Abstract
Field studies of mechanisms involved in population regulation have tended to focus on the roles of either intrinsic or extrinsic factors, but these are rarely mutually exclusive and their interactions can be crucial in determining dynamics. Experiments on red grouse Lagopus lagopus scoticus have shown that population instability can be caused both by the effects of a parasitic nematode, Trichostrongylus tenuis, on host production or by changes in testosterone influencing aggressive behaviour and recruitment. We experimentally tested for an interaction between testosterone and T. tenuis in free-living male grouse. A total of 123 grouse were caught in autumn, treated with an anthelmintic to remove parasites, and then given either testosterone or empty, control, implants. After one month grouse were re-infected with a standard dose of parasites. We show that males with increased testosterone levels had greater parasite intensities than controls after one year. We discuss possible physiological and behavioural mechanisms linking testosterone and increased parasite intensity, and the implications for our understanding of complex, unstable population dynamics.
Keywords: red grouse, population cycles, aggressiveness, testosterone, nematode, interactions
1. Introduction
A central issue in studies of population dynamics lies in identifying the mechanisms involved in regulation. Considerable research effort has focused on populations exhibiting unstable dynamics and workers have applied modelling, time-series analysis and experimentation to identify the putative mechanisms (see Berryman 2003; Turchin 2003). Recent modelling and time-series analyses have shown that a combination of direct and delayed density dependence is part of the signature of multi-annual cycles and that interactions between mechanisms may generate a wide range of dynamical outcomes (e.g. Lafferty & Holt 2003; Packer et al. 2003; Turchin 2003). However, relatively few field studies have considered how different mechanisms interact to influence dynamics (Krebs et al. 1995; Hansson 1999; Klemola et al. 2000). This is highlighted by the considerable effort devoted to exploring the relative roles of intrinsic and extrinsic factors in population cycles (e.g. Stenseth 1999; Moss & Watson 2001; Berryman 2003; Turchin 2003). These reviews illustrate that researchers have tended to concentrate either on extrinsic or intrinsic factors, but potential interactions between them have rarely been explored experimentally in the field.
Red grouse (Lagopus lagopus scoticus) have proved an ideal species to investigate the role of both extrinsic and intrinsic mechanisms in driving cycles. There are long and replicated time-series of abundance, individuals can be captured, marked and observed and some of the main processes can be manipulated experimentally in the field. Large scale, population level experiments are achievable and have focused on the role of food quality (Watson et al. 1984), population density (Moss et al. 1996), parasites (Hudson et al. 1998; Laurenson et al. 2003) and aggressiveness (Moss et al. 1994; Mougeot et al. 2003a,b) in relation to population dynamics. This work has led to research on the two factors most likely responsible for red grouse population cycles; the effects of parasitism on breeding productivity and of aggressiveness on recruitment into the territorial population (Hudson et al. 1992; Moss et al. 1996; Moss & Watson 2001; Hudson et al. 2002). How these two mechanisms interact to influence recruitment, however, is not clearly understood.
Within red grouse there is evidence that increased Trichostrongylus tenuis intensities can reduce aggressiveness (Fox & Hudson 2001, Mougeot et al. in press). In this paper we consider the alternative hypothesis that elevated testosterone increases parasite intensities. Work on parasite-mediated sexual selection has highlighted a role for testosterone in reducing immunocompetence and increasing susceptibility to parasites (Folstad & Karter 1992). In grouse, there is evidence that testosterone enhances aggressive behaviour and reduces population density in autumn (Mougeot et al. 2003a,b, 2005a). Moreover, testosterone has immuno-suppressive effects (Mougeot et al. 2004) and so it could also lead to increased parasite intensities (Folstad & Karter 1992; Hughes & Randolph 2001). In this paper we test the hypothesis that elevated testosterone levels lead to higher parasite intensities in individual male red grouse. If this is the case, then the delayed dependent changes in testosterone could have effects on subsequent parasite intensities and thereby affect recruitment, which is the main cause of population change in this species (Moss & Watson 2001).
