Abstract
An increased susceptibility to disease is one hypothesis explaining how inbreeding hastens extinction in island endemics and threatened species. Experimental studies show that disease resistance declines as inbreeding increases, but data from in situ wildlife systems are scarce. Genetic diversity increases with island size across the entire range of an extremely inbred Galápagos endemic bird, providing the context for a natural experiment examining the effects of inbreeding on disease susceptibility. Extremely inbred populations of Galápagos hawks had higher parasite abundances than relatively outbred populations. We found a significant island effect on constitutively produced natural antibody (NAb) levels and inbred populations generally harboured lower average and less variable NAb levels than relatively outbred populations. Furthermore, NAb levels explained abundance of amblyceran lice, which encounter the host immune system. This is the first study linking inbreeding, innate immunity and parasite load in an endemic, in situ wildlife population and provides a clear framework for assessment of disease risk in a Galápagos endemic.
Keywords: disease, Galápagos Islands, genetic diversity, immune function, natural antibodies
1. Introduction
Extinctions of island endemics account for 75% of animal extinctions and 90% of bird extinctions (Myers 1979; Reid & Miller 1989). Several synergistic key factors may be responsible for this high extinction rate, including introduction of exotic animal and human predators (Blackburn et al. 2004), habitat destruction (Rolett & Diamond 2004), demographic stochasticity (Drake 2005), and inbreeding in island endemics and threatened species (Frankham 1998; Spielman et al. 2004a).
The interaction of disease agents with genetically depauperate (Pearman & Garner 2005) and isolated populations is one hypothesis explaining how inbreeding facilitates extinction in small populations (de Castro & Bolker 2005). Parasites evolve more quickly than hosts, so host antiparasite adaptations are perpetually obsolete (Hamilton et al. 1990; Lively & Apanius 1995). Consequently, genetically uniform host individuals (Acevedo-Whitehouse et al. 2003) and populations (Spielman et al. 2004b) are more susceptible to parasitism than genetically diverse hosts. Studies of model laboratory systems (Arkush et al. 2002), captive wildlife (Cassinello et al. 2001), and free-ranging domesticated animal populations (Coltman et al. 1999) support this claim, although other studies do not (Trouvé et al. 2003). Scant evidence of this phenomenon exists from in situ native wildlife populations (Meagher 1999), and no study has examined the effects of inbreeding on parasite load and innate, humoral immunity across bird populations in the wild (Keller & Waller 2002). The intact endemic avifauna of the Galápagos Islands provides a unique opportunity to examine disease ecology and will provide insight into the impact of invasive disease agents that may enter the ecosystem (Lindström et al. 2004; Thiel et al. 2005).
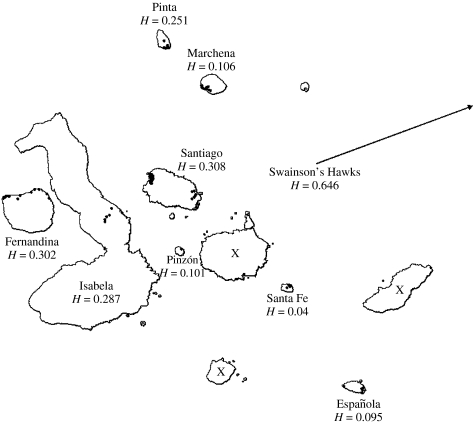
The Galápagos hawk (Buteo galapagoensis), an endemic raptor threatened with extinction (2004 IUCN Red List), breeds on eight islands within the Galápagos National Park, and has been extirpated from several others (figure 1). Island size and genetic diversity are positively related and between-island population structure is high, rendering it an appealing model system in which to examine the effects of inbreeding on disease severity (Bollmer et al. in press a). The basic biology of its two chewing louse species (Insecta: Phthiraptera), an amblyceran (Colpocephalum turbinatum) and an ischnoceran (Degeeriella regalis), has been described (Whiteman & Parker 2004a,b). Thus, we examined the response of each parasite lineage to variance in host inbreeding, using population-level heterozygosity values from the eight island populations of B. galapagoensis and one population of the sister species (Buteo swainsoni; Riesing et al. 2003).
Figure 1.
Map of the Galápagos Archipelago, located approximately 1000 km west of mainland Ecuador, South America. Extant breeding island populations of the Galápagos hawk (Buteo galapagoensis) are named, followed by estimates of island population genetic diversity (H; Stephens heterozygosity values) calculated from multilocus minisatellite data. Small black dots within islands indicate sampling localities. An estimation of H from the mainland Swainson's hawk (the putative sibling species of B. galapagoensis) was included for comparative purposes. Extinct island populations of B. galapagoensis are indicated by an ‘X’ (there is no evidence indicating hawks have ever inhabited Isla Genovesa located in the northeastern part of the archipelago).
We also examined the relationship between immunological host defences, island-level inbreeding effects, and parasite abundance. To assess immunological host defences, we quantified non-specific natural antibody (NAb) titres within seven populations of B. galapagoensis. Quantification of NAbs has several conceptual and methodological advantages over other methods used to assess immune response of wild vertebrates (Matson et al. 2005). NAbs are a product of the innate, humoral immune system and their production is constitutive (stable over time and generally not induced by external antigenic stimulation). Encoded by the germ-line genome, NAbs are present in antigenically naive vertebrates (Ochsenbein & Zinkernagel 2000), form a large percentage of the serum immunoglobulin (Kohler et al. 2003), are capable of recognizing any antigen, and prime the adaptive immune response (Adelman et al. 2004). In chickens, NAbs reacting to ectoparasite-derived antigens have been identified (Wikel et al. 1989) and in lines artificially selected for either high or low levels of specific antibodies, specific and NAb levels covary (Parmentier et al. 2004). NAb response is hypothesized to predict the strength of the adaptive immune response (Kohler et al. 2003). Thus, NAbs form a functional link between the innate and acquired parts of the humoral immune system (Lammers et al. 2004).
