Abstract
This study investigates the consequences of the human foraging niche and multiple dependent offspring on the optimal growth trajectory of humans. We test the hypothesis that the human pattern of slow human growth between age at weaning and puberty helps defer the compound energetic demand on parents with multiple dependents, by using growth and demographic data from two foraging societies, the Ache of eastern Paraguay and the Dobe Ju/'hoansi of Botswana and Namibia. We run simulations of observed and potential growth trajectories among sub-adults and their consequent energetic demands on parents given profiles of fertility, mortality, consumption and production. We find that either sub-adult production or food subsidies from other people must substantially increase in order to compensate for the dramatic increase in energetic demand on parents if offspring were to grow faster at younger ages. Our conclusion is that slow human growth followed by a rapid adolescent growth spurt may have facilitated rising human fertility rates and greater investments in neural capital.
Keywords: hunter–gatherer, human growth, life history, adolescence, growth spurt
1. Introduction
Primate life histories tend to be ‘slow’ because of low-growth rates, long sub-adult periods, long lifespans and low-fertility rates in comparison to those of other mammals of similar body size (Charnov & Berrigan 1993; Pereira & Fairbanks 2002; Kappeler & Pereira 2003). Among primates, chimpanzees and humans represent one extreme where a prominent growth spurt in body weight only occurs after a significant delay that is much longer than expected for growth-spurting primates of our size (Brody 1945; Leigh 2001). However, humans have diverged from the general primate pattern by weaning infants at earlier ages and thereby shortening inter-birth spacing and increasing fertility rates (Harvey et al. 1987). The greater dependency of a larger number of slow-growing offspring generates high-energetic demands on parents throughout their reproductive lives. How do parents afford the energetic demands of more offspring that are dependent for a longer time?
Whereas weaned primates subsidize most of the energetic demand of their own growth (Pereira & Fairbanks 2002), human parents in all cultures must feed and care for weaned, but still dependent, offspring. Bogin (1988, 1997) proposes that human childhood is a novel-derived life-stage that allows for extra-maternal feeding and care, freeing the mother to redirect reproductive effort to subsequent offspring. The increase in fertility provides a selective pressure that favours the evolution of childhood. However, there are increasing costs involved in the feeding and care of multiple dependent children.
Several models have been proposed for the support of increased fertility rates in humans. One is the Grandmother Hypothesis proposed by Hawkes and colleagues that focuses on provisioning by post-reproductive women of daughters and granddaughters (Hawkes et al. 1998; Blurton Jones et al. 1999; Hawkes 2003). The Embodied Capital Model discussed by Kaplan and colleagues (Kaplan et al. 2000) focuses on energetic inputs by both males and females through hunting and other high-skill extractive foraging strategies. The ubiquity of extra-maternal care provided by fathers, grandparents, older children and other kin has even led humans to be described as effective ‘cooperative breeders’ (Hrdy 2005; Kramer 2005). In terms of total calories and macronutrients provided to a hunter–gatherer diet, difficult-to-acquire hunted and extracted resources appear to be much more important than easily acquired foods (Cordain et al. 2000). High production relatively late in life is made possible by early investments in slow growth and learning. If increased provisioning and care lowers early adult mortality rates (Stearns 1992; Charnov 1993), then these models predict a consequent lengthening of the juvenile period. We do not focus here on the source of extra-maternal provisioning but instead seek to quantify the energetic costs that must be paid in order to compensate for the compounding energetic demand of multiple dependency.
We propose that human fertility is maximized by keeping sub-adults relatively small for the duration of childhood-juvenile growth, with the rapid increase in growth, or ‘adolescent growth spurt’ occurring shortly before reproductive maturation. The timing of the growth spurt relative to age of first reproduction should reflect the extent to which adult economic production is limited by body size, skills and learning. Among most folivorous and frugivorous primates, where performance is highly size-dependent and skills requirements are relatively low, it pays to be bigger early in life, whereas in the complex foraging niche of humans, being bigger early in the juvenile period does not significantly increase food production, and would, therefore, only be more costly for parents. There is substantial evidence that the length of component sub-adult growth phases varies substantially among primate species, and appears to be sensitive to ecological variation (Leigh 2001).
