Abstract
During a two year population ecology study in a cave environment, 15 Eurycea (=Typhlotriton) spelaea were observed ingesting bat guano. Furthermore, E. spelaea capture numbers increased significantly during the time that grey bats (Myotis grisescens) deposited fresh guano. We investigated the hypothesis that this behaviour was not incidental to the capture of invertebrate prey, but a diet switch to an energy-rich detritus in an oligotrophic environment. Stable isotope assays determined that guano may be assimilated into salamander muscle tissue, and nutritional analyses revealed that guano is a comparable food source to potential invertebrate prey items. This is the first report of coprophagy in a salamander and in any amphibian for reasons other than intestinal inoculation. Because many temperate subterranean environments are often energy poor and this limitation is thought to select for increased diet breadth, we predict that coprophagy may be common in subterranean vertebrates where it is not currently recognized.
Keywords: Eurycea spelaea, coprophagy, stable isotope analysis, subterranean food web, bat guano, omnivory
1. Introduction
Many temperate subterranean ecosystems are energy limited, and colonial bat guano has been reported to be the dominant energy resource in many cave ecosystems, influencing trophic dynamics, community structure and even physiological specialization (Harris 1970; Poulson 1972; Gnaspini & Trajano 2000). Densities of the cave-adapted salamander Eurycea spelaea (Bonett & Chippindale 2004) have been suspected to increase in the main rooms of some caves during summer months when grey bats (Myotis grisescens) utilize these caves as maternity roosts (Hendricks & Kezer 1958; Brandon 1971). Invertebrate communities associated with bat faeces (guano) increase in density or ‘pulse’ after the bats appear and deposit fresh guano (Poulson & Lavoie 2000). This invertebrate pulse provides a potentially important food resource for salamanders that are understood to be strictly carnivorous and that are living in an oligotrophic environment. For two years, we studied the community ecology of an oligotrophic cave habitat (January-Stansbury Cave, Delaware County, Oklahoma) to investigate the influence of bat guano on the community ecology and trophic dynamics of the system.
To our surprise, during the course of the study we observed 15 larval E. spelaea eating bat guano or regurgitating it upon capture. We initially considered the ingestion of bat guano by E. spelaea to be incidental to capturing invertebrate prey, because consumption of non-food items is known in amphibians. For example, vegetation has been found in the digestive tracts of the carnivorous siren, Siren lacertina (Ultsch 1973). Sirens feed on small aquatic invertebrates by sucking them into their mouths, bringing debris in with the prey. We observed larval E. spelaea employing a suction mechanism to draw prey into their mouths, which would enhance the likelihood of unintentional ingestion of non-target items like a detritus or silt. Silt (i.e. cave stream sediment) was reported to be a major item in the diet of another subterranean salamander, the Florida blind cave salamander (Haideotriton wallacei) (Lee 1969), although another study argued that the ingested silt reflected failed feeding attempts rather than food (Peck 1973). A third cave-adapted salamander, the European olm (Proteus anguinus), has been reported to thrive on a diet of mud and associated microflora (Vandel & Bouillon 1959; Vandel 1964). More specifically to this study, Bogart (J. P. Bogart 1967, unpublished M.A. thesis) and Chippindale (2005) both suspected groundwater salamanders of guano feeding but did not have evidence to document the event. Our repeated observations of larval E. spelaea feeding on bat guano led us to investigate this behaviour as a hypothetically deliberate action and its potential role as a dietary supplement of E. spelaea, utilizing both stable isotope analyses and dietary metrics.
