Abstract
One of the outstanding and poorly understood examples of cooperation between species is found in corals, hydras and jellyfish that form symbioses with algae. These mutualistic algae are mostly acquired infectiously from the seawater and, according to models of virulence evolution, should be selected to parasitize their hosts. We altered algal transmission between jellyfish hosts in the laboratory to examine the potential for virulence evolution in this widespread symbiosis. In one experimental treatment, vertical transmission of algae (parent to offspring) selected for symbiont cooperation, because symbiont fitness was tied to host reproduction. In the other treatment, horizontal transmission (infectious spread) decoupled symbiont fitness from the host, potentially allowing parasitic symbionts to spread. Fitness estimates revealed a striking shift to parasitism in the horizontal treatment. The horizontally transmitted algae proliferated faster within hosts and had higher dispersal rates from hosts compared to the vertical treatment, while reducing host reproduction and growth. However, a trade-off was detected between harm caused to hosts and symbiont fitness. Virulence trade-offs have been modelled for pathogens and may be critical in stabilising ‘infectious’ symbioses. Our results demonstrate the dynamic nature of this symbiosis and illustrate the potential ease with which beneficial symbionts can evolve into parasites.
Keywords: evolution of cooperation, experimental evolution, mutualism, symbiosis, virulence evolution, zooxanthellae
1. Introduction
Understanding the evolutionary stability of cooperation remains a problem in biology (Axelrod & Hamilton 1981; Bull & Rice 1991; Frank 1994, 1996a,b; Sachs et al. 2004). Symbioses—intimate interactions between species—provide some paradoxical examples of mutual aid. For symbionts that exchange costly benefits with hosts, we must explain what prevents them from parasitizing their hosts and thus gaining reproductive advantage over beneficial symbionts (Axelrod & Hamilton 1981; Bull & Rice 1991; Frank 1994, 1996a,b; Sachs et al. 2004). Symbionts which undergo horizontal transmission, defined as infectious transfer among unrelated hosts, represent a most perplexing case of mutualism. Unlike with vertical transmission, in which symbionts are inherited from host parent to offspring, horizontally transmitted symbionts are not tied in reproduction with their host. Virulence theory, developed to study pathogen evolution, suggests that horizontal transmission promotes the evolution of harmful symbionts. There are two main predictions: (i) horizontal transmission allows symbionts to adopt selfish strategies such as appropriating resources from their current hosts before moving on to new hosts (Fine 1975; Ewald 1983; Bull 1994); and (ii) under horizontal transmission, unrelated symbionts can infect the same host, compete for host resources and harm the host in the process (Frank 1994, 1996a,b). Paradoxically, horizontally transmitted mutualists are common in nature, including algal symbionts of marine invertebrates (Trench 1993), mammalian gut-symbionts (Savage 1977), nitrogen-fixing bacteria in plant roots (Sprent et al. 1987) and bioluminescent bacteria in fish and squids (Ruby 1996).
Detailed research studying beneficial symbioses suggests that symbionts do not invariably provide benefits to their hosts (Douglas 1995, 1998). For example, many plants benefit from infection by bacterial and/or fungal root symbionts that donate nitrogen (rhizobia) or phosphorus (mycorrhizae) to their hosts, respectively. However, apparent parasites exist within these populations of beneficial symbionts: ‘ineffective’ strains delivering little or no nutrients to plants have been characterized (Smith & Smith 1996; Quigley et al. 1997). The prevalence of such strains in nature remains unclear.
In algal–invertebrate symbioses, the algae are understood to benefit their hosts, including corals, hydras and jellyfish (Trench 1993) by providing photosynthates (Balderston & Claus 1969) in exchange for nitrogen and inorganic nutrients (Muscatine 1990). However, it is unclear whether these symbionts invariably offer a net benefit to hosts (Douglas & Smith 1983; Douglas 1995, 1998; Muller-Parker & Davy 2001), or if they can evolve to parasitize their hosts. Numerous cnidarian hosts acquire symbiotic algae infectiously at each sexual generation (Fadlallah 1983), and theory suggests that such horizontal transmission selects for symbiont virulence (Fine 1975; Ewald 1983; Bull 1994).
