Abstract
Shoaling behaviour in fish is influenced by numerous factors, such as familiarity, kinship, group size and shoal composition. Grouping decisions are based on both olfactory and visual cues. The visual system of many vertebrates is extended into the ultraviolet (UV) wave range as in three-spined sticklebacks (Gasterosteus aculeatus, L.). We investigated whether the presence or absence of UV wavelengths has an influence on shoaling behaviour in this species. Reproductively non-active three-spined sticklebacks were given the choice between two shoals, equal in numbers of individuals, which could be seen either through a UV-transmitting [UV(+)] or a UV-blocking [UV(−)] filter. Test fish preferred to join the shoal seen under UV(+) conditions. Due to differences in quantal flux between the UV(+) and UV(−) filters used, control experiments with neutral-density optical filters were performed in order to clarify the role of luminance. Here, test fish spent significantly more time near shoals that were seen in a darker environment, suggesting a potential trade-off between UV radiation and lower brightness during shoal choice.
To our knowledge, these results demonstrate for the first time that shoaling decisions are influenced by UV wavelengths.
Keywords: UV vision, ultraviolet, three-spined stickleback, Gasterosteus aculeatus, shoaling preference, luminance
1. Introduction
Living in groups not only provides several benefits but also bears costs. In terms of predation risk, group members benefit from earlier detection of potential predators through enhanced vigilance (Kenward 1987). Furthermore, an improved antipredator response can be achieved through coordinated group manoeuvres (Pitcher & Wyche 1983), the dilution effect (Foster & Treherne 1981) and also the confusion effect (Landeau & Terborgh 1986). Advantages for individuals joining a larger group have also been shown in terms of increased foraging success (Pitcher et al. 1982; Street & Hart 1985). On the other hand, living in groups bears several costs. Larger group size, for example, is associated with greater conspicuousness to potential predators (Calvert et al. 1979). Furthermore, in larger shoals there can occur high intraspecific competition for food (Bertram 1978). The disputed risk of horizontal parasite transfer in larger shoals is discussed in Barber et al. (2000).
Shoaling behaviour is not a random phenomenon; in fact, animals evaluate the costs and benefits of joining, staying with, or leaving a group. Different factors, such as group size (Griffiths & Magurran 1997), familiarity (Barber & Ruxton 2000), status of hunger (Krause 1993; Krause et al. 1999) and conspecificity (Ward et al. 2002), have been shown to influence fishes' decisions regarding shoal association. Because predators are known to select conspicuous individuals from shoals (Landeau & Terborgh 1986; Theodorakis 1989), the benefits an individual can gain may be greatest in a phenotypically homogeneous shoal. Therefore, individuals may be expected to join groups that offer the highest degree of inconspicuousness.
Being inconspicuous to the human eye is not necessarily equivalent to what animals can perceive, because visual systems can greatly differ with regard to their spectral sensitivities. In comparison with the spectrum visible to humans, ranging approximately from 400 to 700 nm, the visual sensitivity of many vertebrates, especially lizards (Fleishman et al. 1993), birds (Bennett & Cuthill 1994) and fish (Losey et al. 1999), is extended into the UV wave range (300–400 nm). This visual ability is used in social contexts such as mate choice (e.g. in birds (Bennett et al. 1996, 1997) and fish (Kodric-Brown & Johnson 2002; Boulcott et al. 2005; Rick et al. in press)) and foraging (e.g. in birds; Church et al. 1998).
Hence, in behavioural studies it is important to take into account the potential visual sensitivity to UV wavelengths.
In three-spined sticklebacks, Rowe et al. (2004) identified micro-spectrophotometrically a fourth UV-sensitive visual pigment maximally absorbing (λmax) at around 360 nm, in addition to the three already known photopigments with λmax of 435, 530 and 605 nm (Lythgoe 1979). Reflectance measurements of reproductively active male sticklebacks indicated that they possess UV-reflective regions on their body surface (Rick et al. 2004). Furthermore, female mate choice experiments in sticklebacks demonstrated a significant influence of UV light on mate preferences (Boulcott et al. 2005; Rick et al. in press).
Energetic shortwave light has a considerable negative photooxidative effect on epithelia and the retinal tissue in particular (Sliney 2002). Therefore, a visual system sensitive to UV light bears physiological costs. Despite these costs, the UV-sensitive visual system of some species must have been favoured by selection compared to a system only using the spectrum visible to humans. In the context of shoaling behaviour, some signalling in the UV spectrum could act as a form of intraspecific communication indiscernible by potential predators, because UV light is strongly scattered in water (Losey et al. 1999) and thus, only visible over shorter distances.
Here, we describe an experimental study on reproductively non-active three-spined sticklebacks, a small shoal-forming fish, testing preferences for shoals seen in an UV-rich environment against shoals seen in an UV-lacking environment. To our knowledge, this is the first study demonstrating an effect of UV radiation on shoaling decisions in fish.