We investigate the merits of the human pattern of slow growth rates during the lengthened period from age at weaning until puberty, followed by rapid adolescent growth. The high number of dependent offspring who are net consumers for an extended time because of the difficult human foraging niche typical of human hunter–gatherers creates an increasing energetic demand on parents over time. We compare the expected energetic savings from the observed pattern of human growth with those from a gradual linear increase in growth, and a chimpanzee-like growth pattern which exhibits more rapid growth at younger ages. Others have proposed that energetic limitations may affect growth patterns (e.g. Holmes 1995), or that growth is delayed in order to learn crucial cultural skills (e.g. Watts 1985). However, this is the first study to quantify the energetic demand of human growth among hunter–gatherers, and directly implicates economic production and fertility in understanding growth. We explicitly show how different growth trajectories affect net energetic costs of parents with multiple dependents.
2. Material and methods
The estimation of the age-specific caloric demand on parents requires four ingredients: age-specific fertility, mortality, physical growth and food production. Rates of fertility and mortality are necessary to estimate the expected number of dependents that parents have at each age interval. Growth rates (observed or simulated) are necessary to estimate the age- and sex-specific energetic requirements of sub-adults. Production rates of sub-adults (also observed or simulated) are necessary to estimate the extent to which sub-adults can subsidize their own growth, versus the extent of energetic production that must be provisioned by other individuals.
We choose two hunter–gatherer groups which vary significantly in their demographic parameters to model the extremes of low and high demand on parents. The Ju/'hoansi (!Kung) of Botswana and Namibia with a low total fertility rate (TFR=4), slow physical growth, and relatively high mortality should show a low level of demand on parents relative to the Ache of Paraguay, who show high fertility (TFR=8), faster growth, and higher sub-adult survival. We use forest period (pre-contact) age-specific average fertility and mortality rates for the Ache reported by Hill & Hurtado (1996). Ju/'hoansi mortality and fertility profiles come from Howell (1979). We base our estimates of demand using the female fertility profile, and sex- and age-specific growth and survival of offspring. Age-specific production and consumption for the Ache are from Kaplan et al. (2000). Body mass is estimated using a JPPS (Jolicoeur et al. 1988) parametric model to cross-sectional data. Ache growth (1980–2001) is from Walker et al. (in press), captive chimpanzee (Pan troglodytes) growth is from Leigh & Shea (1996) and Ju/'hoansi growth (1967–1969) is from Howell's (2000) online source.
Mass-specific energetic cost (kcal kg−1) is estimated from age and sex-specific World Health Organization (WHO) recommendations (FAO/WHO/UNU 1985). Caloric consumption is estimated as the product of the mass-specific energetic cost and age-specific body mass. Body mass is estimated under three scenarios: (a) ‘observed’, derived from the JPPS non-linear regression; (b) ‘linear’, derived from linear growth from weaning to age 18; (c) ‘chimpanzee’, where growth follows the chimpanzee trajectory given in Leigh & Shea (1996) (see electronic supplementary material for discussion of wild chimpanzee growth). Our linear or constant growth scenario acts as a neutral baseline for direct comparison with the delayed human and fast chimpanzee growth patterns. Energetic estimates for ‘chimpanzee’ are calculated as the corresponding human requirements (male or female Ache or Ju/'hoansi) plus the size difference at any age between chimpanzee and human multiplied by metabolic costs for humans at age 18 (44 kcal kg−1 for males; 39 kcal kg−1 for females). This is a conservative estimate of a human with chimpanzee-like growth because metabolic costs per kg decrease considerably with age due to the decline in relative brain size (FAO/WHO/UNU 1985).
We assume an age of independence of 18 years for both sexes. We define energetic demand on parents at any age as the sum of the estimated consumption less production for each of n dependents of various ages. Half of the dependents at birth are male. The expected number of dependents of each age and sex for an individual age x is calculated using the estimates of Ju/'hoansi and Ache age-specific fertility and the yearly probability of survival for offspring at each age y.
3. Results
(a) Physical growth
Figure 1 illustrates the observed and hypothetical growth rates for the Ache (see electronic supplementary material for Ju/'hoansi), demonstrating the pattern of fast infant Ache growth followed by slow childhood and juvenile growth until the pronounced adolescent growth spurt. Distance curves for males are shown in the figure inset. Infants are larger than chimpanzees and larger than if growth were linear. However, after weaning, male chimpanzees surpass Ache in body size until roughly age 18. The crossover of growth velocities occurs at age 10 for Ache and 12 for Ju'/hoansi, where observed growth velocity surpasses both the linear and chimpanzee-like growth velocity. Ju/'hoansi growth velocities are considerably slower than their Ache counterparts. The linear growth for Ache boys and girls is about 3.0 kg yr−1, and 2.1 kg yr−1 for the Ju/'hoansi. The Ju/'hoansi growth spurt is also less pronounced than for the Ache (see electronic supplementary material).