Here, we experimentally altered symbiont transmission mode to investigate the evolution of parasitism in a horizontally transmitted algal–invertebrate symbiosis. We conducted our experiments with Symbiodinium microadriaticum, a dinoflagellate algal symbiont of the upside-down jellyfish, Cassiopea xamachana. The jellyfish are born symbiont-free, disperse from their mother as planula larvae, and acquire algae infectiously (from the environment) once they have reached the sessile polyp stage (figure 1). The asexual polyps can reproduce via clonal budding and infected polyps transmit algae to nascent offspring via vertical transmission. Once infected, polyps undergo metamorphosis to the adult medusa stage. Both polyps and medusae expel a proportion of their algal symbionts back into the environment.
Figure 1.
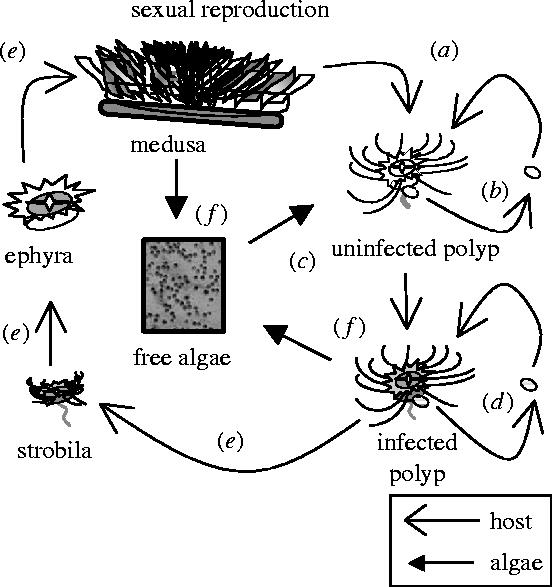
Cassiopea–Symbiodinium symbiosis life cycle. Algae are transmitted horizontally between adult host generations. Female medusae release planula larvae (a) that disperse and settle as uninfected polyps. Uninfected polyps bud to produce clonal offspring (b) ultimately becoming infected by environmental algal symbionts (c). Infected polyps bud, producing clonal offspring that inherit algae via vertical transmission (d). Once infected, polyps undergo metamorphosis (e). Both medusa and infected polyps release algae into the environment (f) and may be the source of new infections (c).
2. Material and methods
(a) Synopsis of experimental evolution
We gathered a mix of algae from wild upside-down jellyfish medusae, infected them into a clone of symbiont-free polyps and experimentally manipulated transmission mode. In one treatment, we enforced vertical transmission: buds produced by infected, isoclonal polyps were used as offspring for new host generations. In the other treatment, horizontal transmission was enforced by infecting a new generation of uninfected host polyps (from the same isoclonal line) with algae expelled from the previous generation of hosts. The vertical transmission treatment maximizes retention of mutualistic symbionts, since symbiont and host reproduction are directly tied. However, the horizontal transmission treatment should favour highly infectious symbionts potentially to the detriment of the host (Fine 1975; Ewald 1983; Bull 1994). Both treatments were replicated threefold, and two rounds of experimental transmission followed initial infection, with seven weeks between transmission rounds (figure 2). To determine the effects of selection, we estimated the fitness of both hosts infected with symbionts and the fitness of symbionts themselves. To assess whether fitness effects of experimental selection generalized across host genotypes, we also infected the experimental algal populations into three novel host genotypes.
Figure 2.
