Finally, research in these previous three areas can pay substantial dividends when applied to agricultural systems, particularly with regard to conservation biological control (Snyder et al. in press). More studies are needed that address the mechanisms underlying whether, and under what circumstances, adjacent matrix habitat functions as a source or sink for natural enemies. Also, we have barely scratched the surface in exploring how pest and enemy abundance, diversity and interactions are influenced by landscape context across multiple spatial scales, yet the limited evidence to date suggests that parasitism and predation within a field can be strongly related to habitat complexity at scales extending well beyond the crop margins (e.g. Kruess 2003; Thies et al. 2003, 2005). The predictive ability of pest–enemy models and the success of management strategies can only be improved by exploring pest–enemy interactions at the landscape level.
The future of research on predator–prey spatial ecology is very bright. Spurred on by recent empirical data, worldwide availability of high-resolution satellite imagery, computational power of desktop computers and emerging spatially realistic landscape models, we foresee a continued growth of research in this area. We also anticipate that research with host–parasitoid systems will lead the way.
Acknowledgments
Support was provided by National Science Foundation grant DEB-0211359 to J.T.C. and a cooperative agreement with the Southern Research Station, USDA Forest Service to J.D.R.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
References
- Althoff D.M, Thompson J.N. Comparative geographic structures of two parasitoid–host interactions. Evolution. 1999;53:818–825. doi: 10.1111/j.1558-5646.1999.tb05375.x. [DOI] [PubMed] [Google Scholar]
- Antonovics J. Long-term study of a plant–pathogen metapopulation. In: Hanski I, Gaggiotti O.E, editors. Ecology, genetics, and evolution of metapopulations. Elsevier Academic Press; Boston, MA: 2004. pp. 471–488. [Google Scholar]
- Aune K, Jonsson B.G, Moen J. Isolation and edge effects among woodland key habitats in Sweden: is forest policy promoting fragmentation? Biol. Conserv. 2005;124:89–95. doi:10.1016/j.biocon.2005.01.015 [Google Scholar]
- Aviron S, Burel F, Baudry J, Schermann N. Carabid assemblages in agricultural landscapes: impacts of habitat features, landscape context at different spatial scales and farming intensity. Agric. Ecosyst. Environ. 2005;108:205–217. doi:10.1016/j.agee.2005.02.004 [Google Scholar]
- Baggen L.R, Gurr G.M. The influence of food on Copidosoma koehleri (Hymenoptera: Encyrtidae), and the use of flowering plants as a habitat management tool to enhance biological control of potato moth, Phthorimaea operculella (Lepidoptera: Gelechiidae) Biol. Control. 1998;11:9–17. doi:10.1006/bcon.1997.0566 [Google Scholar]
- Bailey V.A, Nicholson A.J, Williams E.J. Interaction between hosts and parasites when some host individuals are more difficult to find than others. J. Theor. Biol. 1962;3:1–18. [Google Scholar]
- Barratt B.I.P, Evans A.A, Ferguson C.M, Barker G.M, McNeill M.R. Laboratory nontarget host range of the introduced parasitoids Microctonus aethiopoides and M. hyperodae (Hymenoptera, Braconidae) compared with field parasitism in New Zealand. Environ. Entomol. 1997;26:694–702. [Google Scholar]
