Abstract
A climatic regime shift during the mid-1970s in the North Pacific resulted in decreased availability of lipid-rich fish to seabirds and was followed by a dramatic decline in number of kittiwakes breeding on the Pribilof Islands. Although production of chicks in the mid-1970s was adequate to sustain kittiwake populations in the early 1980s, the disappearance of birds from breeding colonies apparently exceeded recruitment. No mechanism has been proposed to explain why recruitment would differ among fledglings fed lipid-rich or lipid-poor fish during development. Here we show that diets low in lipids induce nutritional stress and impair cognitive abilities in young red-legged kittiwakes, Rissa brevirostris. Specifically, growth retardation, increased secretion of stress hormones and inferior ability to associate food distribution with visual cues were observed in individuals fed lipid-poor diets. We conclude that lipid-poor diets during development affect the quality of young seabirds, which is likely to result in their increased mortality and low recruitment.
Keywords: climate change, cognition, corticosterone, kittiwake, nutritional stress
1. Introduction
During the past three decades, precipitous declines in breeding populations of seabirds have occurred at the Pribilof Islands in the southeastern Bering Sea (Hunt & Byrd 1999). Red-legged kittiwakes were affected the most—starting with a sharp decrease in the early 1980s, their populations at the Pribilof Islands have declined by 50%. The cause of this decline is not well understood. It is unlikely that reproductive failure alone was responsible for the decrease in numbers. Long-lived kittiwakes do not recruit to breeding populations until they are 4 years old (Byrd & Williams 1993). Although production of chicks by kittiwakes in the mid-1970s was adequate to sustain their population numbers in the early 1980s, the disappearance of kittiwakes from breeding populations apparently exceeded recruitment (Hunt & Byrd 1999). The decline of kittiwake populations coincided with a climatic regime shift that transpired in the North Pacific and Bering Sea ecosystems in the mid-1970s (Anderson & Piatt 1999; Hare & Mantua 2000). The regime shift resulted in a decreased abundance of lipid-rich forage fish available to kittiwakes nesting at the Pribilof Islands (Decker et al. 1995; Hunt et al. 1996a,b). Thus, population declines may have resulted from a decrease in post-fledging survival of kittiwakes reared when lipid-rich fish were scarce. However, no mechanism has been proposed to explain why recruitment would differ among fledglings fed lipid-rich or lipid-poor fish during development.
Variations in fitness of animals can be related to their nutritional history at the juvenile stage of life (reviewed in Metcalf & Monaghan 2001). Somatic growth and development is an energetically demanding process, and individuals that were nutritionally stressed during development might suffer retarded growth, smaller size and reduced quality as adults (e.g. Boag 1987; Kitaysky 1999; Nowicki et al. 2000; van der Ziel & Visser 2001). Although temporary nutritional deficits during development do not necessarily result in impaired morphological development of individuals later in life (Schew & Ricklefs 1998), other phenotypic, including cognition-based traits that are directly related to fitness of birds, e.g. in passerines, song learning (Nowicki et al. 1998) and hippocampal structure and spatial memory (Pravosudov et al. in press), might be affected. Experimental evidence for this in seabirds is missing. Earlier studies have shown that in seabird chicks nutritional deficits result in an elevated secretion of stress hormones (Kitaysky et al. 1999, 2001a). We have also reported experimental evidence that exogenous stress hormones damage cognitive functions of young seabirds (Kitaysky et al. 2003). However, until this current study direct evidence that nutritional stress per se causes changes in cognition of young seabirds was not available.
Most young seabirds are independent of their parents after fledging and must quickly learn to forage on their own. The distribution of food resources in oceanic environments is patchy (Lack 1968; Hunt & Schneider 1987), which makes the task of learning even more difficult for young, inexperienced birds. Most often, seabirds forage on patches of food concentrated within visually distinctive oceanographic features such as fronts, eddies and upwelling (Hunt & Schneider 1987; Irons 1992). Thus, the ability of the young to associate visual cues (water colour, surface structure and topographic landmarks) with the presence of food is essential for their foraging success and survival. Here we used a colour discrimination paradigm, previously proved to be appropriate for testing seabirds' ability to form associations between cues and presence of food in a choice situation (Kitaysky et al. 2003), to test cognitive functions of birds in relation to their nutritional history during development. We present the first experimental evidence that a temporary nutritional deficit for seabird chicks affects cognitive abilities later in life, which may account for low recruitment of young raised during years with low availability of high-lipid fish.