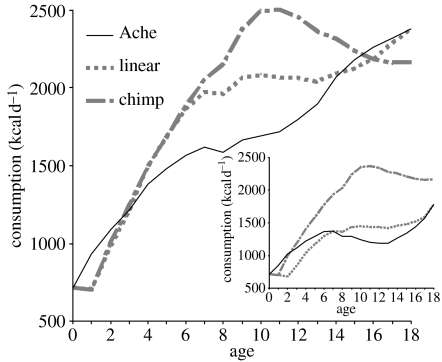
Figure 1.
Growth velocity based on three potential growth trajectories: observed Ache, linear or constant growth and chimpanzee. Inset shows ‘distance curve’ (body size estimates by age) for Ache males compared to linear and chimpanzee growth trajectories.
(b) Per-capita caloric demand
Figure 2 displays the daily age-specific caloric requirements of an average Ache and Ju/'hoansi sub-adult under the observed and hypothetical growth regimes of figure 1. Ache and Ju/'hoansi consumption estimates for observed growth hover above 1500 and 1200 kcals day−1 for a full decade from age 5 to 15.
Figure 2.
Consumption estimates by age for an Ache and Ju/'hoansi (inset) undergoing observed, chimpanzee and linear growth. These estimates are averages for males and females.
Table 1 provides the cumulative per-capita consumption estimates for Ache and Ju'/hoansi males and females under each growth regime. The cumulative consumption of an Ache and Ju/'hoansi sub-adult under normal growth from conception to age 18 is 11–12 Gcals (gigacalories, 1 Gcal=kcal×106) and 8–9 Gcals, respectively. A Gcal can be conceptualized as the approximate consumption of a 60 kg adult male for an entire year (2740 kcal day−1×365 days=1.0 Gcal). If an Ache or Ju/'hoansi girl were to undergo constant growth, we estimate that the cumulative demand would increase by an additional 0.3 and 0.2 Gcals over their lives. This is a modest increase of only 3%. If the same girls were to grow with a chimpanzee-like trajectory of fast early growth, energetic costs increase by 9 and 38%, respectively. For Ache and Ju/'hoansi males, the equivalent shift to chimpanzee growth incurs an increased demand of 25 and 54%, respectively, above observed patterns.
Table 1.
Net energetic consumption of an average grower from conception to age 18.
| growth | cumulative consumption (Gcal) | increase over observed (Gcal) | percent increase over observed (%) |
|---|---|---|---|
| female Ache observed | 11.2 | ||
| female Ache linear | 11.5 | 0.3 | 2.6 |
| female ‘chimp-like’ | 12.1 | 1.0 | 8.6 |
| male Ache observed | 11.9 | ||
| male Ache linear | 13.3 | 1.5 | 12.5 |
| male ‘chimp-like’ | 14.9 | 3.0 | 25.2 |
| female Ju/'hoansi obs. | 8.0 | ||
| female Ju/'hoansi linear | 8.2 | 0.2 | 2.5 |
| female ‘chimp-like’ | 11.0 | 3.0 | 38.1 |
| male Ju/'hoansi obs. | 9.4 | ||
| male Ju/'hoansi linear | 9.4 | 0.0 | 0.0 |
| male “chimp-like” | 14.5 | 5.1 | 54.3 |
(c) Compound caloric demand
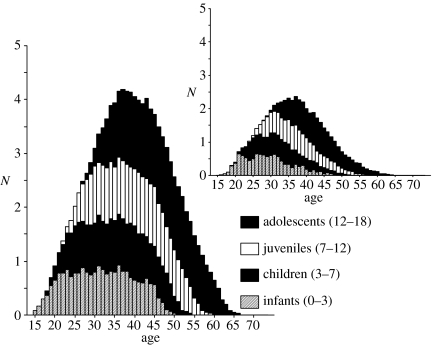
Figure 3 shows the average cumulative number of offspring for a Ju/'hoansi and Ache woman. Offspring are classified as infants (age 0–2), children (3–6), juveniles (7–11), and adolescents (12–18) (sensu Bogin 1988). The higher fertility of Ache compared with Ju/'hoansi results in higher dependency across the lifespan. For example, an Ache woman age 35–45 has on average about one infant, one child, one juvenile and one adolescent dependent. A Ju/'hoansi woman has on average about one-half as many total dependents during the same period. By age 50, a Ju/'hoansi mother has less than one dependent, but an Ache mother has almost three.