Inbreeding may negatively impact phytohaemagglutinin (PHA) induced swelling within wild bird populations (Reid et al. 2003), and reductions in population size reduce overall within-population genetic variation, including variation at loci of immunological import in vertebrates (Miller & Lambert 2004). Since variation in NAb levels responds to artificial selection in chickens (Parmentier et al. 2004), it is reasonable to predict that variation in NAb levels will covary with variation in wild bird population genetic diversity. However, the impact of natural microevolutionary processes on circulating levels of NAbs is unknown in wild vertebrates.
Amblyceran lice (e.g. C. turbinatum) directly encounter host immune defences because they feed on blood and living skin (Marshall 1981). Conversely, bird ischnocerans (e.g. D. regalis) generally feed on the keratin of feathers and dead skin (Marshall 1981) and mainly encounter the mechanical host defences (e.g. preening). Feeding by ectoparasites on skin and blood elicits immune responses (Wikel 1982) that vary from cell-mediated (Prelezov et al. 2002) to humoral (i.e. antibodies; Pfeffer et al. 1997) and from innate (Wikel et al. 1989) to acquired (Ben-yakir et al. 1994). Host antibodies reduce louse fecundity and survivorship, and regulate population growth rate (Ben-yakir et al. 1994). Across bird species, variation in PHA-induced swelling was directly related to amblyceran but not ischnoceran species richness (Møller & Rózsa 2005). However, whether NAbs regulate ectoparasite populations, and louse populations in particular, is unknown.
We measured host inbreeding, parasite abundance and NAb response, and made three predictions: (i) at the island-level, higher inbreeding results in lower average humoral immune response relative to outbred populations; (ii) also at the island-level, higher inbreeding results in reduced variation in humoral immune response relative to outbred populations and (iii) birds with high humoral immune responses harbour fewer parasites (amblyceran lice) relative to birds with lower immune responses.
2. Material and methods
(a) Host sampling
We live-captured a total of 211 Buteo hawk individuals on eight of the Galápagos Islands (n=202 B. galapagoensis; figure 1) and near Las Varillas, Córdoba, Argentina (n=9 B. swainsoni; Whiteman & Parker 2004a), from May–August 2001 (Islas Española, n=8; Isabela, n=25; Marchena, n=26; Santa Fe, n=13), May–July 2002 (Isla Santiago, n=58), January 2003 (Argentina, n=9), and May–July 2003 (Islas Fernandina, n=28; Pinta, n=31; Pinzón, n=10). Birds were sampled following Bollmer et al. (in press a) from multiple locations throughout each island. The University of Missouri-St Louis Animal Care Committee and the appropriate governmental authorities approved all procedures and permits.
(b) Parasite sampling
We quantitatively sampled parasites from birds via dust ruffling with pyrethroid insecticide (non-toxic to birds; Zema Z3 Flea and Tick Powder for Dogs, St John Laboratories, Harbor City, California; Whiteman & Parker 2004a,b). Dust-ruffling provides excellent measures of relative louse intensity (Clayton & Drown 2001).
(c) Blood collection
From each bird, we collected two 50 μl blood samples via venipuncture of the brachial vein for genetic analyses. Samples were immediately stored in 500 μl of lysis buffer (Longmire et al. 1988). For immune assay, whole blood samples were collected from a subsample of birds (n=46) in heparinized tubes, centrifuged in the field and plasma was stored in liquid nitrogen. Due to logistical constraints, no plasma was collected from the Pinzón population of B. galapagoensis or from B. swainsoni.
(d) Innate humoral immunity
We used the general haemolysis–haemagglutination assay protocol (Matson et al. 2005) with two minor modifications (we used plates from Corning Costar #3798, instead of #3795 and Dulbecco's PBS, #D8662, Sigma, St Louis, MO). Sample sizes from Galápagos hawk island populations were as follows: Española, n=3; Fernandina, n=15; Isabela, n=3; Marchena, n=5; Pinta, n=7; Santa Fe, n=5; Santiago, n=8. In each plate, we ran the assay on six hawk samples and two positive controls (pooled chicken plasma, #ES1032P, Biomeda, Foster City, CA). Using digitized images of the assay plates, all samples were blindly scored twice to individual, plate number and position. To demonstrate positive standard reliability, assay variation never exceeded 6.8 and 5.6% coefficient of variation (in all cases, CV was calculated using the sample size correction; Sokal & Rohlf 1995) for agglutination titres among and within plates, respectively. Mean NAb agglutination titres and CV were then calculated for each island population from which plasma was collected. CV is a useful measure in studies such as these, since island population means varied widely and CV is dimensionless and relatively stable compared to standard deviation (Snedecor & Cochran 1989).
(e) DNA fingerprinting
To determine island-level population genetic diversity, we performed phenol–chloroform DNA extraction on a subset of hawks from each population comprising a total of 118 individuals (Galápagos hawks: Española, n=7; Fernandina, n=20; Isabela, n=10; Marchena, n=20; Pinta, n=10; Pinzón, n=10; Santa Fe, n=10; Santiago, n=23; Swainson's hawks: n=8), followed by multi-locus minisatellite (VNTR) fingerprinting using the restriction endonuclease Hae III and Jeffreys' probe 33.15 (Jeffreys et al. 1985) and following procedures described elsewhere for birds generally (Parker et al. 1995) and Galápagos hawks (Bollmer et al. in press a). Estimates of island-level population genetic diversity were obtained by calculating multilocus VNTR heterozygosity values (referred to as H; Stephens et al. 1992) for each island population and for the population of Swainson's hawks using Gelstats v. 2.6 (Rogstad & Pelikan 1996). These markers yield an excellent measure of relative genetic diversity in small, isolated vertebrate populations (Gilbert et al. 1990; Stephens et al. 1992; Parker et al. 1998; Bollmer et al. in press a) but do not measure individual heterozygosity values.