2. Material and methods
(a) Subjects
A total of 20 red-legged kittiwakes were used, all of which hatched in captivity from partially incubated eggs of free-living kittiwakes collected on St George I. (Pribilof Islands, south-eastern Bering Sea). Chicks were raised in individual ‘nests’ in 12D : 12L photoperiod, at 30 °C and 80% relative humidity (newly hatched to 7 days post-hatch), at 25 °C and 70% relative humidity (7–25 days post-hatch) and at outdoor ambient photoperiod, temperatures and humidity thereafter. All chicks were kept in the same room in physical, but not in visual or acoustic isolation from each other. After fledging (at ca 47 days of age), kittiwakes remained in the same aviary where they had been raised since 25 days post-hatch. During their time in the facilities of University of Washington, kittiwakes were exposed to the same personnel and their contact with other people was limited. When birds were 100 days old, they were donated to the Alaska Sea Life Center (Seward, Alaska) for captive breeding programs and permanent exhibition.
(b) Dietary treatments
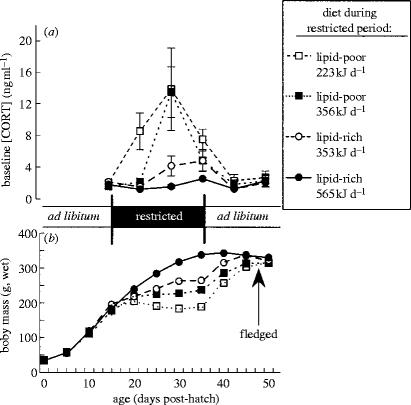
Upon hatching each chick was randomly assigned to one of the four treatments (n=5 individuals in each treatment). All chicks were hand fed a mixture of high-lipid forage fish given ad libitum until two weeks of age. Parent red-legged kittiwakes provide their single chick at the rate of ca 72 g d−1 (an estimate based on data reported in Lance & Roby 1998, 2000). In this study, we experimentally simulated two possible scenarios that were likely to occur at the Pribilof Islands during the regime shift: a decrease in abundance of forage fish; and a decrease in lipid content of fish. Starting at day 15 post-hatch either lipid-rich fish, silverside Menidia menidia (lipid-to-protein ratio, LPR=1.47), or lipid-poor fish, rainbow smelt Osmerus mordax (LPR=0.61) were fed to chicks for 21 days (figure 1a). During this period chicks received either 565 kJ d−1 of lipid-rich fish (80 g d−1, wet fish biomass), 353 kJ d−1 of lipid-rich fish (50 g d−1), 356 kJ d−1 of lipid-poor fish (80 g d−1), or 223 kJ d−1 of lipid-poor fish (50 g d−1). An iso-energetic low-lipid treatment for 80 g silverside was not possible, because the two-week-old red-legged kittiwake chicks physically could not consume 126 g of rainbow smelt per day. Daily food intake of captive chicks fed silverside ad libitum was 84 g d−1, thus, during the food-restricted period chick energy intake was at ca 100, 65 and 40% of the ad libitum ration. Multi-vitamin/mineral supplement was given to all chicks on daily basis to control for nutritional effects other than energy and lipid contents of food. Starting at age 35 days post-hatch and until 47 days post-hatch (average age at fledging), chicks were fed the same lipid-rich or lipid-poor fish, but given ad libitum. Starting at age 47 days post-hatch and until the end of experiments all birds were fed the same lipid-rich fish given ad libitum. Body mass of post-absorptive (after overnight fast) chicks was measured every 5 days. A blood sample of undisturbed chicks (collected immediately after taking a chick from the nest) was taken on a weekly basis starting at the beginning of the experiment. Plasma concentrations of corticosterone were measured with radioimmunoassay (Kitaysky et al. 2001a).
Figure 1.