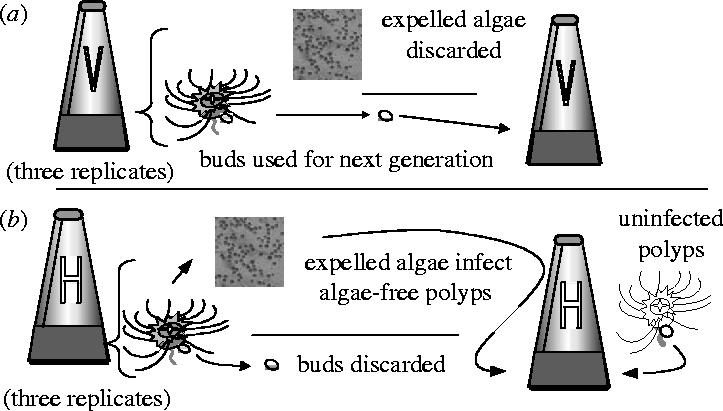
Experimental design. (a) Vertical treatment (V): buds released from infected polyps are saved in a separate flask, where they settle into infected polyps. After seven weeks of infection, 30 polyps are randomly selected from the newly settled pool. These settled polyps represent the next generation. (b) Horizontal treatment (H): buds are discarded. After seven weeks, infected polyps are put into a new flask with ASW for 48 h of algal expulsion. Thirty isoclonal uninfected polyps are infected with the expelled algae. The newly infected polyps represent the next generation. In both treatments there are two rounds of transmission after initial infection.
(b) Host and symbiont collection
Hosts for experimental evolution originated from a planula larva of a female jellyfish collected at Keys Marine Laboratory, Long Key (N 24°49′, W 80°49′) that was grown up to a clone of symbiont-free polyps in the laboratory. Alternate hosts for re-infection experiments were half-sibling groups of polyps from a single mother, and unknown father(s), gathered at Grassy Key (N 24°45′, W 80°59′), Upper-Matecumbe Key (N 24°54′, W 80°38′) and Key Largo (N 25°05′, W 80°27′). Host planulae were rinsed of maternal tissue and raised into algae-free polyps. Algae were collected from two medusae (one large, one small) at each of 10 sites along a 120 mile transect ranging from Key Largo in the northeast (N 25°05′, W 80°27′) to Geiger key in the southwest (N 24°35′, W 81°39′). Previous genetic analysis has shown that multiple strains of one species, S. microadriaticum, infect C. xamachana at these sites (Wilcox et al. 1999).
(c) Infection and experimental selection
An equal density mix of algal isolates was added to 180 isoclonal polyps in artificial seawater (ASW), at a final concentration of 103 algae ml−1. Infection lasted 48 h before the polyps were divided into six flasks: two treatment lines—horizontal and vertical transmission, three replicates per line and 30 polyps per replicate. All infected polyps were fed tri-weekly to repletion on Artemia salina nauplii, and incubated at 21 °C on a 12 : 12 h light–dark cycle. Cassiopea polyps will not metamorphose to medusae at 21 °C, but continue to produce asexual buds. The ASW was changed on each feeding, and flasks were replaced weekly to minimize free algae.
Polyp lines were maintained until algal expulsion was detected (observation of swimming Symbiodinium in the ASW—seven weeks in all cases). Transmission of symbionts to the next generation of hosts was then initiated. In the vertical treatment, polyp buds were collected in separate flasks, and new lines were established using 30 randomly chosen polyps arising from the buds. These polyps inherited their algae from the parent polyp. This new generation of polyps were then placed in a clean flask with fresh ASW and maintained as previously described.
In the horizontal treatment, the next generation of hosts were to acquire their symbionts via new infection. Therefore, buds were discarded. After seven weeks the polyps in each line were placed in clean flasks containing filtered ASW and left undisturbed in the incubator for 48 h. The polyps were then removed from the flasks, while the ASW that contained the algae expelled from the polyps was retained. Into this water were placed 30 uninfected polyps (from the original isoclonal polyp line). The new polyps were allowed to acquire algae for 48 h before a normal feeding and water change. The polyp lines were then maintained as previously described.
During selection, the density of algae within polyps may have diverged between treatments. To control for this during our fitness assays, we created new populations of infected polyps by: (i) extracting the algae from experimental polyps, (ii) separately exposing uninfected isoclonal polyps to equal densities of the algal populations from each replicate, and (iii) waiting 90 days to allow symbionts to fully populate hosts. For each replicate, algal populations were extracted from hosts by grinding. The density of algae within the resulting slurry was estimated by dilution and manual counting on a haemocytometer. New host lines were created by adding equal densities of algae to flasks containing 30 uninfected polyps from the original isoclonal line. In parallel, the three alternate hosts were also infected. After 90 days of infection, the fitnesses of hosts and symbionts were estimated.