We manipulated testosterone in grouse using implants while standardizing initial parasite intensities in all birds. Grouse establish territories in autumn and levels of testosterone at this time of year influence aggressiveness and subsequent breeding density (Mougeot et al. 2003a,b, 2005a). We thus timed our experiment to coincide with this period of territory establishment.
2. Material and methods
(a) Study areas and experimental protocol
This experiment was conducted on three sites in northeast Scotland during 2000/2001 (Edinglassie, Invermark and Invercauld estates) and repeated on three sites in northern England during 2002/2003 (Feldom, Catterick and Moorhouse). A total of 123 males were used in this experiment: 58 in Scotland and 65 in England. Males were caught in autumn t, treated with an anthelmintic to remove parasites, implanted with either testosterone or empty implants (control), at random, and re-caught one month later and re-infected with a standard dose of parasites. Males were re-caught twice over the following year (spring t+1 and autumn t+1), boxed and faecal samples taken to estimate their intensity of infection.
Grouse were captured at night using standard lamping techniques (Hudson & Newborn 1995). On first capture (4th September–16th October), males were aged as either young (<1 year) or old from plumage and morphology (Hudson & Newborn 1995), ringed and fitted with a radio-collar (TW3-necklace radio-tags, Biotrack). All birds were implanted with two silastic tubes (each one 20 mm long, 0.62 mm of inner and 0.95 mm of outer diameter) sealed with silastic glue. Implants were inserted between skin and breast muscles on the flank, under local anaesthesia and were either empty (control males) or filled with crystalline testosterone proprionate (Sigma Aldrich Co Ltd, Poole, Dorset, UK). The length of the tubing was determined during trials on captive grouse so that testosterone implants would last for up to three months.
In the morning following first capture, birds were orally treated with the broad-spectrum anthelmintic, Levamisole hydrochloride, to kill gastrointestinal nematodes. This technique is highly effective at removing T. tenuis (Hudson 1986, Mougeot & Redpath 2004). Males were re-caught after one month and challenged with ca 3000 T. tenuis infective larvae, presented through a single oral dose, before being released. This procedure aimed to standardize parasite infections between males at the start of the experiment. Details on the method for cultivating, counting and storing infective T. tenuis larvae are given in Shaw (1988).
(b) Parasite counts
T. tenuis infections of males were determined in autumn t (prior to treatment), spring t+1 (19th March–31st April) and autumn t+1 (4–25th September). We used either faecal egg counts, from samples collected from live grouse, or direct worm counts, from guts of euthanized or shot males (Seivwright et al. 2004). Direct worm counts are most accurate, but faecal egg concentrations provide reliable estimates of parasite intensity in spring and autumn (Seivwright et al. 2004). To standardize parasite intensities, we used the number of worms per host, estimated from faecal egg concentrations where necessary, using the equations provided by Seivwright et al. (2004).
(c) Testosterone assays
We collected plasma samples for testosterone assays from birds sampled from the Scottish sites only, in 2000–2001. At capture, approximately 0.5–1 ml of blood was taken from the brachial vein and stored in heparinized, haematocrit capillary tubes. Blood samples were centrifuged on site for 5 min at 5000 rpm, plasma was separated from the packed cells, stored in a cold box, taken to the laboratory within 3 h after collection and kept frozen at −40 °C for subsequent analyses. Within two months of collection, plasma testosterone concentrations were measured using a direct double antibody radio-immuno-assay. Duplicate 20 μl plasma samples were assayed. The standards, serially diluted in charcoal-stripped chicken serum, were assayed in triplicate. Both unknown samples and standards were heated to 80 °C for 2 min to denature binding proteins. The primary antibody (8680-1419 Biogenesis, Poole, UK) was used at a dilution of 1:3500 and the tracer was [1,2,6,7-3H] testosterone (Amersham Pharmacia Biotech, Bucks, UK). After 24 h incubation the second antibody (donkey anti-rabbit) was added and bound and free hormone were separated after a further 24 h by centrifugation at 5000g. The sensitivity of the assay was 0.2 nmol l−1. Intra- and inter-assay coefficients of variance were 8.2 and 12.4%, respectively.