The effects of diet quality (lipid-rich versus lipid-poor fish) and daily energy intake on: (a) plasma concentrations (mean±s.e.m.) of corticosterone [CORT]; and (b) growth of body mass (mean±s.e.m.) in red-legged kittiwake chicks.
(c) Learning experiment
Animals' motivation or willingness to cooperate during experiments is important for studies of learning behaviour. Hand-rearing guaranteed that the kittiwakes in this experiment were tame and cooperative. During training all birds were habituated to the experimental conditions until they showed no visible signs of fear or anxiety. An isolated kittiwake behaved calmly only if it was able to hear the rest of the birds. Thus, during experiments, the focal bird was visually but not acoustically isolated from the rest of the group.
(i) Apparatus
The experimental room (2.4 m long×1.8 m wide×2 m high) was adjacent to the common aviary. Walls of the room were covered with black tarpaulin to visually isolate experimental animals from an observer and the rest of the group. Transparent and solid-coloured dishes used during experiments (see below) were identical plastic cylinders 9.5 cm in diameter and 1.8 cm in height. Dishes identical in colour and size were used as lids and bottoms. Dishes were placed (20 cm apart) in a chessboard pattern and locations of dishes were fixed during experiments. Dishes were all smeared with fish on the lid to control for possible effects of odour.
(ii) Procedure: learning
During training (days 47–56 post-hatch) and all subsequent experiments, all birds were self-feeding on high-lipid fish provided ad libitum. They were habituated to being picked up by an observer, placed alone in the experimental room, and eating from a transparent dish (with no lid) placed on the floor in the centre of the experimental room. Experimental trials started when kittiwakes were 57 days old. During experimental trials, kittiwakes were post-absorptive (after overnight fast). During a trial, each subject was placed in the experimental room with dishes. First, birds learned to open (by pushing off the lid) a closed transparent dish containing food. Then, presence of food in the dish was associated with the colour of a solid-coloured opaque dish: black dishes contained food (a single 0.5 g piece of silverside in each dish) and white dishes were empty. Kittiwakes exhibited no preference for either colour during a pre-trial with two (one black and one white) solid-coloured dishes (nine individuals first opened a black dish and 11 individuals first opened a white dish). Number of solid-coloured dishes (with equal number of dishes with and without food) increased from 4 to 12 during three consecutive trials (trial 1, four dishes; trial 2, eight dishes; trial 3, 12 dishes).
(iii) Procedure: memory
To test whether kittiwakes remembered the task, we kept birds in the common aviary without exposure to dishes for 7 days after the last learning trial (trial 3) and then ran the 12-dish trial again (trial 4). Finally, because the location of dishes was fixed, birds could have memorized the location of dishes with food rather than have learned to associate the colour with dish content. To examine this possibility we changed the position of dishes in a random pattern and repeated the 12-dish trial (trial 5) on the next day.
Each bird participated in an experiment on a daily basis, but no more than a single 10 min trial per day was performed on the same individual. The same personnel conducted trials on all birds. Time was recorded starting immediately after a bird was placed in the experimental room. Behaviour of a bird, number of dishes opened and sequence in which a bird opened dishes were continuously recorded on video camera for later analyses. There were Nt correct dish choices available (Nt=2, 4, 6, 6 and 6 for trials 1–5) in each trial. The success of a bird in learning to associate the colour of a dish with the dish content was defined as a proportion (success=Nc/Nt, where Nc is the number of correct choices in the first Nt choices made). Thus, birds that first opened all dishes containing food received the highest score, regardless of how many dishes they opened in total during a trial. We chose this index because the ability of kittiwakes to make an immediate correct decision is essential for their foraging success. In the wild, kittiwakes forage on highly mobile and elusive fish in the surface layer of the ocean. A prey item is usually available to a kittiwake only for several seconds, and a foraging bout continues for a few minutes.