- Bascompte J, Rodriguez M.A. Habitat patchiness and plant species richness. Ecol. Lett. 2001;4:417–420. doi:10.1046/j.1461-0248.2001.00242.x [Google Scholar]
- Berendonk T.U, Bonsall M.B. The phantom midge and a comparison of metapopulation structures. Ecology. 2002;83:116–128. [Google Scholar]
- Bonsall M.B, Hastings A. Demographic and environmental stochasticity in predator–prey metapopulation dynamics. J. Anim. Ecol. 2004;73:1043–1055. doi:10.1111/j.0021-8790.2004.00874.x [Google Scholar]
- Bonsall M.B, French D.R, Hassell M.P. Metapopulation structures affect persistence of predator–prey interactions. J. Anim. Ecol. 2002;71:1075–1084. doi:10.1046/j.1365-2656.2002.00670.x [Google Scholar]
- Bowne D.R, Bowers M.A. Interpatch movements in spatially structured populations: a literature review. Landscape Ecol. 2004;19:1–20. doi:10.1023/B:LAND.0000018357.45262.b9 [Google Scholar]
- Braschler B, Lampel G, Baur B. Experimental small-scale grassland fragmentation alters aphid population dynamics. Oikos. 2003;100:581–591. doi:10.1034/j.1600-0706.2003.12220.x [Google Scholar]
- Briggs C.J, Hoopes M.F. Stabilizing effects in spatial parasitoid–host and predator–prey models: a review. Theor. Popul. Biol. 2004;65:299–315. doi: 10.1016/j.tpb.2003.11.001. doi:10.1016/j.tpb.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Brown J.H, Kodric-Brown A. Turnover rates in insular biogeography: effects of immigration on extinction. Ecology. 1977;58:445–449. [Google Scholar]
- Cantrell R.S, Cosner C. Spatial ecology via reaction–diffusion equations. Wiley; West Sussex: 2003. [Google Scholar]
- Casady G.M, Hanley R.S, Seelan S.K. Detection of leafy spurge (Euphorbia esula) using multidate high-resolution satellite imagery. Weed Technol. 2005;19:462–467. [Google Scholar]
- Childs D.Z, Bonsall M.B, Rees M. Periodic local disturbance in host–parasitoid metapopulations: host suppression and parasitoid persistence. J. Theor. Biol. 2004;227:13–23. doi: 10.1016/S0022-5193(03)00293-5. doi:10.1016/S0022-5193(03)00293-5 [DOI] [PubMed] [Google Scholar]
- Comins H.N, Hassell M.P, May R.M. The spatial dynamics of host–parasitoid systems. J. Anim. Ecol. 1992;61:735–748. [Google Scholar]
- Costamagna A.C, Menalled F.D, Landis D.A. Host density influences parasitism of the armyworm Pseudaletia unipuncta in agricultural landscapes. Basic Appl. Ecol. 2004;5:347–355. doi:10.1016/j.baae.2004.04.009 [Google Scholar]
- Crist T.O, Guertin D.S, Wiens J.A, Milne B.T. Animal movement in hetergeneous landscapes: an experiment with Eleodes beetles in shortgrass prairie. Funct. Ecol. 1992;6:536–544. [Google Scholar]
- Cronin J.T. Matrix heterogeneity and host–parasitoid interactions in space. Ecology. 2003a;84:1506–1516. [Google Scholar]
- Cronin J.T. Movement and spatial population structure of a prairie planthopper. Ecology. 2003b;84:1179–1188. [Google Scholar]
- Cronin J.T. Host–parasitoid extinction and recolonization in a fragmented prairie landscape. Oecologia. 2004;139:503–514. doi: 10.1007/s00442-004-1549-8. doi:10.1007/s00442-004-1549-8 [DOI] [PubMed] [Google Scholar]
- Cronin J.T, Haynes K.J. An invasive plant promotes unstable host–parasitoid patch dynamics. Ecology. 2004;85:2772–2782. [Google Scholar]
- Cronin J.T, Reeve J.D, Wilkens R, Turchin P. The pattern and range of movement of a checkered beetle predator relative to its bark beetle prey. Oikos. 2000;90:127–138. doi:10.1034/j.1600-0706.2000.900113.x [Google Scholar]