A large study on Galápagos hawk population genetics (Bollmer et al. in press a) used the same multilocus minisatellite markers to estimate population genetic diversity (and included all of the individuals genotyped here). Bollmer et al. (in press a) strongly support the pattern of genetic diversity that we found among these hawk populations. Nearly 90% of the variation in hawk population genetic diversity was explained by island area, and the latter correlates with hawk population size (Bollmer et al. in press a). The four smallest islands with hawk populations had the highest reported levels of minisatellite uniformity of any wild, relatively unperturbed bird species.
As in Bollmer et al. (in press a), we randomly selected individuals sampled within each population to assess the relative amount of genetic diversity within each population. We prioritized samples from adults in territorial breeding groups (groups are comprised of unrelated adults; Faaborg et al. 1995). On Isla Pinzón, we sampled only from non-territorial birds from multiple geographic locales because we were unable to capture adults there. However, these birds were likely offspring of multiple breeding groups given that many were of the same age cohort (based on plumage characteristics), and that hawks usually produce only one offspring per breeding attempt. Moreover, marked, non-territorial birds disperse from the natal territory following fledging and roam over their entire natal islands (de Vries 1975; Faaborg 1986; Bollmer et al. in press a). To ensure that our sampling of birds was not biased by the possible presence of within-island population genetic structure, we sampled and multilocus genotyped birds from multiple geographic locales. For example, on Islas Española and Santiago (which harbour hawk populations with among the lowest and highest genetic diversity, respectively), we sampled territorial birds from the extreme eastern and western portions of the islands (figure 1). On the smaller islands, we sampled birds from a greater proportion of island area than on the larger islands (figure 1). Due to the low genetic diversity within the four smallest hawk populations (Española, Santa Fe, Pinzón, and Marchena), sampling from relatively fewer individuals on the smallest islands was sufficient to characterize their population genetic diversity (Bollmer et al. in press a). Bollmer et al. (in press a) found only four multilocus genotypes within Isla Santa Fe in the 15 birds sampled from both multiple years and geographic locations throughout the island (the entire population of hawks on Santa Fe is likely to be ∼30 birds). Bollmer et al. (in press a) further found that populations from Islas Santa Fe, Española, Pinzón, and Marchena were all relatively inbred compared to more variable (but still inbred) populations from Islas Pinta, Fernandina, Isabela and Santiago. Our samples from Swainson's hawks (n=8) and from Isla Isabela (n=10) were small relative to the larger Galápagos hawk population sample sizes, yet both were relatively outbred based on H estimated from the minisatellites. Given this, our estimation of relative genetic diversity within each hawk population sampled is representative of the standing genetic diversity within each population and is not an artifact of sampling bias or within-population genetic structure.
(f) Statistical analyses
For all statistical analyses except the overall comparison of prevalence between louse species which utilized Quantitative Parasitology v. 2.0 (Reiczigel & Rózsa 2001), louse abundance data were ln +1 transformed and Stephen's heterozygosity values were arcsine square root transformed to meet assumptions of normality.
We performed a Pearson's correlation analysis in SPSS v. 11.0 (2004) to assess the strength of the relationship between host population genetic diversity (H) and average host population parasite abundance from nine hawk populations (eight B. galapagoensis and one B. swainsoni). The correlation analyses were one-tailed given our a priori predictions about the direction of the relationship between the variables. We then examined the relationship between average louse abundance and H for the eight Galápagos hawk populations to determine if the relationship was being driven by the relatively outbred Swainson's hawks.
Next, we examined the relationship between innate humoral immunity (NAb agglutination titres) and H on the entire subset of individuals (n=46) for which plasma was collected. The relationship between average island NAb agglutination titres and H was not linear. Thus, we used the GLM procedure in Spss to determine if there was a significant effect of island-level H (a fixed factor) on NAb agglutination titres (the dependent variable) instead (Española, n=3; Fernandina, n=15; Isabela, n=3; Marchena, n=5; Pinta, n=7; Santa Fe, n=5; Santiago, n=8).
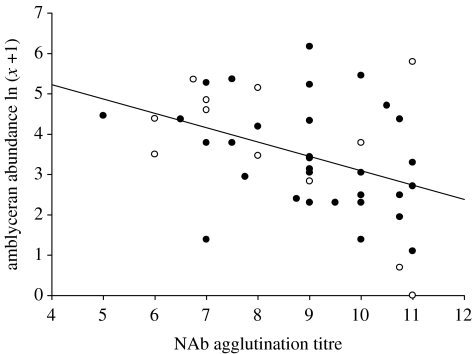
Finally, we performed a GLM analysis in SPSS using a subset of data that included all 43 birds sampled for both plasma and parasites to determine if antibodies and louse abundances were correlated. In order to control for the effect of island inbreeding we used the GLM procedure as in the preceding analysis (NAb agglutination titres of the 43 hawks dependent on island as a fixed factor) except that louse abundance for each of the 43 individuals was included as a covariate in the model (Española n=3; Fernandina n=14; Isabela n=3; Marchena n=5; Pinta n=7; Santa Fe n=4; Santiago n=7). One analysis was performed for each louse species. A scatterplot of the louse abundance data and NAb agglutination titres was created to show the relationships between the two variables before the analyses and individuals were labelled as either inhabiting a relatively inbred (Española, Marchena or Santa Fe) or outbred (Fernandina, Isabela, Pinta or Santiago) island (see figure 3).
Figure 3.