(d) Statistical analyses
All computations were performed with Systat and Statistica statistical packages. During statistical analyses, data were tested for assumptions required by statistical tests according to Sokal & Rohlf (1981). If data violated assumptions for parametric tests, they were examined with non-parametric equivalents. Statistical significance was assumed at p<0.05 (two-tailed). The effects of dietary treatments on corticosterone concentrations (42 days post-hatch) and body mass (50 days post-hatch) were examined with analysis of variance (ANOVA) and Kruskal–Wallis ANOVA, respectively. The effects of dietary treatment on cognitive abilities were tested using repeated-measures ANOVAs (followed by post hoc LSD planned comparison tests), where treatments during development (high versus low-lipid content of food and daily food intake) were used as factors and consecutive trials as a repeated measure.
3. Results
(a) Effects of temporary nutritional deficit on growth and corticosterone secretion
During food-restricted period, chicks raised on energy-restricted diets grew slowly and increased secretion of the stress hormone corticosterone (figure 1). Among chicks that were fed iso-caloric diets, nutritional stress was manifested more strongly in individuals fed a lipid-poor diet than a lipid-rich diet (figure 1).
Within two weeks after food restriction was lifted, all previously undernourished chicks reached similar body mass as controls (effect of the dietary treatments on body mass at age 50 days post-hatch: ANOVA F3,16=0.933, p=0.448; figure 1b). Their corticosterone secretion also normalized within a week after food restriction was lifted (effect of the dietary treatment on baseline levels of corticosterone at age 42 days post-hatch: Kruskal–Wallis ANOVA , p=0.280; figure 1a).
(b) Effects of temporary nutritional deficit on learning abilities
The energy and lipid contents of fish fed to the chicks during development were both determinants of learning abilities of red-legged kittiwakes (figure 2). All birds successfully learned to open plastic dishes in experimental trials and the total number of dishes they opened did not differ among treatments (repeated-measures ANOVA: effect of high- versus low-lipid content of food F1,16=0.264, p=0.614; daily food intake F1,16=0.949, p=0.344; figure 2a). However, the ability of kittiwakes to associate the colour of a dish with the presence of food decreased proportionally with the magnitude of nutritional stress they experienced during development (repeated-measures ANOVA: effect of high versus low-lipid content of food F1,16=16.390, p=0.001; daily food intake F1,16=5.136, p=0.038; figure 2b). The group which was always fed lipid-rich fish given ad libitum (565 kJ d−1) and had never experienced nutritional deficit learned to associate the colour of a dish with its contents faster than birds in the other treatments. Starting at the second trial of experiment, controls consistently made between 83 and 95% of correct choices among dishes they opened first (figure 2b). Kittiwakes which were fed a restricted amount (353 kJ d−1; 65% of an ad libitum energy intake) of lipid-rich fish during development did not show a significant improvement in their performance until the third experimental trial (figure 2b). The individuals that were fed an iso-caloric amount (356 kJ d−1; at 65% of an ad libitum energy intake) of lipid-poor fish hardly learned the task (figure 2b). Finally, kittiwakes that experienced the most severe nutritional deficit during development (fed 223 kJ d−1 lipid-poor fish at 40% of ad libitum energy intake) did not associate the colours of dishes with the presence of food—their performance did not improve during the experiment at all (figure 2b).
Figure 2.
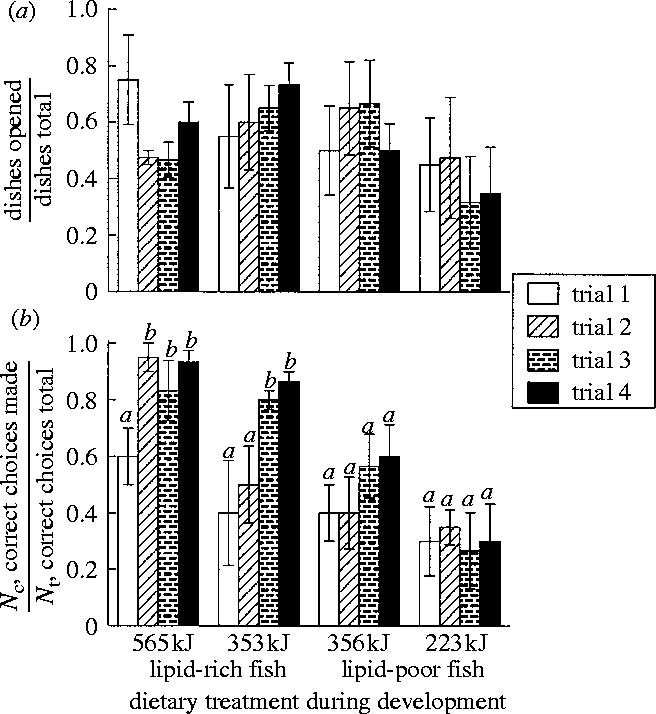
Proportion (mean±s.e.m.) of (a) dishes opened and (b) correct choices made (see §2 for details) per trials 1–4 by two-month-old red-legged kittiwakes in relation to their diet during 15–35 days post-hatch. A significant change in birds' performances (within a treatment) during subsequent trials is indicated by a change of lettering (from a to b) above the s.e.m. bars (corresponding LSD post hoc p-values: p=0.005 and 0.012 for lipid-rich 565 kJ and 353 kJ diet treatments, respectively).