- Cronin J.T, Haynes K.J, Dillemuth F. Spider effects on planthopper mortality, dispersal and spatial population dynamics. Ecology. 2004;85:2134–2143. [Google Scholar]
- Debinski D.M, Holt R.D. A survey and overview of habitat fragmentation experiments. Conserv. Biol. 2000;14:342–355. doi:10.1046/j.1523-1739.2000.98081.x [Google Scholar]
- Dempster J.P, Atkinson D.A, Cheesman O.D. The spatial population dynamics of insects exploiting a patchy food resource. I. Population extinctions and regulation. Oecologia. 1995a;104:340–353. doi: 10.1007/BF00328370. doi:10.1007/BF00328370 [DOI] [PubMed] [Google Scholar]
- Dempster J.P, Atkinson D.A, French M.C. The spatial population dynamics of insects exploiting a patchy food resource. II. Movements between patches. Oecologia. 1995b;104:354–362. doi: 10.1007/BF00328371. doi:10.1007/BF00328371 [DOI] [PubMed] [Google Scholar]
- Dias P.C. Sources and sinks in population biology. Trends Ecol. Evol. 1996;11:326–330. doi: 10.1016/0169-5347(96)10037-9. doi:10.1016/0169-5347(96)10037-9 [DOI] [PubMed] [Google Scholar]
- Donahue M.J, Holyoak M, Feng C. Patterns of dispersal and dynamics among habitat patches varying in quality. Am. Nat. 2003;162:302–317. doi: 10.1086/377185. doi:10.1086/377185 [DOI] [PubMed] [Google Scholar]
- Dunning J.B, Danielson B.J, Pulliam H.R. Ecological processes that affect populations in complex landscapes. Oikos. 1992;65:169–175. [Google Scholar]
- Eber S. Multitrophic interactions: the population dynamics of spatially structured plant–herbivore–parasitoid systems. Basic Appl. Ecol. 2001;2:27–33. doi:10.1078/1439-1791-00033 [Google Scholar]
- Eber S, Brandl R. Ecological and genetic spatial patterns of Urophora cardui (Diptera, Tephritidae) as evidence for population structure and biogeographical processes. J. Anim. Ecol. 1994;63:187–199. [Google Scholar]
- Eber S, Brandl R. Metapopulation dynamics of the tephritid fly Urophora cardui: an evaluation of incidence–function model assumptions with field data. J. Anim. Ecol. 1996;65:621–630. [Google Scholar]
- Eber S, Brandl R. Genetic differentiation of the tephritid fly Urophora cardui in Europe as evidence for its biogeographical history. Mol. Ecol. 1997;6:651–660. doi:10.1046/j.1365-294X.1997.00236.x [Google Scholar]
- Elliott N.C, Kieckhefer R.W, Michels G.J, Giles K.L. Predator abundance in alfalfa fields in relation to aphids, within-field vegetation, and landscape matrix. Environ. Entomol. 2002;31:253–260. [Google Scholar]
- Ellner S.P, et al. Habitat structure and population persistence in an experimental community. Nature. 2001;412:538–543. doi: 10.1038/35087580. doi:10.1038/35087580 [DOI] [PubMed] [Google Scholar]
- Fahrig L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Entomol. 2003;34:487–515. [Google Scholar]
- Gering J.C, Crist T.O, Veech J.A. Additive partitioning of species diversity across multiple spatial scales: implications for regional conservation of biodiversity. Conserv. Biol. 2003;17:488–499. doi:10.1046/j.1523-1739.2003.01465.x [Google Scholar]
- Godfray H.C.J. Parasitoids: behavioral and evolutionary ecology. Princeton University Press; Princeton, NJ: 1994. [Google Scholar]
- Grez A.A, Zaviezo T, Rios M. Ladybird (Coleoptera: Coccinellidae) dispersal in experimental fragmented alfalfa landscapes. Eur. J. Entomol. 2005;102:209–216. [Google Scholar]