(d) Statistical analyses
We used SAS v. 8.02 for all analyses. Data were unbalanced as some individuals died during the experiment and not all individuals or variables were measured at each stage. Testosterone concentration data were log transformed (loge) for normalization and were fitted to models using a normal error distribution and an identity link function (General Linear Mixed Models; SAS 2001). Site was included as a random effect and models that examined changes over time also included individual nested within site as a random effect. Parasite data (number of T. tenuis worms) were aggregated, and are expressed as geometric means ×/÷ Standard Deviation. Parasite intensities (worms per host) were fitted to models using a negative binomial error distribution and a log link function (Genmod procedure; SAS 2001). Worm intensity was log-transformed (loge worms +1) when included in models as explanatory variable.
3. Results
(a) Effects of treatment on plasma testosterone levels
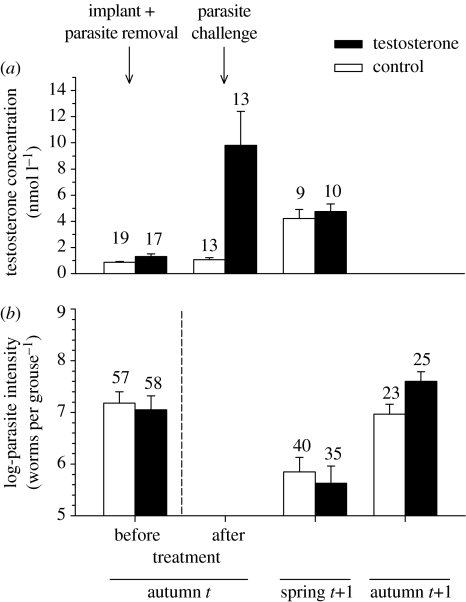
Prior to implant, in autumn t, plasma testosterone levels in Scottish birds did not differ between age groups (F1,31=1.28; p=0.27) or between treatment groups (F1,31=2.29; p=0.14; figure 1a). One month after implanting, testosterone levels increased significantly and differed between treatment groups (F1,22=118.82, p<0.001; figure 1a). The large increase seen in testosterone treated males over that month (F1,7=79.48, p<0.001) was not observed in the control males, although there was a tendency for them to have more (F1,11=4.18, p=0.07; figure 1a). In spring, six months after hormone treatment, testosterone levels did not differ between treatment and control groups (F1,15=0.01, p=0.91; figure 1a), but were significantly higher than they had been at the start of the experiment in both groups (testosterone males: F1,4=44.44, p<0.01; control males F1,6=108.19, p<0.001).
Figure 1.
Variation in time (autumn t, spring t+1 and autumn t+1) and according to treatment (open bars: control; black bars; testosterone treated) in mean±SED. (a) Levels of circulating testosterone, in nmol l−1, and (b) parasite intensity (log-number of T. tenuis worms per grouse). Sample size, above bars, refers to number of males. Based on raw data.
(b) Effects of treatment on parasite infection
At the time of initial capture, T. tenuis intensities varied significantly between sites (Genmod: F5,107=24.97; p<0.001) and age groups (F1,107=13.80; p<0.001; older birds had more parasites), but did not differ between control males and testosterone treated males (F1,107=0.82; p=0.36; figure 1b). Prior to manipulation, T. tenuis intensity was independent of plasma testosterone concentration (model controlling for site and age: F1,28=0.01; p=0.96).