Variation in symbiont benefit to hosts could potentially exist in the initial pool of algae or emerge via mutation over the course of the experiment. We assume a genetically diverse initial pool of symbionts as they were sampled over a wide geographical range that has been shown to be genetically diverse (Wilcox et al. 1999). Opportunity for generation of novel mutants during the experiment was minimal: based on the fastest within-host doubling-time reports of 6–7 days for related algae, there were maximally 34–40 doublings during the experiment (Wilkerson et al. 1983; Fitt 2000).
(d) Fitness estimation
Two types of fitness assays were performed on the host and three on the symbionts. Host fitness was estimated by counting buds released from polyps (asexual reproduction) and measuring growth rate of polyps (a predictor of time to maturation) over two-week periods. Symbiont fitness was estimated by measuring mitotic index (MI, the proportion of algal cells undergoing cytokinesis within the host—a measure of proliferation within the host), the rate that algae were expelled from hosts (a measure of infectiousness, presuming that expelled algae are functional) and algal density within hosts (a measure of symbiont effectiveness).
To measure host growth and reproduction, 12 polyps were randomly chosen from each replicate line and separated into six-well culture dishes with 5 ml of ASW. The oral diameter of each polyp was measured using a drawing tube mounted on a dissecting microscope and tracing the polyp with a computer- linked drawing board (Wacom). All measurements were made while the polyps were undisturbed for 10 min and were assumed to be normally expanded. Polyps were monitored for two weeks, during which time released buds were counted and growth assessed. Growth was measured as change in polyp diameter, and units were transformed to mg protein biomass using a curve standardized to uninfected polyps: .
We subsequently measured algal expulsion, density and division rate in the same 12 hosts. Polyps were separated into 1.5 ml microfuge tubes with 1 ml of ASW, returned to the incubator for 48 h, then removed. The tube was then centrifuged to pellet expelled algae, and the pellet was re-suspended in 50 μl ASW for manual counting of algae on a haemocytometer. Polyps were then frozen and stored at −20 °C for the remaining analyses. Within-host density was estimated by counting algae from whole ground hosts (re-suspended in 100 μl of ASW) and dividing by host biomass. Finally, a squash preparation from each polyp was used to estimate MI (the proportion of algal cells with division plates). All polyps were frozen between 10.00 and 11.00 to minimize diurnal variation in MI measures. Data were analysed using nested ANOVAs, with polyp line as the nested effect and treatment as the main effect. Data were transformed to meet the normality criteria: polyp growth, algal density and expulsion rate were log(x+1) transformed, and MI was transformed.
3. Results
As predicted by theory (Fine 1975; Ewald 1983; Bull 1994), experimental enforcement of horizontal transmission selected for an evolutionary shift to symbiont parasitism. Algae that were selected under horizontal transmission caused striking reductions in host growth (ANOVA, p<0.001, n=52) and budding (ANOVA, p<0.001, n=52: table 1) compared to the vertical treatment. In the host growth assay (with four re-infected host genotypes), algae selected under the horizontal treatment caused 30% of these hosts to shrink by 10% or more in size, while only 35% grew more than 10%. By contrast, in the vertical treatment only 4% shrunk more than 10%, while 88% grew greater than 10%. Treatment effects were consistent across the three alternate host genotypes (table 2; full factorial ANOVA, p<0.001, n=18). This shift to parasitism was linked to algal proliferation within hosts. The horizontal treatment algae had significantly higher division rates within their hosts (ANOVA, p=0.003, n=51), attained higher densities within hosts (ANOVA, p=0.03, n=51), and had significantly higher expulsion rates (with host biomass as a covariate) from their hosts (ANCOVA, p<0.05, n=52).
Table 1.