Performance of all kittiwakes (regardless of treatment during development) in the memory trial (trial 4) was not different from their performance in the learning trial (trial 3) conducted 7 days earlier (figure 2b). Also, performance of all individuals in the trial where dishes were arranged in a random pattern (trial 5) was not different from their performance in the memory trial (trial 4; repeated-measures ANOVA on the performances during trials 4 and 5: F1,16=0.769, p=0.393), and all individuals demonstrated a high repeatability in their performance (Pearson correlation on the performance of the individuals during trials 4 and 5: r=0.83, p<0.001, n=20).
4. Discussion
Kittiwake chicks raised on energy-restricted diets grow slowly and increase secretion of the stress hormone corticosterone (Kitaysky et al. 1999, 2001a). A short-term release of corticosterone in hungry chicks allows them to restore depleted energy reserves by facilitating begging for food and, thereby, increasing parental food provisioning (Kitaysky et al. 2001b). However, if parents are not able to increase energy delivery to chicks, elevated secretion of corticosterone becomes chronic (Kitaysky et al. 2001a). Low-lipid content of fish exacerbates these effects: among food-restricted chicks that were fed iso-caloric diets, nutritional stress was manifested more strongly in individuals fed a lipid-poor diet than in those fed a lipid-rich diet (Kitaysky et al. 1999, 2001a, figure 1). The digestive efficiency of growing kittiwakes is higher when they are fed lipid-rich diets compared to lipid-poor diets (Romano 2000), which would explain the differences in growth and corticosterone secretion observed among treatments in this study.
Should foraging conditions improve, however, red-legged kittiwake chicks are able to compensate for temporary nutritional stress with accelerated growth and normalized secretion of corticosterone (figure 1). Thus, red-legged kittiwakes are developmentally plastic and individuals that experienced an episode of nutritional stress during development might be indistinguishable morphologically from individuals that never experienced a food-related stress. However, a temporary nutritional deficit might have long-lasting effect on learning abilities of affected individuals later in life (Nowicki et al. 2000; Pravosudov et al. in press). Young kittiwakes are independent of their parents after fledging and must learn to forage by relying on making associations between visually distinct oceanographic features and the presence of food. Does an episode of nutritional stress and elevated corticosterone secretion during development compromise the ability of newly fledged red-legged kittiwakes to associate visual cues with the presence of food in a choice situation?
We found that the energy and lipid contents of fish consumed by chicks during development are both determinants of learning abilities of newly fledged red-legged kittiwakes (see §3 and figure 2). The ability of kittiwakes to associate the colour of a dish with the presence of food decreased proportionally with the magnitude of nutritional stress they had experienced during development. Striking differences were also found between the two treatments where kittiwakes were restricted at the same level of daily energy intake (65% of an ad libitum energy intake) with either high or low in lipid content. Specifically, kittiwakes which were fed a restricted amount of lipid-rich fish during development showed a significant albeit slow improvement in their performance during the experiment (figure 2b). In contrast to this, the individuals that were fed an iso-energetic amount of lipid-poor fish did not learn the task (figure 2b). The amount of food given to chicks with this lipid-poor diet (80 g d−1) matches or exceeds a maximum capacity in food delivery by parent red-legged kittiwakes in the wild (ca 72 g d−1, an estimate based on data reported in Lance & Roby 1998, 2000). Therefore, it is unlikely that parent kittiwakes could compensate for the decrease in food quality by delivering sufficient amounts of poor-quality fish. Thus, change in diet composition observed in kittiwakes breeding on the Pribilof Islands (Hunt et al. 1996a) has certainly induced nutritional stress in growing red-legged kittiwakes similar to the experimentally induced stress in this study.