- Gurr G.M, Wratten S.D, Luna J.M. Multi-function agricultural biodiversity: pest management and other benefits. Basic Appl. Ecol. 2003;4:107–116. doi:10.1078/1439-1791-00122 [Google Scholar]
- Gyllenberg M, Hanski I, Hastings A. Structured metapopulation models. In: Hanski I, Gilpin M.E, editors. Metapopulation biology. Academic Press; San Diego, CA: 1997. pp. 93–122. [Google Scholar]
- Hanski I. Population dynamics of shrews on small islands accord with the equilibrium theory. Biol. J. Linn. Soc. 1986;28:23–36. [Google Scholar]
- Hanski I. A practical model of metapopulation dynamics. J. Anim. Ecol. 1994;63:151–162. [Google Scholar]
- Hanski I. Metapopulation dynamics—from concepts and observations to predictive models. In: Hanski I, Gilpin M.E, editors. Metapopulation biology. Academic Press; San Diego: 1997. pp. 69–91. [Google Scholar]
- Hanski I. Metapopulation ecology. Oxford University Press; New York: 1999. [Google Scholar]
- Hanski I. Metapopulations of animals in highly fragmented landscapes and population viability analysis. In: Beissinger S.R, McCullough D.R, editors. Population viability analysis. University of Chicago Press; Chicago, IL: 2002. pp. 86–108. [Google Scholar]
- Hanski I, Ovaskainen O. The metapopulation capacity of a fragmented landscape. Nature. 2000;404:755–758. doi: 10.1038/35008063. doi:10.1038/35008063 [DOI] [PubMed] [Google Scholar]
- Hanski I, Foley P, Hassell M. Random walks in a metapopulation—how much density dependence is necessary for long-term persistence? J. Anim. Ecol. 1996a;65:274–282. [Google Scholar]
- Hanski I, Moilanen A, Gyllenberg M. Minimum viable metapopulation size. Am. Nat. 1996b;147:527–541. doi:10.1086/285864 [Google Scholar]
- Harrison S. Local extinction in a metapopulation context: an empirical evaluation. Biol. J. Linn. Soc. 1991;42:73–88. [Google Scholar]
- Harrison S, Taylor A.D. Empirical evidence for metapopulation dynamics. In: Hanski I, Gilpin M.E, editors. Metapopulation biology. Academic Press; San Diego, CA: 1997. pp. 27–42. [Google Scholar]
- Hassell M.P. The spatial and temporal dynamics of host–parasitoid interactions. Oxford University Press; New York: 2000. [Google Scholar]
- Hassell M.P, May R.M. Aggregation of predators and insect parasites and its effect on stability. J. Anim. Ecol. 1974;43:567–594. [Google Scholar]
- Hassell M.P, Comins H.N, May R.M. Spatial structure and chaos in insect populations. Nature. 1991;353:255–258. doi:10.1038/353255a0 [Google Scholar]
- Hawkins B.A. Patterns and process in host–parasitoid interactions. Cambridge University Press; London: 1994. [Google Scholar]
- Haynes, K. J. 2004. Herbivore movement and spatial population dynamics in a heterogeneous landscape. Ph.D. thesis, Louisiana State University, Louisiana.
- Haynes K.J, Cronin J.T. Confounding of patch quality and matrix effects in herbivore movement studies. Landscape Ecol. 2004;19:119–124. [Google Scholar]
- Heino M, Kaitala V, Ranta E, Lindstrom J. Synchronous dynamics and rates of extinction in spatially structured populations. Proc. R. Soc. B. 1997;264:481–486. doi:10.1098/rspb.1997.0069 [Google Scholar]
- Holt R.D. Food webs in space: an island biogeographic perspective. In: Polis G.A, Winemiller K.O, editors. Food webs—integration of patterns and dynamics. Chapman & Hall; New York: 1996. pp. 313–323. [Google Scholar]