Fitness comparisons of hosts and symbionts by treatmenta.
| fitness measure | units | treatment | values (±1 s.e.) | N | p |
|---|---|---|---|---|---|
| polyp growth rate | mg d−1 | H | 0.041 (0.037, 0.046) | 52 | <0.001 |
| V | 0.090 (0.085, 0.094) | ||||
| polyp budding rate | buds d−1 | H | 1.440 (1.343, 1.541) | 52 | <0.05 |
| V | 2.235 (2.079, 2.398) | ||||
| algal MI | percentage of cells dividing | H | 1.61 (1.48, 1.73) | 51 | 0.003 |
| V | 1.05 (0.94, 1.18) | ||||
| algal expulsion | cells h−1 | H | 996.7 (770.3, 1290) | 52 | <0.05 |
| V | 353.0 (265.4, 469.3) |
Replicate means of horizontal (H) and vertical (V) treatments are shown. All treatment effects were significant and analysed using nested ANOVAs, except algal expulsion rate which was analysed with a nested ANCOVA using host biomass as a covariate. MI is mitotic index.
Table 2.
Fitness comparisons of alternate host genotypes by treatmenta.
| fitness measure | units | host | treatment | values (±1 s.e.) |
|---|---|---|---|---|
| growth rate | mg d−1 | CCP | H | −0.002 (−0.006, 0.001) |
| V | 0.014 (0.011, 0.017) | |||
| GKQ | H | 0.008 (0.005, 0.011) | ||
| V | 0.022 (0.019, 0.025) | |||
| MTK | H | 0.002 (−0.001, 0.005) | ||
| V | 0.025 (0.022, 0.028) |
Alternate hosts were gathered from three disparate sites and infected separately with the algal populations from each treatment line: Coco-Plum (CCP), Grassy Key Quarry (GKQ) and Matecumbe Key (MTK). A full factorial ANOVA of horizontal (H) and vertical (V) treatments showed a significant effect of the treatments, p<0.001, n=18.
Although the algae in the horizontal treatment proliferated within hosts, the fitness costs that these algae imposed upon hosts may have ultimately hindered their spread. Two pieces of evidence suggest that a trade-off exists between the harm an algal symbiont causes its host and its own dissemination. First, there is a strong negative correlation between within-host algal division rate (MI) and host growth (R2=0.834, p=0.011); this is evidence that faster growing symbionts stunt host growth. Second, while algal expulsion rate per unit of host biomass was significantly higher in the horizontal treatment, total expulsion did not differ between treatments (ANOVA, p=0.39, n=52). This results from a positive correlation between host size and algal expulsion rate (R2=0.34, p<0.001) and the smaller size (lower growth rate) of hosts infected with horizontally propagated algae. Thus, fast symbiont division within hosts caused host growth deficits, and small hosts expelled fewer algae. Just as optimal virulence is predicted to be driven by fitness trade-offs in pathogens (Fine 1975; Ewald 1983; Bull & Rice 1991; Bull 1994), evolution of symbiont parasitism may be limited by a trade-off against the symbiont's spread to new hosts.
4. Discussion
Marine algal symbioses are classic examples of symbiotic cooperation (Douglas 1995). However, the symbionts may not always be beneficial. Algae are known to engender costs to their hosts, at least because they take up space and nutrition in host tissue (Douglas & Smith 1983). If algal benefits are diminished so that they engender a net fitness cost to the host, the relationship becomes parasitic by definition (Douglas & Smith 1983). Early experiments showed that hosts infected with symbiotic algae outgrew uninfected hosts in many situations (Muscatine & Lenhoff 1965), leading to the general belief that algal symbionts are invariably beneficial. However, subsequent research suggests that algae can be costly to hosts, potentially acting as parasites. For example, when hydras that host Chlorella algae were grown in the dark, uninfected hosts were found to outgrow infected hosts (Douglas & Smith 1983). Such reduced benefits to hosts may also occur in nature: in some environments algal symbionts grow and proliferate within hosts during winter, though minimal potential exists for photosynthesis (Muller-Parker & Davy 2001). Our results confirm ideas that algal symbionts can parasitize their hosts (Douglas 1995). When the algae from our horizontal treatment were experimentally infected into hosts, almost half of those jellyfish shrunk, consistent with reduced host fitness.