2. Material and methods
January-Stansberry Cave is located 6 km north of the town of Colcord, and is a typical Ozark cave formed from the dissolution of fractures in Mississippian-aged, cherty limestone bedrock of the Boone Formation. The study area was limited to the first 440 m of the cave system (total mapped passage is approximately 1800 m), beginning with the cave mouth where the subterranean stream ‘January River’ resurges and ending in the ‘Moonshine Room’. The average passage dimensions are 5 m wide and 2 m high. Terrestrial habitats within the cave include mud banks, cobble, bedrock, ceiling breakdown, precipitating formations (speleothems) and bat guano piles (ranging in diameter from 3 to 7 and 0.1 to 2 m in depth). A maternity population of approximately 15 000 grey bats (M. grisescens) inhabits the cave from late April to October (Fenolio et al. 2005). January River has an average depth of 1.0 m, but some pools are as deep as 2 m and riffles as shallow as 2 cm; the predominant substrate is chert cobble, but others include clastic sediment and bedrock. Outside of the cave, January River flows 300 m as a surface stream until it joins Spavinaw Creek, a tributary of the Neosho River.
We visually surveyed E. spelaea once a month from September 2001 to October 2003. Visitation was limited by the United States Fish and Wildlife Service because of the federally protected M. grisescens and the presence of a state protected, cave-adapted crayfish Cambarus tartarus. Summer surveys were only conducted at night after the endangered bats had left the cave to forage. Headlamps and hand-held flashlights were used to survey for aquatic larval salamanders and terrestrial adults. Note that E. spelaea has a distinctly biphasic life cycle involving an aquatic larval period of 1–3 years (Brandon 1971; D. C. Rudolph 1980, unpublished Ph.D. dissertation) followed by metamorphosis into a terrestrial adult. Behavioural observations of E. spelaea were recorded on diving slates. We grouped population counts by season (winter—January, February, March; spring—April, May, June; summer—July, August, September; and autumn—October, November, December), and employed the Pearson chi-squared test to test the null hypothesis that salamander counts were evenly distributed by season and by period.
Stable isotope analyses are now widely used in trophic studies of freshwaters (Fry 1999) and cave stream ecosystems (Graening & Brown 2003). Because naturally occurring carbon (13C) and nitrogen (15N) isotopes persist and accumulate in food chains, the technique can be used to decipher both diet and trophic position of a given organism (Peterson & Fry 1987). Mass spectrometry can detect small (one part per thousand—‰), but predictable changes in ratios of carbon and nitrogen stable isotopes as organisms are assimilated into subsequent trophic levels (Gearing 1991); an organism can be linked to its diet by the similarity of stable carbon isotope ratios (13C/12C), and the organism's trophic position can be inferred by the characteristic enrichment of the stable nitrogen isotope (15N/14N) of 3.5‰ per trophic level (DeNiro & Epstein 1981). In January 2003, we collected multiple samples of each of the following materials from the study cave, as described in Graening & Brown (2003): M. grisescens guano (faeces), cave stream sediment; whole bodies of the larvae of E. spelaea; and composite samples of whole amphipods (Gammarus minus) from the system. Samples collected in December 1999 from a second, proximal study site, Logan Cave, were also analysed. Logan Cave (Benton County, Arkansas) occurs in the same geologic formation as January-Stansbury Cave and has a similar cave stream and maternity colony of grey bats. We collected all samples in sterile glass vials with Teflon lids and immediately froze them for transport to the university lab. We then pulverized, freeze-dried, and sieved the samples through a No. 30-mesh screen. Both sample sets were processed using standard methods (France 1996) and analysed at the University of Utah Stable Isotope Ratio Facility for Ecological Research using primary standards (Lajtha & Michener 1994), with low analytical variability (±0.1‰), and reported in standard delta notation (McKinney et al. 1950).
For the nutritional analysis, we collected replicate samples of bat guano from different guano piles in January-Stansberry Cave as well as clastic sediment and whole amphipods (G. minus) from the cave stream. Samples were analysed at the University of Arkansas' Central Analytical Laboratory using a bomb calorimeter for caloric density, a gas chromatograph/Dumas combustion method for crude protein, and an acid digest and inductively coupled plasma/atomic absorption spectroscopy for mineral content.