Negative linear relationship between Colpocephalum turbinatum abundance and natural antibody (NAb) titres. The regression line through the raw data (uncorrected for island) is shown (β=−0.355, p<0.01). The relationship was marginally significant after controlling for the effects of island and other host factors (β=−0.342, p=0.05). Open circles, individuals from more inbred island populations (Española, Marchena, Santa Fe); solid circles, individuals from more outbred island populations (Fernandina, Isabela, Pinta, Santiago).
3. Results
(a) Parasite collections
We collected a total of 14 843 individuals of the louse C. turbinatum and 2858 individuals of the louse D. regalis from 199 Galápagos hawks sampled for lice. These lice typically occur on no other birds in the Galápagos, but have been reported from mainland B. swainsoni (Whiteman & Parker 2004a). Overall prevalence (across islands) of C. turbinatum (97.5%) was higher than that of D. regalis (85.4%; Fisher's exact test, p<0.001); both louse species occurred in all eight host populations.
We collected a total of 17 individuals of C. turbinatum, 22 individuals of Laemobothrion maximum and 11 individuals of a Kurodaia sp. from the nine Swainson's hawks. These three species abundances were pooled and constitute the amblyceran lice from Swainson's hawks; C. turbinatum was the only amblyceran collected from Galápagos hawks. No Degeeriella were collected from the nine Swainson's hawks.
(b) Assessment of population genetic diversity
Untransformed values of H for each host population are shown in figure 1. Individuals from the smallest island-populations of the Galápagos hawk had the highest reported levels of minisatellite uniformity of any wild, unperturbed bird species and these results are consistent with those of Bollmer et al. (in press a). As in Bollmer et al. (in press a), we found >50% of all bands were fixed within these populations (Santa Fe, 13/16 bands fixed; Española, 10/16 bands fixed; Pinzón, 11/20 bands fixed; Marchena, 11/18 bands fixed). The four most inbred populations contained multiple individuals or sets of individuals that were genetically identical at all loci, whereas no identical individuals were found within the four larger islands populations or within Swainson's hawks (Bollmer et al. in press a).
(c) Effects of genetic diversity and other host factors on parasite load
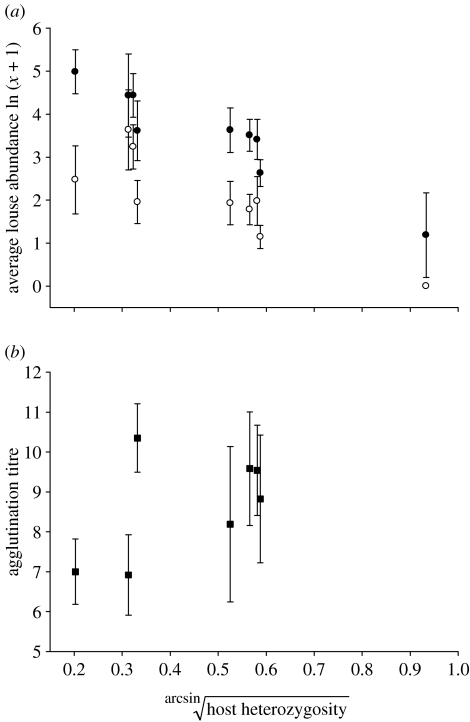
Among Buteo populations (n=208 total individuals sampled for lice by population: Española, n=8; Fernandina, n=28; Isabela, n=25; Marchena, n=26; Pinta, n=31; Pinzón, n=10; Santa Fe, n=13; Santiago, n=58; Swainson's hawks n=9), average amblyceran louse abundance within populations and H were significantly and negatively related across populations (figure 2a; C. turbinatum; Pearson's r=−0.949, n=9, p<0.0001; D. regalis; r=−0.854, n=9, p<0.01). When limited to the eight Galápagos hawk island populations only, similar negative relationships were found for C. turbinatum (r=−0.875, n=8, p<0.01) and D. regalis (r=−0.69, n=8, p<0.05).
Figure 2.
Scatterplot of two disease susceptibility variables versus estimated host population genetic diversity (heterozygosity) values. (a) Louse abundance versus host population genetic diversity. Closed circles, average amblyceran abundance ±95% confidence intervals (Colpocephalum turbinatum, Laemobothrion maximum, and Kurodaia sp.; r=−0.949, n=9, p<0.0001); open circles, average ischnoceran abundance ±95% confidence intervals (Degeeriella regalis; r=−0.854, n=9, p<0.01). Dyads with heterozygosity values greater than 0.9 represent a mainland B. swainsoni population and the remaining values represent eight island populations of B. galapagoensis. Island populations reading left to right are as follows: Santa Fe, Española, Pinzón, Marchena, Pinta, Isabela, Fernandina, Santiago; (b) average agglutination titres (NAbs)±SDM from 46 B. galapagoensis individuals versus estimated host population genetic diversity (the relationship between NAb agglutination titres and genetic diversity was not linear, although significant differences existed in average NAb agglutination titres among island-populations, one-way ANOVA: F6,39, p<0.01). Island populations reading left to right are as follows: Santa Fe, Española, Marchena, Pinta, Isabela, Fernandina, Santiago.