- Holt R.D. From metapopulation dynamics to community structure—some consequences of spatial heterogeneity. In: Hanski I, Gilpin M.E, editors. Metapopulation biology. Academic Press; San Diego, CA: 1997. pp. 149–164. [Google Scholar]
- Holyoak M, Lawler S.P. Persistence of an extinction-prone predator–prey interaction through metapopulation dynamics. Ecology. 1996a;77:1867–1879. [Google Scholar]
- Holyoak M, Lawler S.P. The role of dispersal in predator–prey metapopulation dynamics. J. Anim. Ecol. 1996b;65:640–652. [Google Scholar]
- Huffaker C.B. Experimental studies on predation: dispersion factors and predator–prey oscillations. Hilgardia. 1958;27:343–383. [Google Scholar]
- Ims R.A, Yaccoz N.G. Studying transfer processes in metapopulations: emigration, migration, and colonization. In: Hanski I, Gilpin M.E, editors. Metapopulation biology. Academic Press; San Diego, CA: 1997. pp. 247–265. [Google Scholar]
- Johannesen J, Seitz A. Comparative population genetic structures of the fruit fly Urophora cardui and its primary parasitoid Eurytoma robusta. Entomol. Exp. Appl. 2003;108:149–157. doi:10.1046/j.1570-7458.2003.00077.x [Google Scholar]
- Kankare M, van Nouhuys S, Gaggiotti O, Hanski I. Metapopulation genetic structure of two coexisting parasitoids of the Glanville fritillary butterfly. Oecologia. 2005;143:77–84. doi: 10.1007/s00442-004-1782-1. doi:10.1007/s00442-004-1782-1 [DOI] [PubMed] [Google Scholar]
- Kareiva P. Habitat fragmentation and the stability of predator–prey interactions. Nature. 1987;326:388–390. doi:10.1038/326388a0 [Google Scholar]
- Keeling M.J, Bjørnstad O.N, Grenfell B.T. Metapopulation dynamics of infectious diseases. In: Hanski I, Gaggiotti O.E, editors. Ecology, genetics, and evolution of metapopulations. Boston; Elsevier Academic Press: 2004. pp. 415–445. [Google Scholar]
- Komonen A, Penttila R, Lindgren M, Hanski I. Forest fragmentation truncates a food chain based on an old-growth forest bracket fungus. Oikos. 2000;90:119–126. doi:10.1034/j.1600-0706.2000.900112.x [Google Scholar]
- Kruess A. Effects of landscape structure and habitat type on a plant–herbivore–parasitoid community. Ecography. 2003;26:283–290. doi:10.1034/j.1600-0587.2003.03402.x [Google Scholar]
- Kruess A, Tscharntke T. Habitat fragmentation, species loss, and biological control. Science. 1994;264:1581–1584. doi: 10.1126/science.264.5165.1581. [DOI] [PubMed] [Google Scholar]
- Kruess A, Tscharntke T. Species richness and parasitism in a fragmented landscape: experiments and field studies with insects on Vicia sepium. Oecologia. 2000;122:129–137. doi: 10.1007/PL00008829. [DOI] [PubMed] [Google Scholar]
- Landis D.A, Wratten S.D, Gurr G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000;45:175–201. doi: 10.1146/annurev.ento.45.1.175. doi:10.1146/annurev.ento.45.1.175 [DOI] [PubMed] [Google Scholar]
- Lei G.C, Hanski I. Metapopulation structure of Cotesia melitaearum, a specialist parasitoid of the butterfly Melitaea cinxia. Oikos. 1997;78:91–100. [Google Scholar]
- Levins R. Extinction. In: Gerstenhaber M, editor. Some mathematical problems in biology. American Mathematical Society; Providence, Rhode Island, USA: 1970. pp. 75–107. [Google Scholar]
- Lillesand T.M, Kiefer R.W, Chipman J.W. Remote sensing and image interpretation. 5th edn. Wiley; New York: 2003. [Google Scholar]