Young red-legged kittiwakes remembered the association between the colours and the presence of food for at least one week after the learning part of experiment was completed. Specifically, performance of all birds did not change between the trials 3 and 4 (figure 2b), which were conducted one week apart. Birds also performed similarly during the trial where dishes were arranged in a random pattern. This evidence rules out the possibility that birds memorized the location of dishes with food rather than learned to associate the colour with the content of dishes. A similar behaviour was observed in black-legged kittiwakes, Rissa tridactyla, tested under the same experimental paradigm (Kitaysky et al. 2003), suggesting that foraging kittiwakes, in general, rely on associative cues rather than on an exact spatial location of food resources. This appears to be an adaptive trait considering that under natural conditions exact locations of food patches exploited by kittiwakes vary greatly on small spatial scales, whereas association of food with visually distinct oceanographic features is relatively constant (Hunt & Schneider 1987).
Malnutrition imposed early in life is known to alter morphological, neuro-physiological and functional aspects of the developing brain, which might affect learning and memory formation in mammals. A chronic elevation of corticosterone may also cause atrophy of hippocampal processes and neuron loss in mammals (Sapolsky et al. 2000). In this study, poorly performing red-legged kittiwakes could have been less capable of learning either because they were exposed to a chronically elevated secretion of corticosterone during development or because they experienced a temporary developmental retardation (figure 1a). In a study of black-legged kittiwakes, we found that exposure of young chicks (fed lipid-rich fish ad libitum) to experimentally elevated levels of corticosterone for one week had similar detrimental effects on their learning abilities later in life, and these effects were permanent (Kitaysky et al. 2003). Indeed, average levels of corticosterone in red-legged kittiwakes during the food-restricted period were significantly negatively correlated with their average performance during the four trials of experiment (correlation coefficient: r=−0.49, p=0.027, n=20). Thus, it seems likely that cognition of young red-legged kittiwakes in this study was directly (and probably permanently) affected by chronically elevated secretion of corticosterone associated with an episode of nutritional deficit exacerbated by lipid-poor content of fish.
The phenotype of an individual can reflect its exposure to environmental perturbations during development (Metcalf & Monaghan 2001). Recent studies of kittiwakes have shown substantial heterogeneity in phenotype, as manifested in reproductive performance and survivorship among individuals nesting at the same colony (Cam & Monnat 2000). Numerous ecological studies identified a direct link between dramatic changes in abundance and/or quality of food and seabird population processes in the North Pacific and Atlantic regions (e.g. Hunt et al. 1996a; Frederiksen et al. 2004; Wanless et al. 2005). Our study demonstrates that the phenotype of red-legged kittiwakes is affected by their nutritional history during development. Specifically, the results of our study suggest that declines in availability of lipid-rich forage fish—caused by climate change or human-induced—are likely to result in an inferior quality of seabirds later in life. Reduced cognitive abilities are likely to translate into decreased survival and reduced fitness of birds that, in turn, would further exacerbate declines of seabird populations. Thus, chick diet composition during development is likely to be an important mechanistic link between climate variability and dynamics of recruitment in seabird populations. It remains to be assessed whether impaired cognition as a result of food stress affects seabird populations via a strong effect on the immediate survival of fledglings and/or weaker effects on survival and reproductive performance of affected individuals later in their life. If the latter is the case, early nutritional history might be an important factor in determining the survival of adult individuals, which is the most important cause of decline in seabirds (Frederiksen et al. 2004).