3. Results
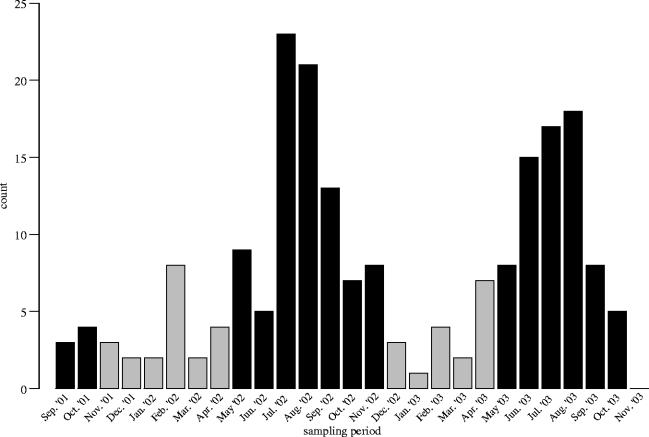
Figure 1 presents the results of the monthly salamander surveys in relation to the presence or absence of the grey bats. Statistical analysis of the data revealed that the surveys were significantly different by season, with spring and summer (the grey bat maternity seasons) having the highest salamander counts (Pearson , p<0.0001).
Figure 1.
Numbers of Eurycea spelaea from September 2001 to October 2003. The black bars indicate the period of time in which the maternity colony of Myotis grisescens occupied the cave and deposited guano into the habitat.
The results of the stable isotope analysis are presented in table 1 using standard delta notation (McKinney et al. 1950). Carbon isotopic signatures (13C/12C) of M. grisescens guano in January-Stansbury Cave and Logan Cave were almost identical, and were very similar to those reported in other studies (Mitzutani et al. 1992). Carbon isotopic signatures of E. spelaea and guano were similar, suggesting that bat guano could be a dietary item of this salamander. Nitrogen stable isotope analysis reinforced this supposition, because the E. spelaea samples were also enriched in 15N compared to the M. grisescens guano in both caves, which places the salamander higher in trophic position.
Table 1.
Stable isotopic signatures of carbon (13C/12C) and nitrogen (15N/14N) of Eurycea spelaea muscle (n=5) and Myotis grisescens guano (n=4) in two similar Ozark cave stream ecosystems, January-Stansbury Cave (Delaware County, Oklahoma) and Logan Cave (Benton County, Arkansas), presented in standard delta notation (δ) on a per mil (‰) basis (Lajtha & Michener 1994).
| sample type | δ15N (‰) | δ13C (‰) |
|---|---|---|
| January-stansbury Cave | ||
| E. spelaea muscle # 1 | 14.3 | −21.8 |
| E. spelaea muscle # 2 | 13.9 | −22.1 |
| E. spelaea muscle # 3 | 11.0 | −25.4 |
| E. spelaea muscle # 4 | 11.5 | −22.8 |
| mean | 12.7 | −23.0 |
| M. grisescens guano # 1 | 11.5 | −24.6 |
| M. grisescens guano # 2 | 13.2 | −22.9 |
| M. grisescens guano # 3 | 9.7 | −25.4 |
| mean | 11.5 | −24.3 |
| Logan Cave | ||
| E. spelaea muscle | 11.0 | −24.0 |
| M. grisescens guano | 9.5 | −24.0 |
Nutritional analyses of bat guano reveal that it contains nutrients roughly equivalent to those that would be found in a potential prey item in this ecosystem, amphipods (table 2). Bat guano's surprisingly high crude protein content (54%), caloric density (4124 cal g−1) and essential mineral content (parts per thousand) exceeded those of gammarid amphipods, a suitable syntopic prey item of larval salamanders. Conversely, cave stream sediment, a far more abundant potential food source, had almost no detectable protein or caloric content.
Table 2.