(d) Innate antibody levels, genetic diversity and parasite load
We found a significant (and nonlinear) effect of island on average NAb agglutination titres (figure 2b; one-way ANOVA; n=46, F6,39=3.41, p<0.01). The Marchena population, the third most inbred population, exhibited the highest average titre and Española and Santa Fe, the most inbred populations, exhibited the lowest (figure 2b). The more outbred island populations had intermediate NAb titres. The variance in NAb titres was lower within the inbred populations than the more outbred populations (figure 2b). The CV of the inbred populations (Santa Fe, Española, Marchena) was 12% within and 25.5% among islands, whereas the CV of the more outbred islands (Fernandina, Isabela, Pinta, Santiago) was 17.8% within and 4.7% among islands. Furthermore, C. turbinatum abundance was negatively related to NAb agglutination titres (marginally significant) when individual birds were considered (controlling for the effects of island in a GLM; corrected model F7,35=4.05, p<0.01; island effect F=2.50, p<0.05, C. turbinatum abundance parameter estimate β=−0.342, F=4.10, p=0.05; figure 3). The scatterplot yielded a triangular pattern whereby birds with low NAb titres consistently harboured high C. turbinatum abundances, but birds with high NAb titres harboured both low and high louse abundances. As predicted, no significant relationship was found between the ischnoceran, feather-feeding D. regalis and NAb agglutination titres (controlling for the effects of island in a GLM; corrected model F7,35=3.01, p<0.05; island effect F=2.60, p<0.05, D. regalis abundance parameter estimate β=−0.259, F=1.68, p>0.05).
4. Discussion
We have shown that variation in host population genetic diversity is correlated negatively with average parasite load and positively with variation in NAb levels across populations of the Galápagos Hawk. Smaller, more inbred host populations had higher parasite loads, lower average immune responses (generally) and lower variation in within-population immune response than more outbred populations. NAb levels were negatively correlated with the abundance of a skin and blood feeding amblyceran louse, further linking inbreeding, immune response and parasite burden.
As a result of lower within-population genetic variability and lower and less variable within-population NAb levels, most of the peripheral, inbred and highly differentiated island populations of the Galápagos hawk are vulnerable to disease agents. This result may not be surprising, but few studies have evaluated this relationship in wildlife populations. These populations contained more among-island variability in NAb levels than the larger island-populations, possibly due to the strong effects of genetic drift (Spielman et al. 2004b; Pearman & Garner 2005) or local coevolutionary dynamics (Thompson 1999). Protection of the highly differentiated peripheral hawk populations should be prioritized as the variation they contain is essential for the long-term viability of this species (Lesica & Allendorf 1995). Conversely, the large amount of within-population genetic and immunological variation within the largest hawk island populations is also important from a conservation perspective. Since tradeoffs exist between the humoral and cellular immune response (Lindström et al. 2004), these populations may be better able to respond to multiple invasions of pathogens than the smaller, more isolated populations. Notably, breeding populations within three large islands (Islas Floreana, San Cristóbal and Santa Cruz) are now likely extinct (Bollmer et al. in press a,b) and each of these is geographically proximal to one or several of the most inbred island populations. Thus, if metapopulation dynamics were operating in this system (Thompson 1999; Templeton et al. 2001), the potential for the introduction of novel alleles (e.g. resistance alleles) by recurrent gene flow among populations has now been reduced given that only 8 out of 11 island populations remain intact. Thus, managers of the Galápagos National Park may consider restricting travel to the smallest island populations of the hawk, given that invasive avian disease vectors have established within several human-inhabited islands that serve as a base of operations for the tourism industry (Wikelski et al. 2004; Whiteman et al. 2005).
As a potential mechanism underlying the relationship between host genetic diversity and average parasite load, we showed that NAb agglutination titres were negatively related to abundance of native parasites that feed on skin and blood (C. turbinatum), although the correlational nature of this analysis and its marginal significance, after correcting for the effects of island, indicate that this result be accepted with caution and requires confirmation. However, strength of the PHA-induced immune response in birds was directly related to amblyceran species richness, indicating that amblycerans and their avian hosts are engaged in coevolutionary arms races (Møller & Rózsa 2005). Thus, our finding of a potential relationship between host immune response and amblyceran but not ischnoceran abundance at the individual host level is in accord with this macroevolutionary trend.
The influence of another unmeasured factor correlating with population genetic diversity may also explain the results, although we know of no such factor. Nearly 90% of the variation in hawk genetic diversity is explained by island size, and these hawk populations are genetically isolated from one another (Bollmer et al. in press a,b). Given that larger island populations typically had lower parasite loads, a simple relationship between host population size and parasite load is unlikely here (Lindström et al. 2004). Specific mechanisms underlying the relationship between H and disease susceptibility may include the exposure of deleterious recessive alleles (Keller & Waller 2002), the fixation of slightly deleterious alleles through genetic drift (Johnson & Seger 2001), other microevolutionary processes associated with founder events and maintenance of small population sizes over time, or a combination of these. Generalized inbreeding depression may also lead to physical and behavioural changes that affect preening efficiency and this may be particularly germane for D. regalis, which mainly encounters mechanical host defences (Clayton et al. 1999; Whiteman & Parker 2004b).
Extinction and disease ecology are ‘by their nature cryptic and difficult to study in natural communities’ (de Castro & Bolker 2005). Clearly, however, this information is of basic biological interest and offers insight into how populations will respond to invasions of alien pathogens, which is underway in most previously isolated ecosystems. Future studies examining host immunogenetics, parasite population genetics and transmission dynamics are necessary for fully assessing the threat of pathogens to this island endemic.
Acknowledgments
N.K.W. and P.G.P. were supported by a Dissertation Enhancement Grant from the National Science Foundation (NSF; INT-030759), grants from the Field Research for Conservation Program (FRC) of the Saint Louis Zoo, International Center for Tropical Ecology (UM-St Louis), Sigma Xi, and funds from the E. Desmond Lee and Family Fund's Collaborative Vision in Zoological Studies. K.D.M. was supported by grants from the NSF (IBN-0212587) and the FRC from the Saint Louis Zoo. For the Galápagos sampling and permits, we thank the Servicio Parqué Nacional de Galápagos and the Estación Científica Charles Darwin (Dr David Wiedenfeld), Isla Santa Cruz, Galápagos, Ecuador and Dr Tjitte de Vries and students, Pontificia Universidad Catolica del Ecuador for help with fieldwork. TAME provided discounted roundtrip air-travel within Ecuador. For sampling in Argentina, we thank José H. Sarasola and Dr Juan J. Negro (Estación Biológica de Doñana, Seville, Spain), Mark Beilstein (University of Missouri-St Louis) and Donna Eakman (Bothel, WA), and for collection and export permits, Dr Sandra Aliscioni (Instituto de Botánica Darwinion, Buenos, Aires, Argentina). We thank Drs Dale H. Clayton (University of Utah), Kirk C. Klasing (University of California, Davis), Robert J. Marquis (University of Missouri-St Louis), Robert E. Ricklefs (University of Missouri-St Louis), Alex Scheuerlein (University of Missouri-St Louis), and two anonymous reviewers for helpful comments on a previous version of the manuscript. Finally, we thank Dr Rebecca E. Forkner (University of South Florida) for assistance with statistical analyses in a previous version of this manuscript.