In spring, parasite intensity differed between sites (F5,68=18.22; p<0.01) but did not differ between treatment groups (F1,68=0.08; p=0.77; figure 1b). However, parasite intensity increased during spring, from March to April, and this increase differed between treatment groups (model controlling for site; month: F1,63=3.98; p=0.05; treatment F1,63=4.78; p<0.05; month × treatment: F1,63=4.15; p<0.05). In control males, parasite intensity decreased from a geometric mean of 547 (×/÷1.44) worms in March to 242 (×/÷1.89) worms in April. In testosterone males, parasite intensity increased during spring, from a geometric mean of 172 (×/÷2.05) worms per grouse in March to 512 (×/÷1.66) worms in April.
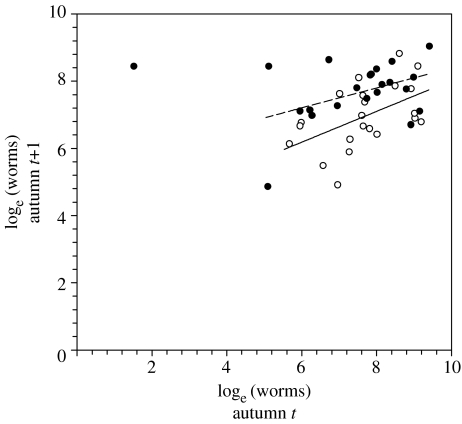
In autumn t+1, T. tenuis intensities did not differ between sites (F5,41=3.67; p=0.60) but differed between treatment groups (F1,41=3.98; p<0.05). Testosterone implanted males had about twice as many worms as controls (geometric means of 1069×/÷0.93 worms, n=23, and of 2013×/÷0.93, n=25, for control and testosterone males, respectively; figure 1b). Variation in T. tenuis intensity in autumn t+1 was also significantly explained by initial parasite intensity (parasite intensity before parasite removal and challenge) and by the interaction between initial parasite intensity and testosterone treatment (Genmod: site: F5,35=0.91; p=0.47; initial worm burden: F1,35=0.83; p=0.36; treatment: F1,35=5.78; p<0.05; interaction F1,35=5.04; p<0.05; figure 2). When excluding an obvious outlier (figure 2), variation in parasite intensity in autumn t+1 was explained by initial intensity (F1,34=6.07; p<0.05) and treatment (F1,34=6.41; p<0.05), but was no longer explained by the interaction between initial intensity and treatment (F1,34=1.59; p=0.20).
Figure 2.
Relationship between initial parasite intensity (autumn t, prior to implant, parasite removal and challenge) and parasite intensity in the next year (autumn t+1) in testosterone treated (filled circle; dashed line) and control males (open circle; solid line). The regression line for testosterone treated males is that obtained when excluding an obvious outlier (X=1.52; Y=8.44; top left corner of the graph).
4. Discussion
In red grouse, high testosterone in autumn resulted in increased parasite intensity one year later. This is an important new finding since it shows that the intrinsic and extrinsic factors capable of causing unstable dynamics in red grouse populations interact within individuals.
Parasite intensities did not differ between treatment groups in spring, six months after implanting testosterone. Mougeot et al. (2004) also found no response in T. tenuis levels one month after implanting with testosterone in autumn. We suspect that the delay in the response time may be due to a seasonal effect, as there is little recruitment to the adult worm population during the winter months (Hudson & Dobson 1995). Ingested T. tenuis larvae arrest their development in late autumn or winter and the re-emergence of arrested larvae accounts for the increased recruitment into the adult worm population in the following spring (Shaw 1988). The recorded time of de-arrestment varies from February to April (Moss et al. 1993; Hudson & Dobson 1997), so we might not have detected differences because we sampled before de-arrestment. We did, however, detect an increase in parasite intensity during spring that was greater in testosterone treated males than in control males. This is consistent with the idea that testosterone treated males then had more arrested larvae developing into worms than control males.