Host biology may be an important factor in algal parasitism. Hosts are often assumed to have some control over endogenous algae: by regulating algal growth and/or expulsion from the host (Muscatine & Pool 1970; Baghdasarian & Muscatine 2000; Fitt 2000) hosts may curtail algal parasites. In our experiment, harmful algae grew to higher densities within hosts than beneficial algae, and this argues against host control. We conducted our experiments on polyps and not mature hosts, as it is at the polyp stage in which infection initiates. Thus, if hosts have mechanisms of ‘partner choice’, in which beneficial symbionts are selected by the host over harmful ones (Bull & Rice 1991; Sachs et al. 2004; Simms et al. in press), we would expect such mechanisms to be active in the polyp stage.
Symbiont populations may vary widely with respect to the level of benefit provided to hosts, with some symbionts acting as parasites (Douglas 1998). Here, we specifically addressed the question of when parasitic algae might spread in nature. Virulence theory predicts that horizontal transmission of symbionts promotes the evolution of parasitism (Fine 1975; Ewald 1983; Bull 1994), and our results are in line with this theory. With repeated horizontal transmission, we uncovered symbionts that—relative to vertically transmitted algae—grew faster within their hosts, attained higher densities within hosts and were expelled at higher rates (per-host mass), at a marked cost to host growth and reproduction. However, the horizontal treatment did not unleash an epidemic of highly infectious algae, as some theory would predict (Ewald 1983). The rapid proliferation of symbionts within hosts in the horizontal treatment was associated with growth deficits in those hosts, ultimately causing the harmful symbionts to be hindered in their infectious spread to new hosts. Such trade-offs have been modelled for pathogens and may be a critical factor stabilizing cooperation in horizontally transmitted symbioses.
Acknowledgements
We thank A. Sloan and C. Rosel for assistance in the field and laboratory, respectively. J. Bull, R. Heineman and W. Harcombe provided helpful comments on the manuscript. J.L.S. was supported by NSF DEB-0308780.
References
- Axelrod R, Hamilton W.D. The evolution of cooperation. Science. 1981;211:1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- Baghdasarian G, Muscatine L. Preferential expulsion of dividing algal cells as a mechanism for regulating algal-cnidarian symbiosis. Biol. Bull. 2000;199:278–286. doi: 10.2307/1543184. [DOI] [PubMed] [Google Scholar]
- Balderston W.L, Claus G. A study of the symbiotic relationship between Symbiodinium microadriaticum Freudenthal, a zooxanthella and the upside-down Jellyfish, Cassiopeia sp. Nova Hedwigia. 1969;17:373–382. [Google Scholar]
- Bull J.J. Perspective: virulence. Evolution. 1994;48:1423–1437. doi: 10.1111/j.1558-5646.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Bull J.J, Rice W.R. Distinguishing mechanisms for the evolution of co-operation. J. Theor. Biol. 1991;149:63–74. doi: 10.1016/s0022-5193(05)80072-4. [DOI] [PubMed] [Google Scholar]
- Douglas A.E. The ecology of symbiotic micro-organisms. Adv. Ecol. Res. 1995;26:69–103. [Google Scholar]
- Douglas A.E. Host benefit and the evolution of specialization in symbiosis. Heredity. 1998;81:599–603. [Google Scholar]
- Douglas A.E, Smith D.C. The costs of symbionts to the host in green hydra. In: Schenk H.E.A, Schwemmler W, editors. Endocytobiology 2. Walter de Gruyter; Berlin: 1983. pp. 631–647. [Google Scholar]
- Ewald P.W. Host parasite relations, vectors, and the evolution of disease severity. Ann. Rev. Ecol. Syst. 1983;14:465–485. doi:10.1146/annurev.es.14.110183.002341 [Google Scholar]