References
- Acevedo-Whitehouse K, Gulland F, Greig D, Amos W. Inbreeding-dependent pathogen susceptibility in California sea lions. Nature. 2003;422:35. doi: 10.1038/422035a. 10.1038/422035a [DOI] [PubMed] [Google Scholar]
- Adelman M.K, Schluter S.F, Marchalonis J.J. The natural antibody repertoire of sharks and humans recognizes the potential universe of antigens. Protein J. 2004;23:103–118. doi: 10.1023/b:jopc.0000020077.73751.76. 10.1023/B:JOPC.0000020077.73751.76 [DOI] [PubMed] [Google Scholar]
- Arkush K.D, Giese A.R, Mendonca H.L, McBride A.M, Marty G.D, Hedrick P.W. Resistance to three pathogens in the endangered winter-run chinook salmon (Oncorhynchus tshawytscha): effects of inbreeding and major histocompatibility complex genotypes. Can. J. Fish. Aquat. Sci. 2002;59:966–975. 10.1139/f02-066 [Google Scholar]
- Ben-yakir D, Mumcuoglu K.Y, Manor O, Ochanda J, Galun R. Immunization of rabbits with a midgut extract of the human body louse (Pediculus humanus humanus): the effect of induced resistance on the louse population. Med. Vet. Entomol. 1994;8:114–118. doi: 10.1111/j.1365-2915.1994.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Blackburn T.M, Cassey P, Duncan R.P, Evands K.L, Gaston K.J. Avian extinction and mammalian introductions on oceanic islands. Science. 2004;305:1955–1958. doi: 10.1126/science.1101617. 10.1126/science.1101617 [DOI] [PubMed] [Google Scholar]
- Bollmer, J. L., Whiteman, N. K., Bednarz, J. C., DeVries, Tj. & Parker, P. G. In press a Population genetics of the Galápagos hawk: genetic monomorphism within isolated populations. Auk
- Bollmer, J. L., Kimball, R. T., Whiteman, N. K., Sarasola, J. H. & Parker, P. G. In press b Phylogeography of the Galápagos Hawk (Buteo galapagoensis): a recent arrival to the Galápagos Islands. Mol. Phylogenet. Evol [DOI] [PubMed]
- Cassinello J, Gomendio M, Roldan E.R.S. Relationship between coefficient of inbreeding and parasite burden in endangered gazelles. Conserv. Biol. 2001;15:1171–1174. 10.1046/j.1523-1739.2001.0150041171.x [Google Scholar]
- Clayton D.H, Drown D.M. Critical evaluation of five methods for quantifying chewing lice (Insecta: Phthiraptera) J. Parasitol. 2001;87:1291–1300. doi: 10.1645/0022-3395(2001)087[1291:CEOFMF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Clayton D.H, Lee P.L.M, Tompkins D.M, Brodie E.D., III Reciprocal natural selection on host–parasite phenotypes. Am. Nat. 1999;154:261–270. doi: 10.1086/303237. 10.1086/303237 [DOI] [PubMed] [Google Scholar]
- Coltman D.W, Pilkington J.G, Smith J.A, Pemberton J. Parasite-mediated selection against inbred Soay sheep in a free-living, island population. Evolution. 1999;53:1259–1267. doi: 10.1111/j.1558-5646.1999.tb04538.x. [DOI] [PubMed] [Google Scholar]
- de Castro F, Bolker B. Mechanisms of disease-induced extinction. Ecol. Lett. 2005;8:117–126. 10.1111/j.1461-0248.2004.00693.x [Google Scholar]
- de Vries T. The breeding biology of the Galápagos Hawk, Buteo galapagoensis. Le Gerfaut. 1975;65:29–57. [Google Scholar]
- Drake J.M. Density-dependent demographic variation determines extinction rate in experimental populations. PLoS Biol. 2005;3:e222. doi: 10.1371/journal.pbio.0030222. 10.1371/journal.pbio.0030222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faaborg J. Reproductive success and survivorship of the Galápagos hawk (Buteo galapagoensis): potential costs and benefits of cooperative polyandry. Ibis. 1986;128:337–347. [Google Scholar]
- Faaborg J, Parker P.G, DeLay L, de Vries T, Bednarz J.C, Maria Paz S, Naranjo J, Waite T. Confirmation of cooperative polyandry in the Galápagos hawk (Buteo galapagoensis) Behav. Ecol. Sociobiol. 1995;36:83–90. 10.1007/s002650050127 [Google Scholar]
- Frankham R. Inbreeding and extinction: island populations. Conserv. Biol. 1998;12:665–675. 10.1046/j.1523-1739.1998.96456.x [Google Scholar]
- Gilbert D.A, Lehman N, O'Brien S.J, Wayne R.K. Genetic fingerprinting reflects population differentiation in the California Channel Island fox. Nature. 1990;344:764–767. doi: 10.1038/344764a0. 10.1038/344764a0 [DOI] [PubMed] [Google Scholar]
- Hamilton W.D, Axelrod R, Tanese R. Sexual reproduction as an adaptation to resist parasites. Proc. Natl Acad. Sci. USA. 1990;87:3566–3573. doi: 10.1073/pnas.87.9.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A.J, Wilson V, Thein S.L. Hypervariable ‘minisatellite’ regions in human DNA. Nature. 1985;314:67–73. doi: 10.1038/314067a0. 10.1038/314067a0 [DOI] [PubMed] [Google Scholar]
- Johnson K.P, Seger J. Elevated rates of nonsynonymous substitution in island birds. Mol. Biol. Evol. 2001;18:874–881. doi: 10.1093/oxfordjournals.molbev.a003869. [DOI] [PubMed] [Google Scholar]