In control males, parasite intensity in autumn t+1 was positively correlated with that in the previous autumn, despite treatment with anthelmintic and re-infection with a constant number of infective stages. This agrees with previous observations that grouse develop little acquired immunity to T. tenuis (Shaw & Moss 1989; Hudson & Dobson 1997). It also suggests that there is considerable variation between individuals in either their susceptibility or exposure to this parasite. Elevated testosterone appeared to have a larger effect on parasite intensities in those birds with relatively few worms at the start of the experiment, but this finding depended on the effect of an outlier, and was thus not robust. Our experiment showed that parasite intensity after a year was explained by previous parasite intensities, but was greater than expected from previous intensities in testosterone treated males.
There are two broad, non-exclusive hypotheses to explain why testosterone leads to higher parasite intensities, one related to susceptibility and one to exposure. First, if testosterone were immuno-suppressive, then increased testosterone would increase susceptibility to infection (Hillgarth & Wingfield 1997). This hypothesis is supported by a growing body of evidence in birds (e.g. Zuk et al. 1995; Verhulst et al. 1999; Duffy et al. 2000; Peters 2000). Indeed our own work has shown that male grouse with experimentally elevated testosterone had reduced cell-mediated immunity after one month (Mougeot et al. 2004). As grouse show little evidence of acquired adaptive immunity this suggests that elevated testosterone might interact with innate immunity by influencing complement production, cytokine production or simply the production of mucus (Onah & Nawa 2000). Alternatively, susceptibility may be increased by resources being allocated away from parasite defence to territorial behaviour (e.g. Sheldon & Verhulst 1996).
Second, the alternative hypothesis to testosterone increasing susceptibility is high testosterone leading to behavioural changes that increase an individual's exposure to parasite infective stages (Hughes & Randolph 2001). Grouse with high levels of testosterone lost condition faster, attracted more females than control birds and defended larger territories (Moss et al. 1994; Mougeot et al. 2004; Redpath et al. in press). These changes may have led to increased exposure to infective larvae of the parasite through increased feeding rates, or increased exposure to larvae from competitors or females.
We cannot, yet, clearly distinguish between these hypotheses or identify the relative roles of susceptibility and exposure. However, irrespective of the precise mechanism these results have important implications. Previous studies of red grouse have focused on the instability in population dynamics caused by either parasitic infections on host breeding production or aggressiveness (e.g. Hudson et al. 1998; Moss & Watson 2001). It is clear from the present study and others (Fox & Hudson 2001; Mougeot et al. 2005b) that these processes interact within individuals and that these interactions appear to work in both directions; parasites limit aspects of aggressiveness and testosterone leads to higher parasite intensity.
Interactions between these two destabilizing mechanisms may affect the dynamics of cyclic populations. High aggressiveness can lead to population declines and reduced recruitment (Mougeot et al. 2003a,b). The interaction with parasites may further increase the rate of decline by leading to higher worm intensities, thereby reducing productivity and increasing mortality (Hudson et al. 2002). By increasing parasite intensities, elevated testosterone may also reduce aggregation of T. tenuis, causing further instability (Dobson & Hudson 1992; Jaenike 1996). Furthermore, the seasonality in this system and the time delay between high aggressiveness and increased parasite intensities will further destabilize the system (Hudson et al. 2002). Given that climate is an important feature influencing parasite transmission rates (Hudson et al. 1992), it seems plausible that these additive effects may be particularly apparent in years when climate favours high parasite transmission rates (Hudson et al. 1992, Cattadori et al. 2005). In contrast, in sites where parasites dominate, high parasite intensities may reduce aggressiveness, aiding recruitment of new individuals in subsequent years. Thus, it is plausible that these interactions may influence the amplitude and period of cycles. As Stenseth et al. (1996) pointed out for microtine cycles, intrinsic and extrinsic processes are intertwined. The challenge now is to explore the dynamical consequences of these interactions through modelling and further experimentation.