- Marino P.C, Landis D.A. Effect of landscape structure on parasitoid diversity and parasitism in agroecosystems. Ecol. Appl. 1996;6:276–284. [Google Scholar]
- May R.M. Host–parasitoid systems in patchy environments: a phenomenological model. J. Anim. Ecol. 1978;47:833–843. [Google Scholar]
- McCauley E, Wilson W.G, de Roos A.M. Dynamics of age-structured predator–prey populations in space: asymmetrical effects of mobility in juvenile and adult predators. Oikos. 1996;76:485–497. [Google Scholar]
- Mehner H, Cutler M, Fairbairn D, Thompson G. Remote sensing of upland vegetation: the potential of high spatial resolution satellite sensors. Global Ecol. Biogeogr. 2004;13:359–369. doi:10.1111/j.1466-822X.2004.00096.x [Google Scholar]
- Meiners T, Obermaier E. Hide and seek on two spatial scales—vegetation structure effects herbivore oviposition and egg parasitism. Basic Appl. Ecol. 2004;5:87–94. doi:10.1078/1439-1791-00182 [Google Scholar]
- Menalled F.D, Marino P.C, Gage S.H, Landis D.A. Does agricultural landscape structure affect parastism and parasitoid diversity? Ecol. Appl. 1999;9:634–641. [Google Scholar]
- Menalled F.D, Costamagna A.C, Marino P.C, Landis D.A. Temporal variation in the response of parasitoids to agricultural landscape structure. Agric. Ecosyst. Environ. 2003;96:29–35. [Google Scholar]
- Moilanen A, Hanski I. On the use of connectivity measures in spatial ecology. Oikos. 2001;95:147–151. doi:10.1034/j.1600-0706.2001.950116.x [Google Scholar]
- Morrison J.A. Infection of Juncus dichotomus by the smut fungus Cintractia junci: an experimental field test of the effects of neighbouring plants, environment, and host plant genotype. J. Ecol. 1996;84:691–702. [Google Scholar]
- Murdoch W.W, Swarbrick S.L, Luck R.F, Walde S, Yu D.S. Refuge dynamics and metapopulation dynamics: an experimental test. Am. Nat. 1996;147:424–444. doi:10.1086/285859 [Google Scholar]
- Murphy D.D, Freas K.E, Weiss S.B. An environment–metapopulation approach to population viability analysis for a threatened invertebrate. Conserv. Biol. 1990;4:41–51. doi:10.1111/j.1523-1739.1990.tb00266.x [Google Scholar]
- Nee S, May R.M, Hassell M.P. Two-species metapopulation models. In: Hanski I, Gilpin M.E, editors. Metapopulation biology. Academic Press; San Diego, CA: 1997. pp. 123–147. [Google Scholar]
- Okubo A, Hastings A, Powell T. Population dynamics in temporal and spatial domains. In: Okubo A, Levin S.A, editors. Diffusion and ecological problems: modern perspectives. Springer; New York: 2001. pp. 298–373. [Google Scholar]
- Ovaskainen O. The effective size of a metapopulation living in a heterogeneous patch network. Am. Nat. 2002;160:612–628. doi: 10.1086/342818. doi:10.1086/342818 [DOI] [PubMed] [Google Scholar]
- Ovaskainen O. Habitat-specific movement parameters estimated using mark–recapture data and a diffusion model. Ecology. 2004;85:242–257. [Google Scholar]
- Ovaskainen O, Cornell S.J. Biased movement at boundary and conditional occupancy times for diffusion processes. J. Appl. Prob. 2003;40:557–580. doi:10.1239/jap/1059060888 [Google Scholar]
- Ovaskainen O, Hanski I. Extinction threshold in metapopulation models. Ann. Zool. Fenn. 2003;40:81–97. [Google Scholar]