Acknowledgments
We thank K. Brownbridge for help with raising chicks, R. Kitaysky for help with the experiment, G. Hunt, M. Shultz and M. Benowitz-Fredericks for suggestions on earlier drafts of this manuscript. This work was supported by NPMR and EPSCoR University Alaska Fairbanks, by the Exxon Valdez Oil Spill Trustee Council and by the US National Science Foundation. The experimental manipulations with birds were conducted according to the rules of Laboratory Animal Care and Use Protocol of the University of Washington, and under federal and state collection permits.
References
- Anderson P.J, Piatt J.F. Community reorganization in the Gulf of Alaska following ocean climate regime shift. Mar. Ecol. Prog. Ser. 1999;189:117–123. [Google Scholar]
- Boag P.T. Effects of nestling diet on growth and adult size of zebra finches (Poephilla guttata) Auk. 1987;104:413–441. [Google Scholar]
- Byrd G.V, Williams J.C. Red-legged kittiwake (Rissa brevirostris) In: Poole A, Gill F, editors. The birds of North America. No. 60. The Academy of Natural Sciences/The American Ornithologists' Union; Philadelphia/Washington, DC: 1993. [Google Scholar]
- Cam E, Monnat J.-V. Apparent inferiority of first-time breeders in the kittiwake: the role of heterogeneity among age classes. J. Anim. Ecol. 2000;69:380–394. doi:10.1046/j.1365-2656.2000.00400.x [Google Scholar]
- Decker M.B, Hunt G.L, Byrd G.V. The relationships between sea-surface temperature, the abundance of juvenile walleye pollock (Theragra chalcogramma) and the reproductive performance and diets of seabirds at the Pribilof Islands, southeastern Bering Sea. In: Beamish R.J, editor. Climate change and northern fish populations. vol. 121. 1995. pp. 425–437. (Canadian Special Publication in Fisheries and Aquatic Science.) [Google Scholar]
- Frederiksen M, Wanless S, Harris M.P, Rothery P, Wilson L. The role of industrial fisheries and oceanographic change in the decline of North Sea black-legged kittiwakes. J. Appl. Ecol. 2004;41:1129–1139. doi:10.1111/j.0021-8901.2004.00966.x [Google Scholar]
- Hare S.R, Mantua N.J. Empirical evidence for North Pacific regime shifts in 1977 and 1989. Prog. Oceanogr. 2000;47:103–145. doi:10.1016/S0079-6611(00)00033-1 [Google Scholar]
- Hunt G.L, Byrd G.V. Marine bird populations and carrying capacity of the eastern Bering Sea. In: Loughlin T.R, Otani K, editors. Dynamics of the Bering Sea. University of Alaska Sea Grant; Fairbanks, AK: 1999. pp. 631–650. [Google Scholar]
- Hunt G.L, Jr, Schneider D.C. Scale-dependent processes in the physical and biological environment of marine birds. In: Croxall J.P, editor. Seabirds feeding ecology and role in marine ecosystems. Cambridge University Press; Cambridge, UK: 1987. pp. 7–42. [Google Scholar]
- Hunt G.L, Jr, Decker M.B, Kitaysky A.S. Fluctuations in the Bering Sea ecosystem as reflected in the reproductive ecology and diets of kittiwakes on the Pribilof Islands, 1975 to 1991. In: Greenstreet S.P.R, Tasker M.L, editors. Aquatic predators and their prey. Fishing News Books; Oxford, UK: 1996a. pp. 142–153. [Google Scholar]
- Hunt G.L, Jr, Kitaysky A.S, Decker M.B, Dragoo D.E, Springer A.M. Changes in the distribution and size of juvenile walleye pollock, Theragra chalcogramma, as indicated by seabird diets at the Pribilof Islands, and by bottom trawl surveys in the eastern Bering Sea, 1975 to 1993. NOAA Tech. Rep. Ser. 1996b;126:133–147. [Google Scholar]
- Irons, D. B. 1992 Aspects of foraging behavior and reproductive biology of the black-legged kittiwake. Ph.D. dissertation, University of California, Irvine.