- Fadlallah T.H. Sexual reproduction, development and larval biology in scleractinian corals. A review. Coral Reefs. 1983;2:129–150. doi:10.1007/BF00336720 [Google Scholar]
- Fine P.E.M. Vectors and vertical transmission: an epidemiological perspective. Ann. NY Acad. Sci. 1975;266:173–194. doi: 10.1111/j.1749-6632.1975.tb35099.x. [DOI] [PubMed] [Google Scholar]
- Fitt W.K. Cellular growth of host and symbiont in a cnidarian–zooxanthellar symbiosis. Biol. Bull. 2000;198:110–120. doi: 10.2307/1542809. [DOI] [PubMed] [Google Scholar]
- Frank S.A. Genetics of mutualism: the evolution of altruism between species. J. Theor. Biol. 1994;170:393–400. doi: 10.1006/jtbi.1994.1200. doi:10.1006/jtbi.1994.1200 [DOI] [PubMed] [Google Scholar]
- Frank S.A. Host control of symbiont transmission: the separation of symbionts into germ and soma. Am. Nat. 1996a;148:1113–1124. doi:10.1086/285974 [Google Scholar]
- Frank S.A. Host–symbiont conflict over mixing of symbiotic lineages. Proc. R. Soc. B. 1996b;263:339–344. doi: 10.1098/rspb.1996.0052. [DOI] [PubMed] [Google Scholar]
- Muscatine L. The role of symbiotic algae in carbon and energy flux in coral reefs. In: Dubinsky Z, editor. Ecosystems of the World: coral reefs. Elsevier; Amsterdam: 1990. pp. 75–81. [Google Scholar]
- Muscatine L, Lenhoff H.M. Symbiosis and algae. II. Effects of limited food and starvation on growth of symbiotic and aposymbiotic hydra. Biol. Bull. 1965;129:316–328. [Google Scholar]
- Muscatine L, Pool R.R. Regulation of numbers of intracellular algae. Proc. R. Soc. B. 1970;204:131–139. doi: 10.1098/rspb.1979.0018. [DOI] [PubMed] [Google Scholar]
- Muller-Parker G, Davy S.K. Temperate and tropical algal–sea anenome symbioses. Invertebr. Biol. 2001;120:104–123. doi:10.1111/j.1744-7410.2001.tb00115.x [Google Scholar]
- Quigley P.E, Cunningham P.J, Hannah M, Ward G.N, Morgan T. Symbiotic effectiveness of Rhizobium leguminosaru, bv. trifolii collected from pastures in south-western Victoria. Aust. J. Exp. Agr. 1997;37:623–630. doi:10.1071/EA96089 [Google Scholar]
- Ruby E.G. Lessons from a cooperative, bacterial–animal association: the Vibrio fischeri–Euprymna scolopes light organ symbiosis. Annu. Rev. Microbiol. 1996;50:591–624. doi: 10.1146/annurev.micro.50.1.591. doi:10.1146/annurev.micro.50.1.591 [DOI] [PubMed] [Google Scholar]
- Sachs J.L, Mueller U.G, Wilcox T.P, Bull J.J. The evolution of cooperation. Q. Rev. Biol. 2004;79:135–160. doi: 10.1086/383541. doi:10.1086/383541 [DOI] [PubMed] [Google Scholar]
- Savage D.C. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. doi:10.1146/annurev.mi.31.100177.000543 [DOI] [PubMed] [Google Scholar]
- Simms E.L, Taylor D.L, Povich J, Shefferson R.P, Sachs J.L, Urbina M, Tauczik Y. An empirical test of partner choice mechanisms in a wild legume–rhizobium interaction. Proc. R. Soc. B. In press;273 doi: 10.1098/rspb.2005.3292. doi:10.1098/rspb.2005.3292 Published online 18 October 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F.A, Smith S.E. Mutualism and parasitism: diversity in function and structure in the ‘Arbuscular’ (VA) mycorrhizal symbiosis. Adv. Bot. Res. 1996;22:1–43. [Google Scholar]
- Sprent J.I, Sutherland J.M, Faria S.M. Some aspects of the biology of nitrogen-fixing organisms. Phil. Trans. R. Soc. B. 1987;317:111–129. [Google Scholar]
- Trench R.K. Microalgal–invertebrate symbiosis: a review. Endocyt. Cell Res. 1993;9:135–175. [Google Scholar]
- Wilcox T.P, Hickok J, Sloan A. Genotypic diversity among algal symbionts isolated from Cassiopea xamachana. Am. Zool. 1999;39:717. [Google Scholar]
- Wilkerson F.P, Muller Parker G, Muscatine L. Temporal patterns of cell division in natural populations of endosymbiotic algae. Limnol. Oceanogr. 1983;28:1009–1014. [Google Scholar]