Acknowledgments
We are grateful to the landowners and keepers of all estates. Particular thanks are due to: D. Calder, J. Davidson, D. Caithness, Major T.P.J. Helps, C. McCarthy and J. Adamson for their help with organizing the fieldwork; B. Arroyo, R. Cox, J. Millan, S. Evans and David Tidhar for their help with the fieldwork. We would like to thank Andy Dobson for stimulating discussions. Matthew Evans kindly provided advice and assistance on implanting and Alistair Dawson undertook measures of circulating testosterone. Robert Moss provided helpful comments on the manuscript. This work was funded by a NERC grant (NER/A/S/1999/00074) and carried out under Home Office licence PPL 80/1437.
References
- Berryman A.A. On principles, laws and theory in population ecology. Oikos. 2003;103:695–701. doi:10.1034/j.1600-0706.2003.12810.x [Google Scholar]
- Cattadori I.M, Haydon D.T, Hudson P.J. Parasites and climate synchronize red grouse populations. Nature. 2005;433:737–741. doi: 10.1038/nature03276. doi:10.1038/nature03276 [DOI] [PubMed] [Google Scholar]
- Dobson A.P, Hudson P.J. Regulation and stability of a free-living host-parasite system, Trichostrongylus tenuis in red grouse II. Population models. J. Anim. Ecol. 1992;61:487–498. [Google Scholar]
- Duffy D.L, Bentley G.E, Drazen D.L, Ball G.F. Effects of testosterone on cell-mediated and humoral immunity in non-breeding adult European starlings. Behav. Ecol. 2000;11:654–662. doi:10.1093/beheco/11.6.654 [Google Scholar]
- Folstad I, Karter A.J. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 1992;139:603–622. doi:10.1086/285346 [Google Scholar]
- Fox A, Hudson P.J. Parasites reduce territorial behaviour in red grouse (Lagopus lagopus scoticus) Ecol. Lett. 2001;4:139–143. doi:10.1046/j.1461-0248.2001.00207.x [Google Scholar]
- Hansson L. Intraspecific variation in dynamics: small rodents between food and predation in changing landscapes. Oikos. 1999;86:159–169. [Google Scholar]
- Hillgarth N, Wingfield J.C. Parasite-mediated sexual selection: endocrine aspects. In: Clayton D.H, Moore J, editors. Host–parasite evolution. general principles & avian models. Oxford University Press; Oxford: 1997. pp. 78–104. [Google Scholar]
- Hudson P.J. The effect of a parasitic nematode on the breeding production of red grouse. J. Anim. Ecol. 1986;55:85–92. [Google Scholar]
- Hudson P.J, Dobson A.P. Macroparasites: observed patterns in naturally fluctuating animal populations. In: Grenfell B.T, Dobson A.P, editors. Ecology of infectious diseases in natural populations. Cambridge University Press; Cambridge: 1995. pp. 144–176. [Google Scholar]
- Hudson P.J, Dobson A.P. Transmission dynamics and host-parasite interactions of Trichostrongylus tenuis in red grouse. J. Parasitol. 1997;83:194–202. [PubMed] [Google Scholar]
- Hudson P.J, Newborn D. Game Conservancy Trust; Fordingbridge: 1995. A manual of red grouse and Moorland management. [Google Scholar]
- Hudson P.J, Newborn D, Dobson A.P. Regulation and stability of a free-living host-parasite system: Trichostrongylus tenuis in red grouse. I. Monitoring and parasite reduction experiments. J. Anim. Ecol. 1992;61:477–486. [Google Scholar]
- Hudson P.J, Dobson A.P, Newborn D. Prevention of population cycles by parasite removal. Science. 1998;282:2256–2258. doi: 10.1126/science.282.5397.2256. doi:10.1126/science.282.5397.2256 [DOI] [PubMed] [Google Scholar]