Figure 3.
Number of infants, children, juveniles and adolescents for Ache and Ju/'hoansi (inset) mothers.
In the simplest scenario, where dependents produce no food, the total cost of all dependents for Ache and Ju/'hoansi is 67 and 24 Gcals, respectively (table 2 and electronic supplementary material). We next consider the increased caloric burden of linear and chimpanzee-like growth. Linear growth increases Ache and Ju/'hoansi caloric demand by 5.6 and 0.1 Gcals. Chimpanzee-like growth increases demand by 12.1 and 10.4 Gcals, respectively. This represents a substantial increase above that obtained from observed patterns (18 and 44%, respectively). Several examples help to illustrate the additional burden at specific ages. For example, the estimated demand on a 42 year-old Ache mother would increase 601 kcal day−1 if the growth of all of her offspring were linear. If her children grew in a manner analogous to a chimpanzee, the daily demand would increase another 666 kcal day−1, a total increase of 1267 kcal day−1. Observed cross-sectional Ju/'hoansi growth is very similar to linear growth and so the energetic savings are minor at each age. Nevertheless, the demand on a 37-year-old Ju/'hoansi mother would increase by 1503 kcal day−1 if her offspring grew like chimpanzees.
Table 2.
Compound energetic demand of various fertility/mortality/production profiles with observed (1st line), linear (2nd line) and chimpanzee-like (3rd line) growth. (‘Production factor’ refers to the multiplier of sub-adult production necessary for the net energetic demand on parents to remain constant (i.e. equal to observed, 1st line).)
| fertility/mortality | production | growth | cumulative demand (Gcal) | increase over observed (Gcal) | percent increase over observed (%) | production factor |
|---|---|---|---|---|---|---|
| Ache | none | Ache obs. | 66.9 | |||
| Ache linear | 72.5 | 5.6 | 8.3 | |||
| chimpanzee | 79.0 | 12.1 | 18.0 | |||
| Ache | Ache | Ache obs. | 54.4 | |||
| Ache linear | 60.0 | 5.6 | 10.2 | 1.44 | ||
| chimpanzee | 66.5 | 12.1 | 22.2 | 1.96 | ||
| Ache | high skill | Ache obs. | 37.6 | |||
| Ache linear | 43.2 | 5.6 | 14.8 | 1.19 | ||
| chimpanzee | 49.7 | 12.1 | 32.1 | 1.41 | ||
| Ache | low skill | Ache obs. | 14.0 | |||
| Ache linear | 19.6 | 5.6 | 39.8 | 1.11 | ||
| chimpanzee | 26.1 | 12.1 | 86.3 | 1.28 | ||
| Ju/'hoansi | Ache | Ache obs. | 25.4 | |||
| Ache linear | 27.7 | 2.3 | 8.9 | 1.41 | ||
| chimpanzee | 30.5 | 5.0 | 19.8 | 1.92 | ||
| Ju/'hoansi | Ache | Ju/'hoansi obs. | 18.2 | |||
| Ju/'hoansi linear | 18.3 | 0.1 | 0.4 | 1.03 | ||
| chimpanzee | 28.6 | 10.4 | 57.0 | 2.89 | ||
| Ju/'hoansi | none | Ju/'hoansi obs. | 23.7 | |||
| Ju/'hoansi linear | 23.7 | 0.1 | 0.2 | |||
| chimpanzee | 34.1 | 10.4 | 43.8 | |||
| Ju/'hoansi | high skill | Ju/'hoansi obs. | 12.3 | |||
| Ju/'hoansi linear | 12.4 | 0.1 | 0.5 | 1.02 | ||
| chimpanzee | 22.7 | 10.4 | 84.0 | 1.92 | ||
| Ju/'hoansi | low skill | Ju/'hoansi obs. | 5.2 | |||
| Ju/'hoansi linear | 5.3 | 0.1 | 1.4 | 1.01 | ||
| chimpanzee | 15.6 | 10.4 | 197.5 | 1.56 |
(d) Production rates
We now consider the production of sub-adults and the effect such production can have on the caloric burden estimated in the previous section. There may be considerable variation in the absolute levels of food production by sub-adult hunter–gatherers, depending on the local ecology and the relative and absolute levels of physical (strength) and brain-based capital (skills) required to effectively acquire and process food. Sub-adults can produce a significant percentage of their food in certain environments where foraging is relatively safe, for example among Hadza (Blurton Jones et al. 1989), Meriam (Bliege Bird & Bird 2002) and Mikea (Tucker & Young 2005), and in seasons of the year when easily accessible foods such as fruits or shallow tubers are available. Nonetheless, for a variety of human foraging groups, peak production is not achieved until age 35–45 (Walker et al. 2002; Gurven & Kaplan in press) and production level at age 15 hovers between 10–40% of adult levels. The Ache diet while foraging is roughly 80% meat, and men do most of the hunting. Women and children therefore directly contribute relatively few calories. Ju/'hoansi diet consists of 30–40% meat, also obtained almost exclusively by men. Women collect mongongo nuts, roots, berries and other fruits and contribute about half of the calories in the diet (Lee 1979). Children contribute relatively little to the diet.