- Ovaskainen O, Hanski I. Metapopulation dynamics in highly fragmented landscapes. In: Hanski I, Gaggiotti O.E, editors. Ecology, genetics, and evolution of metapopulations. Elsevier Academic Press; Boston, MA: 2004. pp. 73–104. [Google Scholar]
- Pimentel D, Nagel W.P, Madden J.L. Space–time structure of the environment and the survival of parasite–host systems. Am. Nat. 1963;97:141–166. doi:10.1086/282265 [Google Scholar]
- Pimm S.L. The balance of nature. University of Chicago Press; Chicago, IL: 1991. [Google Scholar]
- Pimm S.L, Lawton J.H. Number of trophic levels in ecological communities. Nature. 1977;268:329–331. doi:10.1038/268329a0 [Google Scholar]
- Polis G.A, Anderson W.B, Holt R.D. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu. Rev. Ecol. Syst. 1997;28:289–316. doi:10.1146/annurev.ecolsys.28.1.289 [Google Scholar]
- Reeve J.D. Environmental variability, migration, and persistence in host–parasitoid systems. Am. Nat. 1988;132:810–836. doi:10.1086/284891 [Google Scholar]
- Reeve, J. D., Haynes, K. J. & Cronin, J. T. In preparation. Diffusion models for planthopper movement incorporating heterogeneity among substrates, individuals and edge behaviours. [DOI] [PubMed]
- Revilla E, Wiegand T, Palomares F, Ferreras P, Delibes M. Effects of matrix heterogeneity on animal dispersal: from individual behavior to metapopulation-level parameters. Am. Nat. 2004;164:E130–E153. doi: 10.1086/424767. doi:10.1086/424767 [DOI] [PubMed] [Google Scholar]
- Ricketts T.H. The matrix matters: effective isolation in fragmented landscapes. Am. Nat. 2001;158:87–99. doi: 10.1086/320863. doi:10.1086/320863 [DOI] [PubMed] [Google Scholar]
- Ricklefs R.E, Schluter D, editors. Species diversity in ecological communities: historical and geographical perspectives. University of Chicago Press; Chicago, IL: 1993. [Google Scholar]
- Ries L, Fagan W.F. Habitat edges as a potential ecological trap for an insect predator. Ecol. Entomol. 2003;28:567–572. doi:10.1046/j.1365-2311.2003.00550.x [Google Scholar]
- Rocchini D, Chiarucci A, Loiselle S.A. Testing the spectral variation hypothesis by using satellite multispectral images. Acta Oecol. 2004;26:117–120. doi:10.1016/j.actao.2004.03.008 [Google Scholar]
- Roland J. Landscape ecology of parasitism. In: Hochberg M.E, Ives A.R, editors. Parasitoid population biology. Princeton University Press; Princeton, NJ: 2000. pp. 83–99. [Google Scholar]
- Roland J, Taylor P.D. Insect parasitoid species respond to forest structure at different spatial scales. Nature. 1997;386:710–713. doi:10.1038/386710a0 [Google Scholar]
- Roschewitz I, Hucker M, Tscharntke T, Thies C. The influence of landscape context and farming practices on parasitism of cereal aphids. Agric. Ecosyst. Environ. 2005;108:218–227. doi:10.1016/j.agee.2005.02.005 [Google Scholar]
- Ryall K.L, Fahrig L. Habitat loss decreases predator–prey ratios in a pine-bark beetle system. Oikos. 2005;110:265–270. doi:10.1111/j.0030-1299.2005.13691.x [Google Scholar]
- Schmidt M.H, Tscharntke T. Landscape context of sheetweb spider (Araneae: Linyphiidae) abundance in cereal fields. J. Biogeogr. 2005;32:467–473. doi:10.1111/j.1365-2699.2004.01244.x [Google Scholar]
- Snyder, W. E., Chang, G. C. & Prasad, R. P. In press. Biodiversity and successful conservation biological control: is there a relationship? In Ecology of predator–prey interactions (ed. P. Barbosa & I. Castellanos). London: Oxford University Press.