- Keller L.F, Waller D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002;17:230–241. 10.1016/S0169-5347(02)02489-8 [Google Scholar]
- Kohler H, Bayry J, Nicoletti A, Kaveri S.V. Natural autoantibodies as tools to predict the outcome of immune response? Scand. J. Immunol. 2003;58:285–289. doi: 10.1046/j.1365-3083.2003.01314.x. 10.1046/j.1365-3083.2003.01314.x [DOI] [PubMed] [Google Scholar]
- Lammers A, Klomp M.E, Nieuwland M.G, Savelkoul H.F, Parmentier H.K. Adoptive transfer of natural antibodies to non-immunized chickens affects subsequent antigen-specific humoral and cellular immune responses. Dev. Comp. Immunol. 2004;28:51–60. doi: 10.1016/s0145-305x(03)00102-2. 10.1016/S0145-305X(03)00102-2 [DOI] [PubMed] [Google Scholar]
- Lesica P, Allendorf F.W. When are peripheral populations valuable for conservation? Conserv. Biol. 1995;9:753–760. 10.1046/j.1523-1739.1995.09040753.x [Google Scholar]
- Lindström K.M, Foufopoulos J, Parn H, Wikelski M. Immunological investments reflect parasite abundance in island populations of Darwin's finches. Proc. R. Soc. B. 2004;271:1513–1519. doi: 10.1098/rspb.2004.2752. 10.1098/rspb.2004.2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lively C.M, Apanius V. Genetic diversity in host–parasite interactions. In: Grenfell B.T, Dobson A.P, editors. Ecology of infectious diseases in natural populations. Cambridge University Press; Cambridge, UK: 1995. pp. 421–449. [Google Scholar]
- Longmire J.L, et al. Isolation and molecular characterization of a highly polymorphic centromeric tandem repeat in the family Falconidae. Genomics. 1988;2:14–24. doi: 10.1016/0888-7543(88)90104-8. 10.1016/0888-7543(88)90104-8 [DOI] [PubMed] [Google Scholar]
- Marshall A.G. Academic Press; London: 1981. The ecology of ectoparasitic insects. [Google Scholar]
- Matson K.D, Ricklefs R.E, Klasing K.C. A hemolysis–hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Dev. Comp. Immunol. 2005;29:275–286. doi: 10.1016/j.dci.2004.07.006. 10.1016/j.dci.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Meagher S. Genetic diversity and Capillaria hepatica (Nematoda) prevalence in Michigan deer mouse populations. Evolution. 1999;53:1318–1324. doi: 10.1111/j.1558-5646.1999.tb04547.x. [DOI] [PubMed] [Google Scholar]
- Miller H.C, Lambert D.M. Genetic drift outweighs balancing selection in shaping post-bottleneck major histocompatibility complex variation in New Zealand robins (Petroicidae) Mol. Ecol. 2004;13:3709–3721. doi: 10.1111/j.1365-294X.2004.02368.x. 10.1111/j.1365-294X.2004.02368.x [DOI] [PubMed] [Google Scholar]
- Møller A.P, Rózsa L. Parasite biodiversity and host defenses: chewing lice and immune response of their avian hosts. Oecologia. 2005;142:169–176. doi: 10.1007/s00442-004-1735-8. 10.1007/s00442-004-1735-8 [DOI] [PubMed] [Google Scholar]
- Myers N. Pergamon Press; New York: 1979. The sinking ark: a new look at the problem of disappearing species. [Google Scholar]
- Ochsenbein A.F, Zinkernagel R.M. Natural antibodies and complement link innate and acquired immunity. Immunol. Today. 2000;21:624–630. doi: 10.1016/s0167-5699(00)01754-0. 10.1016/S0167-5699(00)01754-0 [DOI] [PubMed] [Google Scholar]
- Parker P.G, Waite T.A, Decker M.D. Behavioral association and kinship in communally roosting black vultures. Anim. Behav. 1995;49:395–401. 10.1006/anbe.1995.0052 [Google Scholar]
- Parker P.G, Snow A.A, Schug M.D, Booton G.B, Fuerst P.A. What molecules can tell us about populations: choosing and using a molecular marker. Ecology. 1998;79:361–382. [Google Scholar]
- Parmentier H.K, Lammers A, Hoekman J.J, De Vries Reilingh G, Zaanen I.T, Savelkoul H.F. Different levels of natural antibodies in chickens divergently selected for specific antibody responses. Dev. Comp. Immunol. 2004;28:39–49. doi: 10.1016/s0145-305x(03)00087-9. 10.1016/S0145-305X(03)00087-9 [DOI] [PubMed] [Google Scholar]
- Pearman P.B, Garner T.W.J. Susceptibility of Italian agile frog populations to an emerging strain of Ranavirus parallels population genetic diversity. Ecol. Lett. 2005;8:401–408. 10.1111/j.1461-0248.2005.00735.x [Google Scholar]
- Pfeffer A, Phegan M.D, Bany J. Detection of homocytotropic antibody in lambs infected with the louse, Bovicola bovis, using a basophil-histamine release assay. Vet. Immunol. Immunopathol. 1997;57:315–325. doi: 10.1016/s0165-2427(97)00014-7. 10.1016/S0165-2427(97)00014-7 [DOI] [PubMed] [Google Scholar]
- Prelezov P, Gundasheva D, Groseva N. Haematological changes in chickens, experimentally infected with biting lice (Phthiraptera: Insecta) Bulg. J. Vet. Med. 2002;5:29–38. [Google Scholar]
- Reiczigel, J. & Rózsa, L. 2001 Quantitative Parasitology 2.0 Budapest: distributed by the authors.