Given the paucity of detailed food production profiles across the lifespan of hunter–gatherers, we simulate two different age-specific production curves for Ache and Ju/'hoansi males and females and call them ‘high skill’ and ‘low skill’. We model production using a Cobb-Douglas function with two inputs—strength, s(x), and skill, k(x), where x refers to age. We allow strength to peak early in the lifespan and decline in later ages, while skills increase throughout the lifespan at a decreasing rate. The ‘high-skill’ curve is meant to depict a socioecology where sub-adults sacrifice early production to achieve large caloric gains later in life. In the ‘low-skill’ scenario, sub-adults are greater producers because caloric gains are achieved earlier in life, and peak production is therefore achieved at an earlier age but at a lower absolute level than in the ‘high-skill’ case. Production, P(x), is estimated as s(x)αk(x)β, where α and β signify the relative weight of strength versus skill. For ‘high skill’, α>β, and vice-versa for ‘low skill’. Details on the estimation procedure of production are given in Gurven & Kaplan (in press). Production functions for each population are scaled such that production equals consumption over the lifespan of an individual.
When Ache and Ju/'hoansi sub-adults acquire calories as defined by the ‘high-skill’ profile, they are able to fund 29 and 11 Gcals, or 44 and 48% of their respective baseline demand under observed growth patterns (table 2). With ‘low skill’, Ache and Ju/'hoansi both cover 78–79% of their baseline demand. These are very generous estimates of sub-adult production. Using actual Ache age-specific production, we find that sub-adults can reduce their baseline lifetime demand by only 12 Gcals, or 19%. No age-specific production data are available for Ju/'hoansi. In our analysis, adding production does not change the additional demand required of alternative growth regimes, although the percentage increase from baseline increases significantly because the baseline becomes smaller with greater sub-adult production (table 2).
We have ignored the effect that having a larger body size earlier in the lifespan would have on sub-adult production. We should expect a greater impact on sub-adult production when early gains in physical size exhibit rapid gains in performance. How much must sub-adult production increase in order for the net demand on parents from each growth alternative to remain equal to the observed demand? Under low and high-skill production, we estimate that Ache sub-adult production from ages 4–18 must increase by a factor of 1.3 and 1.4 in order to have the same lifetime net energetic demand on parents if growth were chimpanzee-like rather than the observed delayed pattern. Ju/'hoansi sub-adult production must increase by a factor of 1.6 and 1.9 for low and high-skill production profiles, respectively, for the demand of chimpanzee growth trajectories to equal that of observed growth trajectories. Linear growth requires little adjustment. Assuming observed Ache production, sub-adult production must increase by larger factors (table 2).