- Hudson P.J, Dobson A.P, Cattadori I.M, Newborn D, Haydon D, Shaw D.J, Benton T.G, Grenfell B.T. Trophic interactions and population growth rates: describing patterns and identifying mechanisms. Phil. Trans. R. Soc. B. 2002;357:1259–1271. doi: 10.1098/rstb.2002.1126. doi:10.1098/rstb.2002.1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes V.L, Randolph S.E. Testosterone depresses innate and acquired resistance to ticks in natural rodent hosts: a force for aggregated distributions of parasites. J. Parasitol. 2001;87:49–54. doi: 10.1645/0022-3395(2001)087[0049:TDIAAR]2.0.CO;2. doi:10.1007/s004360000287 [DOI] [PubMed] [Google Scholar]
- Jaenike J. Population-level consequences of parasite aggregation. Oikos. 1996;76:155–160. [Google Scholar]
- Klemola T, Koivula M, Korpimaki E, Norrdahl K. Experimental tests of predation and food hypotheses for population cycles of voles. Proc. R. Soc. B. 2000;267:351–356. doi: 10.1098/rspb.2000.1008. doi:10.1098/rspb.2000.1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs C.J, Boutin S, Boonstra R, Sinclair A.R.E, Smith J.N.M, Dale M.R.T, Martin K, Turkington R. Impact of food and predation on the snowshoe hare cycle. Science. 1995;269:1112–1115. doi: 10.1126/science.269.5227.1112. [DOI] [PubMed] [Google Scholar]
- Lafferty K.D, Holt R.D. How should environmental stress affect the population dynamics of disease. Ecol. Lett. 2003;6:654–664. doi:10.1046/j.1461-0248.2003.00480.x [Google Scholar]
- Laurenson M.K, Norman R.A, Gilbert L, Reid H.W, Hudson P.J. Identifying disease reservoirs in complex systems: mountain hares as reservoirs of ticks and louping-ill virus, pathogens of red grouse. J. Anim. Ecol. 2003;72:177–185. doi:10.1046/j.1365-2656.2003.00688.x [Google Scholar]
- Moss R, Watson A. Population cycles in birds of the grouse family (Tetraonidae) Adv. Ecol. Res. 2001;32:53–110. doi:full_text [Google Scholar]
- Moss R, Watson A, Trenholm I.B, Parr R. Caecal threadworms Trichostrongylus tenuis in red grouse Lagopus lagopus scoticus: effects of weather and host density upon estimated worm burdens. Parasitology. 1993;107:119–209. doi: 10.1017/s0031182000067317. [DOI] [PubMed] [Google Scholar]
- Moss R, Parr R, Lambin X. Effects of testosterone on breeding density, breeding success and survival of red grouse. Proc. R. Soc. B. 1994;258:175–180. [Google Scholar]
- Moss R, Watson A, Parr R. Experimental prevention of a population cycle in red grouse. Ecology. 1996;77:1512–1530. [Google Scholar]
- Mougeot F, Redpath S.M. Sexual ornamentation relates to immune function in male red grouse Lagopus lagopus scoticus. J. Avian Biol. 2004;35:425–433. [Google Scholar]
- Mougeot F, Redpath S.M, Leckie F, Hudson P.J. The effect of aggressiveness behaviour on the population dynamics of a territorial bird. Nature. 2003a;421:737–739. doi: 10.1038/nature01395. doi:10.1038/nature01395 [DOI] [PubMed] [Google Scholar]
- Mougeot F, Redpath S.M, Moss R, Matthiopoulos J, Hudson P.J. Territorial behaviour and population dynamics in red grouse Lagopus lagopus scoticus I. Population experiments. J. Anim. Ecol. 2003b;72:1073–1082. doi:10.1046/j.1365-2656.2003.00781.x [Google Scholar]
- Mougeot F, Irvine J.R, Seivwright L.J, Redpath S.M, Piertney S. Testosterone, immunocompetence and honest sexual signalling in male red grouse. Behav. Ecol. 2004;15:630–637. doi:10.1093/beheco/arh087 [Google Scholar]