- Steffan-Dewenter I. Importance of habitat area and landscape context for species richness of bees and wasps in fragmented orchard meadows. Conserv. Biol. 2003;17:1036–1044. doi:10.1046/j.1523-1739.2003.01575.x [Google Scholar]
- Steffan-Dewenter I, Munzenberg U, Burger C, Thies C, Tscharntke T. Scale-dependent effects of landscape context on three pollinator guilds. Ecology. 2002;83:1421–1432. [Google Scholar]
- Thies C, Tscharntke T. Landscape structure and biological control in agroecosystems. Science. 1999;285:893–895. doi: 10.1126/science.285.5429.893. doi:10.1126/science.285.5429.893 [DOI] [PubMed] [Google Scholar]
- Thies C, Steffan-Dewenter I, Tscharntke T. Effects of landscape context on herbivory and parasitism at different spatial scales. Oikos. 2003;101:18–25. doi:10.1034/j.1600-0706.2003.12567.x [Google Scholar]
- Thies C, Roschewitz I, Tscharntke T. The landscape context of cereal aphid–parasitoid interactions. Proc. R. Soc. B. 2005;272:203–210. doi: 10.1098/rspb.2004.2902. doi:10.1098/rspb.2004.2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.D, Kunin W.E. The spatial structure of populations. J. Anim. Ecol. 1999;68:647–657. doi:10.1046/j.1365-2656.1999.00330.x [Google Scholar]
- Tscharntke T. Parasitoid populations in agricultural landscapes. In: Hochberg M.E, Ives A.R, editors. Parasitoid population biology. Princeton University Press; Princeton, NJ: 2000. pp. 235–253. [Google Scholar]
- Tscharntke T, Brandl R. Plant–insect interactions in fragmented landscapes. Annu. Rev. Entomol. 2004;49:405–430. doi: 10.1146/annurev.ento.49.061802.123339. doi:10.1146/annurev.ento.49.061802.123339 [DOI] [PubMed] [Google Scholar]
- Tscharntke T, Steffan-Dewenter I, Kruess A, Thies C. Contribution of small habitat fragments to conservation of insect communities of grassland–cropland landscapes. Ecol. Appl. 2002;12:354–363. [Google Scholar]
- Turner M.G. Landscape ecology: the effect of pattern on process. Annu. Rev. Ecol. Syst. 1989;20:171–197. doi:10.1146/annurev.es.20.110189.001131 [Google Scholar]
- Tylianakis J.M, Didham R.K, Wratten S.D. Improved fitness of aphid parasitoids receiving resource subsidies. Ecology. 2004;85:658–666. [Google Scholar]
- van der Meijden E, van der Veen-van Wijk C.A.M. Tritrophic metapopulation dynamics: a case study of ragwort, the cinnabar moth, and the parasitoid Cotesia popularis. In: Hanski I, Gilpin M.E, editors. Metapopulation biology. Academic Press; San Diego: 1997. pp. 387–405. [Google Scholar]
- van Nouhuys S, Ehrnsten J. Wasp behavior leads to uniform parasitism of a host available only a few hours per year. Behav. Ecol. 2004;15:661–665. doi:10.1093/beheco/arh059 [Google Scholar]
- van Nouhuys S, Hanski I. Host diet affects extinctions and colonizations in a parasitoid metapopulation. J. Anim. Ecol. 1999;68:1248–1258. doi:10.1046/j.1365-2656.1999.00365.x [Google Scholar]
- van Nouhuys S, Hanski I. Colonization rates and distances of a host butterfly and two specific parasitoids in a fragmented landscape. J. Anim. Ecol. 2002;71:639–650. doi:10.1046/j.1365-2656.2002.00627.x [Google Scholar]
- van Nouhuys S, Tay W.T. Causes and consequences of small population size for a specialist parasitoid wasp. Oecologia. 2001;128:126–133. doi: 10.1007/s004420100635. doi:10.1007/s004420100635 [DOI] [PubMed] [Google Scholar]
- Walde S.J. Immigration and the dynamics of a predator–prey interaction in biological control. J. Anim. Ecol. 1994;63:337–346. [Google Scholar]
- Weisser W.W. Metapopulation dynamics in an aphid–parasitoid system. Entomol. Exp. Appl. 2000;97:83–92. doi:10.1023/A:1004096312093 [Google Scholar]
- Wiens J.A. Metapopulation dynamics and landscape ecology. In: Hanski I, Gilpin M.E, editors. Metapopulation biology. Academic Press; San Diego, CA: 1997. pp. 43–62. [Google Scholar]
- Wilson H.B, Hassell M.P. Host–parasitoid spatial models: the interplay of demographic stochasticity and dynamics. Proc. R. Soc. B. 1997;264:1189–1195. doi:10.1098/rspb.1997.0164 [Google Scholar]
- With K.A. Metapopulation dynamics: perspectives from landscape ecology. In: Hanski I, Gaggiotti O.E, editors. Ecology, genetics, and evolution of metapopulations. Elsevier Academic Press; Boston, MA: 2004. pp. 23–44. [Google Scholar]
- With K.A, Crist T.O. Critical thresholds in species' responses to landscape structure. Ecology. 1995;76:2446–2459. [Google Scholar]