4. Discussion and conclusion
The human pattern of slow human growth between ages at weaning and puberty helps defer rising demand on parents with multiple, overlapping dependents. Our results are consistent with the ‘ecological risk-aversion’ argument made by Janson & Van Schaik (1993), whereby staying small reduces feeding competition with adults and therefore benefits juveniles. We used Ache and !Kung demography and growth trajectories to show that significant energetic savings are possible when childhood-juvenile growth is slow, and adolescent growth is rapid. We assumed that adult body size and age at maturation are optimally set to balance the benefits obtained from growing longer and thus reproducing at a higher rate, with the costs of decreased survivorship as one spends more time growing and not reproducing (Charnov & Berrigan 1993). It is the growth trajectory that occurs between ages at weaning and maturation that is of interest in this study, rather than optimal age at maturation. Slow human growth followed by rapid adolescent growth may have evolved in response to rising human fertility rates in a foraging niche where early increases in body size are sacrificed for early investments in brain growth and immune function. Indeed, human brain size is over three times larger than expected for an anthropoid primate of our size (Falk 1980), and the neocortex is 3.6 times larger than expected (Rilling & Insel 1999). The large size of the human brain relative to the rest of the body consumes about 60% of a newborn's basal metabolic rate (Martin 1981). We have shown that juvenile production would have to increase dramatically to account for the higher demands of larger body size. It is unlikely that larger body size alone at early ages can lead to increases in foraging production enough to compensate for this greater caloric demand (Walker et al. 2002).
One possibility is that the extra calories necessary to offset faster growth could be provided by older individuals. High fertility may only be possible when adult mortality is sufficiently low that enough older individuals are alive to help feed children and grandchildren, thereby decreasing weaning time and child mortality, and increasing fertility. However, additional simulation shows that observed peak adult production occurs when caloric demand of dependents is highest, and so peak production levels would have to increase significantly to fund faster growing children. Instead, higher fertility is achieved from a strategy that emphasizes more slow growing children than fewer faster growing children.
The shape and absolute levels of the age-specific production profile are critical features affecting optimal growth trajectories. When juveniles can easily subsidize their own growth because production performance is heavily dependent on body size, growth should be fairly rapid. This is characteristic of most folivorous and frugivorous monkey species where adolescent growth spurts are mostly absent (Leigh & Park 1998), and to a lesser extent among the apes, where spurts are variable across species and sex (and occur only for weight, not stature). In more difficult foraging niches, greater investment in brain growth and immune function is associated with increased sociality, longevity and dependency of juveniles. Pathogenic environments further shift early energetic investments from growth to immune function (McDade 2003). Higher absolute levels of production and decreased child mortality allow for greater fertility, which places increased pressure on parents to economize by having children who grow more slowly during their least productive years. Thus, slow growth with a later growth spurt is a unique feature of our human life history (see Bogin 1988). There is suggestive evidence from the Turkana fossil that such a growth trajectory was absent in Homo erectus (Smith 1993), and may have appeared only in modern H. sapiens, up to 125 kya (Bogin 1997).
Adolescent growth spurts are robust features of the human life course, and are somewhat absent only in severe circumstances, e.g. hypoxia, heavy workloads and chronic malnutrition among Peruvian Quechua (Frisancho 1977). Adolescent and juvenile growth rates are more similar across advantaged and disadvantaged contexts than infant and child growth (Stinson 2000). Nonetheless, compared with those from well-nourished populations, children from food-limited populations display slower growth over a longer time period, with catch-up growth enabling individuals to recoup early losses in physical growth (Stinson 2000). The growth spurt occurs earlier and over a shorter period in well-fed populations. Increased energetic availability, therefore, favours earlier maturation and at a larger body size. More food does not promote linear growth or very rapid growth early in the lifespan. Instead, a greater energy budget results in a greater quantity and quality of offspring. The greater quality derives from the earlier onset of reproductive maturation and at a higher reproductive rate. Having a greater quantity of multiple dependents with slow growth and adolescent growth spurts may be a more efficient means of supporting more offspring.
Acknowledgments
We thank three anonymous reviewers for their helpful comments. We are also especially grateful to Nancy Howell, Kim Hill and Magdalena Hurtado for conducting demographic studies of the highest quality and for making growth data available.