Nutritional analyses of potential dietary items of Eurycea spelaeus: Myotis grisescens guano, amphipods (Gammarus minus) and cave stream sediment. (Parameters measured (dry matter basis) are mean percentage dry matter content, mean caloric density (cal g−1), mean percentage crude protein, mean percentage crude fat and mineral content (mg kg−1). Nutritional data of a hamburger (McDonald's Corporation Big Mac sandwich) provided for comparison to human diet.)
| grey bat guano | stream amphipods | cave sediment | Big Mac hamburger | |
|---|---|---|---|---|
| sample size (n) | 3 | 2 | 1 | 2 |
| percentage of dry matter | 13 | 14 | 77 | 55 |
| calories (cal g−1) | 4124 | 3600 | <1 | 6139 |
| percentage of protein | 54 | 44 | <1 | 23 |
| percentage of fat | 1 | 8 | <1 | 33 |
| percentage of ash | 15 | — | 97 | 3 |
| minerals (mg kg−1) | ||||
| calcium | 27 552 | 119 221 | 6215 | 1223 |
| copper | 161 | 62 | 15 | 2 |
| iron | 1753 | 320 | 17 321 | 25 |
| magnesium | 2408 | 1356 | 669 | 216 |
| manganese | 169 | 62 | 2247 | — |
| phosphorous | 8951 | 7537 | 1011 | 1499 |
| potassium | 4792 | 11 409 | 1136 | 1988 |
| zinc | 400 | 80 | 55 | 29 |
4. Discussion
Our study demonstrates that E. spelaea numbers increase significantly in the main caverns of the system when and where grey bats deposit fresh guano, and that salamander larvae ingest guano that falls into the cave stream. Terrestrial adults may also utilize guano as a food source because we observed salamanders directly upon guano piles next to the cave stream. Stable isotope analyses indicate that larval E. spelaea may assimilate this guano, and nutritional analyses imply that bat guano could sustain larval E. spelaea if normal prey, such as amphipods, were unavailable. To put the nutritional value of guano into perspective, we concurrently analysed a hamburger with the other samples. Bat guano had nearly twice the crude protein content and almost two-thirds of the calories as the sampled hamburger: McDonald's Corporation Big Mac sandwich contained 23% crude protein and 6139 cal g−1 (dry matter basis). Unlike the faeces of many animals, bat guano is still rich in calories and nutrients. To meet the energetic demands of flight and reproduction, insectivorous bats such as M. grisescens have evolved extremely short digestive tracts and rapid food transit times (Mitzutani et al. 1992). The digestive efficiency of myotid bats is only 69–78%, resulting in the expulsion of unabsorbed nutrients in guano and urine (Webb et al. 1993; Stalinski 1994). Microbial biofilms that form on the guano may boost its nutritional value in much the same way that microbial conditioning of leaves increases their palatability and assimilation by detritivores (Allan 1995). Cummins (1974) used the analogy of enhancing a dry cracker with peanut butter. With regard to amphibians, the notion of deriving nutrition from bacteria is not unknown; Burke (1933) demonstrated that tadpoles could be reared to metamorphosis solely on bacteria.
Our study contradicts the general understanding that salamanders are strictly carnivorous. In fact, coprophagy may be common in subterranean vertebrates. Oligotrophy is thought to select for increased diet breadth in subterranean fauna (Culver 1982, 1994; Holyoak & Sachdev 1998). Other studies determined that Ozark cavefish (Amblyopsis rosae) and black bullhead catfish (Ameiurus melas) feed on bat guano in Ozark caves (Poulson 1963; Black 1971), but the studies did not include nutritional and stable isotope analyses to demonstrate the nutritional benefit and habitual nature of the behaviour. Coprophagy has been reported in larval frogs, but for the purpose of inoculation of their intestines by beneficial microbes (Steinwascher 1978; Beebee 1991; Beebee & Wong 1992). To our knowledge, however, this is both the first reported instance of coprophagy by a salamander and also the first report of its practice by any amphibian for what we believe to be nutritional benefit. In general, the assumption that temperate subterranean vertebrates are strictly carnivores is incorrect, particularly in oligotrophic environments that contain an alternative food resource such as an energy-rich detritus. We predict that other vertebrates inhabiting subterranean environments, currently unknown to consume nutrient-rich detritus, will be found to have shifted their diet from strict carnivory to include coprophagy when faced with starvation in oligotrophic habitats.