- Reid J.M, Arcese P, Keller L.F. Inbreeding depresses immune response in song sparrows (Melospiza melodia): direct and inter-generational effects. Proc. R. Soc. B. 2003;270:2151–2157. doi: 10.1098/rspb.2003.2480. 10.1098/rspb.2003.2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid W.V, Miller K.R. World Resources Institute; Washington, DC: 1989. Keeping options alive: the scientific basis for conserving biodiversity. [Google Scholar]
- Riesing M.J, Kruckenhauser L, Gamauf A, Haring E. Molecular phylogeny of the genus Buteo based on mitochondrial marker sequences. Mol. Phylogenet. Evol. 2003;27:328–342. doi: 10.1016/s1055-7903(02)00450-5. 10.1016/S1055-7903(02)00450-5 [DOI] [PubMed] [Google Scholar]
- Rogstad S.H, Pelikan S. Gelstats: a computer program for population genetics analyses using VNTR multilocus probe data. Biotechniques. 1996;21:1128–1131. doi: 10.2144/96216bc05. [DOI] [PubMed] [Google Scholar]
- Rolett B, Diamond J. Environmental predictors of pre-European deforestation on Pacific Islands. Nature. 2004;431:443–446. doi: 10.1038/nature02801. 10.1038/nature02801 [DOI] [PubMed] [Google Scholar]
- Snedecor G.W, Cochran W.G. Iowa State University Press; Aimes: 1989. Statistical methods. [Google Scholar]
- Sokal R.R, Rohlf F.J. Biometry. 3rd edn. W.H. Freeman & Company; New York: 1995. [Google Scholar]
- Spielman D, Brook B.W, Frankham R. Most species are not driven to extinction before genetic factors impact them. Proc. Natl Acad. Sci. USA. 2004a;101:15 261–15 264. doi: 10.1073/pnas.0403809101. 10.1073/pnas.0403809101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman D, Brook B.W, Briscoe D.A, Frankham R. Does inbreeding and loss of genetic diversity decrease disease resistance? Conserv. Genet. 2004b;5:439–448. 10.1023/B:COGE.0000041030.76598.cd [Google Scholar]
- SPSS, v. 11 2004 SPSS, Inc. Chicago, Illinois.
- Stephens J.C, Gilbert D, Yuhki N, O'Brien S. Estimation of heterozygosity for single-probe multilocus DNA fingerprints. Mol. Biol. Evol. 1992;9:729–743. doi: 10.1093/oxfordjournals.molbev.a040755. [DOI] [PubMed] [Google Scholar]
- Templeton A.R, Robertson R.J, Brisson J, Strasburg J. Disrupting evolutionary processes: the effect of habitat fragmentation on collared lizards of the Missouri Ozarks. Proc. Natl Acad. Sci USA. 2001;101:5426–5432. doi: 10.1073/pnas.091093098. 10.1073/pnas.091093098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel T, Whiteman N.K, Tirapé A, Baquero M.I, Cendeño V, Walsh T, Uzcátequi G.J, Parker P.G. Characterization of canarypox-like viruses infecting the endemic birds in the Galápagos Islands. J. Wildl. Dis. 2005;41:342–353. doi: 10.7589/0090-3558-41.2.342. [DOI] [PubMed] [Google Scholar]
- Thompson J.N. Specific hypotheses on the geographic mosaic of coevolution. Am. Nat. 1999;153:S1–S14. 10.1086/303208 [Google Scholar]
- Trouvé S, Degen L, Renaud R, Goudet J. Evolutionary implications of a high selfing rate in the freshwater snail Lymnaea truncatula. Evolution. 2003;57:2303–2314. [PubMed] [Google Scholar]
- Whiteman N.K, Parker P.G. Effects of host sociality on ectoparasite population biology. J. Parasitol. 2004a;90:939–947. doi: 10.1645/GE-310R. [DOI] [PubMed] [Google Scholar]
- Whiteman N.K, Parker P.G. Body condition and ectoparasite load predict territory ownership in the Galápagos hawk. Condor. 2004b;106:915–921. [Google Scholar]
- Whiteman N.K, Goodman S.J, Sinclair B.J, Walsh T, Cunningham A.A, Kramer L.D, Parker P.G. Establishment of the avian disease vector Culex quinquefasciatus Say, 1823 (Diptera: Culicidae) on the Galápagos Islands, Ecuador. Ibis. 2005;147:844–847. 10.1111/j.1474-919X.2005.00468.x [Google Scholar]
- Wikel S.K. Immune responses to arthropods and their products. Annu. Rev. Entomol. 1982;27:21–48. doi: 10.1146/annurev.en.27.010182.000321. 10.1146/annurev.en.27.010182.000321 [DOI] [PubMed] [Google Scholar]
- Wikel S.K, DeVaney J.A, Augustine P.C. Host immune response to northern fowl mite: immunoblot and lectin blot identification of mite antigens. Avian Dis. 1989;33:558–575. [PubMed] [Google Scholar]
- Wikelski M, Foufopoulos J, Vargas H, Snell H. Galápagos birds and diseases: Invasive pathogens as threats for island species. Ecol. Soc. 2004;9 http://www.ecologyandsociety.org/vol9/iss1/art5 [Google Scholar]