Supplementary Material
References
- Bliege Bird R, Bird D. Constraints of knowing or constraints of growing? Fishing and collecting by the children of Mer. Hum. Nat. 2002;13:239–267. doi: 10.1007/s12110-002-1009-2. [DOI] [PubMed] [Google Scholar]
- Blurton Jones N.G, Hawkes K, O'Connell J. Modeling and measuring costs of children in two foraging societies. In: Standen V, Foley R.A, editors. Comparative socioecology: the behavioral ecology of humans and other mammals. Blackwell Scientific Publications; Oxford, UK: 1989. [Google Scholar]
- Blurton Jones N, Hawkes K, O'Connell J.F. Some current ideas about the evolution of the human life history. In: Lee P.C, editor. Comparative primate socioecology. Cambridge University Press; Cambridge, UK: 1999. pp. 140–166. [Google Scholar]
- Bogin B. Patterns of human growth. Cambridge University Press; Cambridge, UK: 1988. [Google Scholar]
- Bogin B. Evolutionary hypotheses for human childhood. Yrbk. Phys. Anthropol. 1997;104:63–90. 10.1002/(SICI)1096-8644(1997)25+%3C63::AID-AJPA3%3E3.0.CO;2-8 [Google Scholar]
- Brody S. Bioenergetics and growth. Reinhold; New York: 1945. [Google Scholar]
- Charnov E.L. Life history invariants: some explanations of symmetry in evolutionary ecology. Oxford University Press; Oxford, UK: 1993. [Google Scholar]
- Charnov E, Berrigan D. Why do female primates have such long lifespans and so few babies? Evol. Anthropol. 1993;1:191–194. 10.1002/evan.1360010604 [Google Scholar]
- Cordain L, Brand Miller J, Eaton S.B, Mann N, Holt S.H.A, Speth J.D. Plant-animal subsistence ratios and macronutrient energy estimations in hunter–gatherer diets. Am. J. Clin. Nutr. 2000;71:682–692. doi: 10.1093/ajcn/71.3.682. [DOI] [PubMed] [Google Scholar]
- Falk D. Hominid brain evolution: the approach from paleoneurology. Yrbk. Phys. Anthropol. 1980;23:93–107. [Google Scholar]
- FAO/WHO/UNU. WHO technical report series. WHO; Geneva: 1985. Protein and energy requirements. [Google Scholar]
- Frisancho A.R. Human growth and development among high-altitude populations. In: Baker P, editor. The biology of high altitude peoples. Cambridge University Press; Cambridge, UK: 1977. pp. 117–171. [Google Scholar]
- Gurven, M. D. & Kaplan, H. S. In press. Determinants of time allocation to production across the lifespan among the Machiguenga and Piro Indians of Peru. Hum. Nat. [DOI] [PubMed]
- Harvey P.H, Martin R.D, Clutton-Brock T.H. Life histories in comparative perspective. In: Smuts B.B, Cheney D.L, Seyfarth R.M, Wrangham R.W, Struthsaker T.T, editors. Primate societies. University of Chicago; Chicago, IL: 1987. [Google Scholar]
- Hawkes K. Grandmothers and the evolution of human longevity. Am. J. Hum. Biol. 2003;15:380–400. doi: 10.1002/ajhb.10156. 10.1002/ajhb.10156 [DOI] [PubMed] [Google Scholar]
- Hawkes K, O'Connell J.F, Blurton Jones N.G, Alvarez H, Charnov E.L. Grandmothering, menopause, and the evolution of human life histories. Proc. Natl Acad. Sci. USA. 1998;95:1336–1339. doi: 10.1073/pnas.95.3.1336. 10.1073/pnas.95.3.1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Hurtado A.M. Aldine; Hawthorne, NY: 1996. Ache life history: the ecology and demography of a foraging people. [Google Scholar]
- Holmes R. Small is adaptive: nutritional anthropometry of native Amazonians. In: Sponsel L, editor. Indigenous peoples and the future of Amazonia. University of Arizona Press; Tucson, AZ: 1995. pp. 121–148. [Google Scholar]
- Howell N. Demography of the Dobe !Kung. Academic Press; New York: 1979. [Google Scholar]
- Howell N. University of Toronto, Department of Sociology, Data Library Service; Toronto, Ont.: 2000. Bushman demography: demography of the Dobe !Kung, 1963–1973 [machine readable data file] ( http://www.chass.utoronto.ca/datalib/codebooks/utm/howell.htm) [Google Scholar]
- Hrdy S. Comes the child before the man: how cooperative breeding and prolonged post-weaning dependence shaped human potentials. In: Hewlett B.S, Lamb M.E, editors. Hunter–gatherer childhoods. Transactions; Piscataway: 2005. pp. 65–91. [Google Scholar]