Acknowledgments
Funding for this study was provided through the Oklahoma Department of Wildlife Conservation (M. Howery, sponsor), NATURAE—Consultoria Ambiental Ltda., the University of Oklahoma Graduate Student Senate, the Oklahoma Biological Survey, The Department of Zoology, University of Oklahoma and The Nature Conservancy. S. Hensley (US Fish and Wildlife Service) provided access to the cave and assistance in fieldwork. S. Jones, M. Walvoord, C. Deen, M. Gerber, J. Malone, L. Bergey and R. Stark assisted in the field. The following provided constructive criticism in the preparation of this manuscript: E. Bergey, J. Caldwell, M. Conner, C. Deen, S. Green, V. Hutchison, C. Leary, J. Lee, J. O'Reilly, S. Richter, D. Shepard, J. Simmons, L. Sternberg, S. Trauth, C. Vaughn and M. Walvoord. The Texas Agricultural Experiment Station and Texas A&M University System supported B. Collier's contributions to this project. We collected animals under Oklahoma Department of Wildlife Conservation special license number 3086 and University of Oklahoma Animal Care and Use Committee assurance number A3240-01. The authors thank the Fenolio family for their support throughout this project.
References
- Allan J.D. Chapman & Hall; London: 1995. Stream ecology: structure and function of running waters. [Google Scholar]
- Beebee T.J.C. Purification of an agent causing growth inhibition in anuran larvae and its identification as a unicellular unpigmented alga. Can. J. Zool. 1991;69:2146–2153. [Google Scholar]
- Beebee T.J.C, Wong A.L.C. Prototheca-mediated interference competition between anuran larvae operates by resource diversion. Phys. Zool. 1992;65:815–831. [Google Scholar]
- Black J.H. The cave life of Oklahoma. Okla. Underground. 1971;4:2–53. [Google Scholar]
- Bonett R.M, Chippindale P.T. Speciation, phylogeography and evolution of life history and morphology in plethodontid salamanders of the Eurycea multiplicata complex. Mol. Ecol. 2004;13:1189–1203. doi: 10.1111/j.1365-294X.2004.02130.x. doi:10.1111/j.1365-294X.2004.02130.x [DOI] [PubMed] [Google Scholar]
- Brandon R.A. Correlation of seasonal abundance with feeding and reproductive activity in the grotto salamander (Typhlotriton spelaeus) Am. Midl. Nat. 1971;86:93–100. [Google Scholar]
- Burke V. Bacteria as food for vertebrates. Science. 1933;78:194–195. doi: 10.1126/science.78.2018.194. [DOI] [PubMed] [Google Scholar]
- Chippindale P.T. Eurycea tridentifera. In: Lannoo M.J, editor. Declining amphibians: a United States' response to the global phenomenon. University of California Press; Berkeley, CA: 2005. pp. 765–766. [Google Scholar]
- Culver D.C. Harvard University Press; Cambridge, MA: 1982. Cave life: evolution and ecology. [Google Scholar]
- Culver D.C. Species interactions. In: Gibert J, Danielopol D, Stanford J, editors. Groundwater ecology. Academic Press; San Diego, CA: 1994. pp. 271–286. [Google Scholar]
- Cummins K.W. Structure and function of stream ecosystems. Bioscience. 1974;24:631–641. [Google Scholar]
- DeNiro M.J, Epstein S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta. 1981;45:341–351. doi:10.1016/0016-7037(81)90244-1 [Google Scholar]
- Fenolio D.B, Graening G.O, Stout J.F. Seasonal movement pattern of pickerel frogs (Rana palustris) through an Ozark cave and ecological implications supported by stable isotope evidence. Southwest. Nat. 2005;50:385–389. doi:10.1894/00384909(2005)050[0385:SMPOPF]2.0.CO;2 [Google Scholar]
- France R. Carbon-13 conundrums: limitations and cautions in the use of stable isotope analysis in stream ecotonal research. Can. J. Fish. Aquat. Sci. 1996;53:1916–1919. doi:10.1139/cjfas-53-8-1916 [Google Scholar]