- Kitaysky A.S. Metabolic and developmental responses of alcid chicks to experimental variation in food intake. Physiol. Biochem. Zool. 1999;72:469–473. doi: 10.1086/316684. doi:10.1086/316684 [DOI] [PubMed] [Google Scholar]
- Kitaysky A.S, Piatt J.F, Wingfield J.C, Romano M. The adrenocortical stress-response of Black-legged Kittiwake chicks in relation to dietary restrictions. J. Comp. Physiol. B. 1999;169:303–310. doi:10.1007/s003600050225 [Google Scholar]
- Kitaysky A.S, Kitaiskaia E.V, Wingfield J.C, Piatt J.F. Dietary restriction causes chronic elevation of corticosterone and enhances stress response in red-legged kittiwake chicks. J. Comp. Physiol. B. 2001a;171:701–709. doi: 10.1007/s003600100230. doi:10.1007/s003600100230 [DOI] [PubMed] [Google Scholar]
- Kitaysky A.S, Wingfield J.C, Piatt J.F. Corticosterone Facilitates begging and affects resource allocation in the black-legged Kittiwake. Behav. Ecol. 2001b;12:619–625. doi:10.1093/beheco/12.5.619 [Google Scholar]
- Kitaysky A.S, Kitaiskaia E.V, Piatt J.F, Wingfield J.C. Benefits and costs of increased levels of corticosterone in seabird chicks. Horm. Behav. 2003;43:140–149. doi: 10.1016/s0018-506x(02)00030-2. doi:10.1016/S0018-506X(02)00030-2 [DOI] [PubMed] [Google Scholar]
- Lack D. 1st edn. Methuen; London: 1968. Ecological adaptations for breeding in birds. [Google Scholar]
- Lance B.K, Roby D.D. Diet and postnatal growth in red-legged and black-legged kittiwakes: an inter-specific comparison. Colon. Waterbird. 1998;21:375–387. [Google Scholar]
- Lance B.K, Roby D.D. Diet and postnatal growth in red-legged and black-legged kittiwakes: an interspecies cross-fostering experiment. Auk. 2000;117:1016–1028. [Google Scholar]
- Metcalf N.B, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. doi:10.1016/S0169-5347(01)02124-3 [DOI] [PubMed] [Google Scholar]
- Nowicki S, Peters S, Podos J. Song learning, early nutrition and sexual selection in songbirds. Am. Zool. 1998;38:179–190. [Google Scholar]
- Nowicki S, Hasselquist D, Bensch S, Peters S. Nestling growth and song repertoire size in great reed warblers: evidence for song learning as an indicator mechanism in mate choice. Proc. R. Soc. B. 2000;267:2419–2424. doi: 10.1098/rspb.2000.1300. doi:10.1098/rspb.2000.1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravosudov, V. V., Levenex, P. & Omanska, A. In press. Nutritional deficits during early development affect hippocampal structure and spatial memory later in life. Behav. Neurosci [DOI] [PubMed]
- Romano, M. D. 2000 Effects of diet on growth and development of nestling seabirds. MS dissertation, Oregon State University, Corvallis.
- Sapolsky R.M, Romero M.L, Munck A.U. How do glucocorticoids influence stress responses? Integrative, permissive, suppressive, stimulatory, and preparation actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. doi:10.1210/er.21.1.55 [DOI] [PubMed] [Google Scholar]
- Schew W.A, Ricklefs R.E. Developmental plasticity. In: Starck J.M, Ricklefs R.E, editors. Avian growth and development: evolution within the altricial–precocial spectrum. Oxford University Press; New York: 1998. pp. 288–304. [Google Scholar]
- Sokal R.R, Rohlf F.J. 2nd edn. W. H. Freeman & Co; San-Francisco, CA: 1981. Biometry. [Google Scholar]
- van der Ziel C.E, Visser G.H. The effect of food restriction on morphological and metabolic development in two lines of growing Japanese Quail chicks. Physiol. Biochem. Zool. 2001;74:52–65. doi: 10.1086/319314. doi:10.1086/319314 [DOI] [PubMed] [Google Scholar]
- Wanless S, Harris M.P, Redman P, Speakman J.R. Low energy values of fish as a probable cause of a major seabird breeding failure in the North Sea. Mar. Ecol. Prog. Ser. 2005;189:117–123. [Google Scholar]