- Janson C.H, Van Schaik C.P. Ecological risk aversion in juvenile primates: slow and steady wins the race. In: Pereira M, Fairbanks L, editors. Juvenile primates: life history, development and behavior. Oxford University Press; New York: 1993. pp. 57–76. [Google Scholar]
- Jolicoeur P, Pontier J, Pernin M.O, Sempe M. A lifetime asymptotic growth curve for human height. Biometrics. 1988;44:995–1003. [PubMed] [Google Scholar]
- Kaplan H, Hill K, Lancaster J.B, Hurtado A.M. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 2000;9:156–185. 10.1002/1520-6505(2000)9:4%3C156::AID-EVAN5%3E3.0.CO;2-7 [Google Scholar]
- Kappeler P.M, Pereira M.E, editors. Primate life history and socioecology. University of Chicago Press; Chicago, IL: 2003. [Google Scholar]
- Kramer K.L. Harvard University Press; Cambridge, MA: 2005. Maya children: helpers at the farm. [Google Scholar]
- Lee R.B. The !Kung San: men, women, and work in a foraging society. Cambridge University Press; Cambridge: 1979. [Google Scholar]
- Leigh S.R. Evolution of human growth. Evol. Anthropol. 2001;10:223–236. 10.1002/evan.20002 [Google Scholar]
- Leigh S.R, Park P.B. Evolution of human growth prolongation. Am. J. Phys. Anthropol. 1998;107:331–350. doi: 10.1002/(SICI)1096-8644(199811)107:3<331::AID-AJPA9>3.0.CO;2-#. 10.1002/(SICI)1096-8644(199811)107:3%3C331::AID-AJPA9%3E3.0.CO;2-# [DOI] [PubMed] [Google Scholar]
- Leigh S.R, Shea B.T. Ontogeny of body size variation in African apes. Am. J. Phys. Anthropol. 1996;99:43–65. doi: 10.1002/(SICI)1096-8644(199601)99:1<43::AID-AJPA3>3.0.CO;2-0. 10.1002/(SICI)1096-8644(199601)99:1%3C43::AID-AJPA3%3E3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- Martin R.D. Relative brain size and basal metabolic rate in terrestrial vertebrates. Nature. 1981;293:57–60. doi: 10.1038/293057a0. 10.1038/293057a0 [DOI] [PubMed] [Google Scholar]
- McDade T.W. Life history theory and the immune system: steps toward a human ecological immunology. Yrbk. Phys. Anthropol. 2003;46:100–125. doi: 10.1002/ajpa.10398. 10.1002/ajpa.10398 [DOI] [PubMed] [Google Scholar]
- Pereira M.E, Fairbanks L.A, editors. Juvenile primates: life history, development, and behavior. University of Chicago Press; Chicago, IL: 2002. [Google Scholar]
- Rilling J.K, Insel T.R. The primate neocortex in comparative perspective using magnetic resonance imaging. J. Hum. Evol. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. 10.1006/jhev.1999.0313 [DOI] [PubMed] [Google Scholar]
- Smith B.H. Physiological age of KMN-WT 15000 and its significance for growth and development of early Homo. In: Walker A.C, Leakey R.F, editors. The Nariokatome. Belknap Press; Cambridge, MA: 1993. pp. 195–220. [Google Scholar]
- Stearns S.C. The evolution of life histories. Oxford University Press; Oxford, UK: 1992. [Google Scholar]
- Stinson S. Growth variation: biological and cultural factors. In: Stinson S, Bogin B, Huss-Ashmore R, O'Rourke D, editors. Human biology: an evolutionary and biocultural perspective. Wiley-Liss; New York: 2000. pp. 425–463. [Google Scholar]
- Tucker B, Young A.G. Growing up mikea: children's time allocation and tuber foraging in southwestern Madagascar. In: Hewlett B, Lamb M, editors. Hunter–gatherer childhoods. Aldine de Gruyter; New York: 2005. pp. 147–171. [Google Scholar]
- Walker R, Hill K, Kaplan H, McMillan G. Age-dependency in skill, strength and hunting ability among the Ache of eastern Paraguay. J. Hum. Evol. 2002;42:639–657. doi: 10.1006/jhev.2001.0541. 10.1006/jhev.2001.0541 [DOI] [PubMed] [Google Scholar]
- Walker, R., Hill, K., Burger, O. & Hurtado, A. M. In press. Life in the slow lane revisited: ontogenetic separation between chimpanzees and humans. Am. J. Phys. Anthropol. [DOI] [PubMed]
- Watts E.S. Adolescent growth and development of monkeys, apes and humans. In: Watts E.S, editor. Nonhuman primate models for human growth and development. Alan R. Liss; New York: 1985. pp. 41–65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.