- Fry B. Using stable isotopes to monitor watershed influences on aquatic trophodynamics. Can. J. Fish. Aquat. Sci. 1999;56:2167–2171. doi:10.1139/cjfas-56-11-2167 [Google Scholar]
- Gearing J.N. The study of diet and trophic relationships through natural abundance 13C. In: Coleman D.C, Fry B, editors. Carbon isotope techniques. Academic Press; San Diego, CA: 1991. pp. 201–218. [Google Scholar]
- Gnaspini P, Trajano E. Guano communities in tropical caves. In: Wilkens H, Culver D.C, Humphreys W.F, editors. Ecosystems of the world 30: subterranean ecosystems. Elsevier; Amsterdam: 2000. pp. 251–268. [Google Scholar]
- Graening G.O, Brown A.V. Ecosystem dynamics of an Ozark cave stream. J. Am. Water Resour. Assoc. 2003;39:1497–1507. [Google Scholar]
- Harris J.A. Bat-guano cave environment. Science. 1970;169:1342–1343. doi: 10.1126/science.169.3952.1342-a. [DOI] [PubMed] [Google Scholar]
- Hendricks L.J, Kezer J. An unusual population of a blind cave salamander and its fluctuation during one year. Herpetologica. 1958;14:41–43. [Google Scholar]
- Holyoak M, Sachdev S. Omnivory and the stability of simple food webs. Oecologia. 1998;117:413–419. doi: 10.1007/s004420050675. doi:10.1007/s004420050675 [DOI] [PubMed] [Google Scholar]
- Lajtha K, Michener R, editors. Stable isotopes in ecology and environmental science. Blackwell Science Publications; Oxford, UK: 1994. [Google Scholar]
- Lee D.S. A food study of the salamander Haideotriton wallacei Carr. Herpetologica. 1969;25:175–177. [Google Scholar]
- McKinney C, McCrea J, Epstein S, Allen H, Urey H. Improvements in mass spectrometers for the measurement of small differences in isotope abundance ratios. Rev. Sci. Instrum. 1950;21:724–730. doi: 10.1063/1.1745698. doi:10.1063/1.1745698 [DOI] [PubMed] [Google Scholar]
- Mitzutani H, McFarlane D.A, Kabaya Y. Carbon and nitrogen isotopic signatures of bat guanos as record of past environments. Mass Spectrosc. 1992;40:67–82. [Google Scholar]
- Peck S.B. Feeding efficiency in the cave salamander Haideotriton wallacei. Int. J. Speleol. 1973;5:5–15. [Google Scholar]
- Peterson B, Fry B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987;18:293–320. doi:10.1146/annurev.es.18.110187.001453 [Google Scholar]
- Poulson T.L. Cave adaptation in amblyopsid fishes. Am. Midl. Nat. 1963;70:257–291. [Google Scholar]
- Poulson T.L. Bat guano ecosystems. Bull. Natl Speleol. Soc. 1972;34:55–59. [Google Scholar]
- Poulson T.L, Lavoie K.H. The trophic basis of subsurface ecosystems. In: Wilkens H, Culver D.C, Humphreys W.F, editors. Ecosystems of the world 30: subterranean ecosystems. Elsevier; Amsterdam: 2000. pp. 231–249. [Google Scholar]
- Stalinski J. Digestion, defecation and food passage rate in the insectivorous bat Myotis myotis. Acta Theriol. 1994;39:1–11. [Google Scholar]
- Steinwascher K. The effect of coprophagy on the growth of Rana catesbeiana tadpoles. Copeia. 1978;1978:130–134. [Google Scholar]
- Ultsch G.R. Observations on the life history of Siren lacertina. Herpetologica. 1973;29:304–305. [Google Scholar]
- Vandel A. Gauthier-Villars; Paris: 1964. Biospeleologie: la biologie des animaux cavernicoles. [Google Scholar]
- Vandel A, Bouillon M. Le Protee et son interet biologique. Ann. Speleol. 1959;5:369–377. [Google Scholar]
- Webb P.I, Speakman J.R, Racey P.A. Defecation, apparent absorption efficiency, and the importance of water obtained in the food for water balance in captive born long-eared (Plecotus auritus) and Daubenton's (Myotis daubentoni) bats. J. Zool. 1993;230:619–628. [Google Scholar]