- Mougeot F, Dawson A, Redpath S.M, Leckie F. Testosterone and autumn territorial behaviour in male red grouse Lagopus lagopus scoticus. Horm. Behav. 2005a;47:576–584. doi: 10.1016/j.yhbeh.2004.11.021. doi:10.1016/j.yhbeh.2004.11.021 [DOI] [PubMed] [Google Scholar]
- Mougeot F, Evans S.A, Redpath S.M. Interactions between population processes in a cyclic species: parasites reduce autumn territorial behaviour of male red grouse. Oecologia. 2005b;144:289–298. doi: 10.1007/s00442-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Onah D.N, Nawa Y. Mucosal immunity against parasitic gastrointestinal nematodes. Kor. J. Parasitol. 2000;38:209–236. doi: 10.3347/kjp.2000.38.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer C, Holt R.D, Hudson P.J, Lafferty K.D, Dobson A.P. Keeping the heads healthy and alert: implications of predator control for infectious disease. Ecol. Lett. 2003;6:797–802. doi:10.1046/j.1461-0248.2003.00500.x [Google Scholar]
- Peters A. Testosterone treatment is immunosuppressive in superb fairy-wrens, yet free-living males with high testosterone are more immunocompetent. Proc. R. Soc. B. 2000;267:883–889. doi: 10.1098/rspb.2000.1085. doi:10.1098/rspb.2000.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath, S. M., Mougeot, F., Leckie, F. & Evans, S. A. In press. The effect of testosterone on survival and productivity in red grouse. Anim. Behav.
- SAS Institute. SAS Institute Inc.; Cary, NC: 2001. SAS/STAT users' guide, Version 8.01. [Google Scholar]
- Seivwright L.J, Redpath S.M, Mougeot F, Watt L, Hudson P.J. Faecal egg counts provide a reliable measure of Trichostrongylus tenuis intensities in free-living red grouse Lagopus lagopus scoticus. J. Helminthol. 2004;78:69–76. doi: 10.1079/joh2003220. doi:10.1079/JOH2003220 [DOI] [PubMed] [Google Scholar]
- Shaw J.L. Arrested development of Trichostrongylus tenuis as third stage larvae in red grouse. Res. Vet. Sci. 1988;45:256–258. [PubMed] [Google Scholar]
- Shaw J.L, Moss R. Factors affecting the establishment threadworm Trichostrongylus tenuis in red grouse (Lagopus lagopus scoticus) Parasitology. 1989;99:259–264. doi: 10.1017/s0031182000058716. [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. doi:10.1016/0169-5347(96)10039-2 [DOI] [PubMed] [Google Scholar]
- Stenseth N.Chr. Population cycles in voles and lemmings: density dependence and phase dependence in a stochastic world. Oikos. 1999;87:427–461. [Google Scholar]
- Stenseth N.Chr, Bjornstad O.N, Flack W. Is spacing behaviour coupled with predation causing the microtine density cycle? A synthesis of current process-orientated and pattern-orientated studies. Proc. R. Soc. B. 1996;263:1423–1435. doi: 10.1098/rspb.1996.0208. [DOI] [PubMed] [Google Scholar]
- Turchin P. Princeton University Press; Princeton, NJ: 2003. Complex population dynamics: a theoretical/empirical synthesis. [Google Scholar]
- Verhulst S, Dieleman S.J, Parmentier H.K. A tradeoff between immunocompetence and sexual ornamentation in domestic fowl. Proc. Natl Acad. Sci. USA. 1999;96:4478–4481. doi: 10.1073/pnas.96.8.4478. doi:10.1073/pnas.96.8.4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A, Moss R, Parr R. Effects of food enrichment on numbers and spacing behaviour of red grouse. J. Anim. Ecol. 1984;53:663–678. [Google Scholar]
- Zuk M, Johnsen T.S, Maclarty T. Endocrine–immune interactions, ornaments and mate choice in red jungle fowl. Proc. R. Soc. B. 1995;260:205–210. [Google Scholar]