2. Material and methods
(a) Experimental subjects
Several hundred three-spined sticklebacks were caught with minnow traps before the start of the breeding season on 9 and 15 March 2004 from a shallow pond near Euskirchen, Germany (50°38′ N/6°47′ E). The pond is located in a small woodland. Because of only sparse vegetation at the shore line, it is exposed to full sunlight penetration throughout the year. The fish were released into two outdoor stocking tanks (volume 700 l; provided with tap water at a flow rate of 3 l min−1 and air ventilation). To guarantee full penetration of UV-rich sunlight, stocking tanks were cleaned regularly. All fish were fed daily ad libitum on a diet of frozen chironomid larvae. Non-reproductively active sticklebacks with standard lengths (SL) between 30 and 40 mm were selected from the stocking tanks and transferred into group tanks (50×30×30 cm) in the laboratory. Groups consisted of 10 individuals each. Group tanks were provided with fine gravel (diameter of 1–1.5 mm) and aerated, filtered water. In order to avoid fish from different group tanks becoming familiar to each other, opaque partitions between group tanks prevented visual contact. Illumination was provided by fluorescent tubes (True Light, Natural Daylight 5500, 36 W, 1200 mm) hanging 15 cm above the water surface. These lights contain a proportion of UV similar to natural skylight. The fish were kept under a 16 : 8 h light–dark regime at 17±2 °C. As before, fish were fed daily with frozen chironomid larvae ad libitum. The experiment was carried out between 22 March and 14 June 2004.
(b) Choice experiment
The test aquarium (80×40×40 cm) was divided into three sections by perspex partitions, which were transmittive for light in the wave range between 300 and 800 nm (GS-2458, Röhm, Darmstadt, Germany). The aquarium was filled with tap water to a depth of 25 cm. There was no olfactory exchange between the three compartments. To exclude confounding effects between trials, water was totally replaced after each trial.
Each of the two outer sections (16×40×40 cm) formed a shoal compartment and the middle one (48×40×40 cm) the choice arena. The choice arena was divided into two equal-sized preference zones by a black line, which we drew on the bottom of the aquarium. We inserted two optical filters in front of both shoal compartments, which could be lifted with a thin string. One was UV-blocking (GS-233, Röhm, Darmstadt, Germany) and the other one was transmittive for UV-A wavelengths (GS-2458, Röhm, Darmstadt, Germany). Therefore, the test fish could see one shoal in a wavelength range between 400 and 800 nm and the other one in an extended wavelength range including UV-A (300–800 nm).
Again, the aquarium was illuminated by a fluorescent tube, emitting radiation with a proportion of UV light similar to natural skylight. The tube was installed 80 cm above the water surface. All trials were recorded with a webcam from 80 cm above the centre of the tank. To prevent visual disturbance from outside, the walls of the aquarium were covered with grey opaque plastic partitions, which reflected moderately in the UV-A range. Additionally, a black curtain surrounded the whole set-up.
Because shoaling decisions in non-reproductive adult sticklebacks are influenced by familiarity (Frommen & Bakker 2004), trials were performed with unfamiliar fish. Individuals were randomly selected out of nine different group tanks with hand nets and immediately introduced into the test aquarium. One fish, the test fish, was released into the choice arena. The remaining eight individuals were split into two groups of four fish each, which were then transferred to the two shoal compartments. After introduction the recording was started. Fish were not fed on the day of the experiment.
Each trial was divided into two sub-trials. One sub-trial consisted of 30 min acclimatization time followed by 30 min testing time. During the 30 min of acclimatization the test fish section was separated by opaque partitions from the shoal compartments. Then the opaque partitions were lifted with a string and the test time commenced. The test fish could see one shoal through an UV(+) and the other shoal through an UV(−) filter. After 30 min testing time, the opaque partitions were put down again in order to change the positions of the filters. After that, the second sub-trial started with further 30 min of acclimatization and, after lifting up the opaque partitions again, with another 30 min of testing. Now the test fish could see the same shoals but under reversed UV(+) and UV(−) conditions. The positions of the optical filters in the first trial were alternated after each experiment. After the trials, all fish were measured for SL and body mass. Furthermore, a condition factor (CF) was calculated as CF=100×W/SL3 (with W, weight (g); SL, standard length (cm); Bolger & Conolly 1989).
For statistical analyses, we measured the total time that test fish spent in the left half of the choice arena. The shoals on the left side could be seen through both a UV-transmitting (UV+) and UV-blocking (UV−) filter. Measurements lasted for 10 min per sub-trial and started once the entire test fish had passed the line which divided the choice compartment into two equal halves. All films were analysed blind, that is, without knowledge of the positions of the UV(+) and UV(−) filters. All fish were only used once.
(c) Control experiments
(i) Achromatic brightness selection
The two optical filters UV(+) and UV(−) differed in their spectral transmission and consequently also in quantal flux (UV(+) to UV(−): 18% reduction; Rick et al. in press). Thus, test fish perceived the shoals under different brightness conditions during the UV experiment. Therefore, a control experiment was necessary in order to distinguish whether UV wavelengths are used for hue or for brightness discrimination. We performed exactly the same experiment as described above except that now two neutral-density filters (ND1 and ND2, Lee and Cotech Filters, respectively) were used as interchangeable dividers instead of the UV-transmittive and UV-opaque ones (UV(+) and UV(−)). Here, the test fish could see one shoal behind the ND1 and the other shoal behind the ND2 filter in the first sub-trial, and in the second sub-trial both shoals under reversed ND1 and ND2 conditions. Both neutral-density filters were transmittive for wavelengths between 300 and 800 nm and altered luminance independent of hue. The reduction in quantitative transmission from the ND2 filter to the ND1 filter was 34% (Rick et al. in press), that is, nearly twice as large as between the UV treatment filters.
(ii) Habitat selection
This control experiment was designed to determine whether habitat rather than shoaling selection took place. In order to test whether there exists a preference for the habitat seen through any of the four filters used (UV(+), UV(−), ND1 or ND2), additional choice experiments were performed. The experimental procedure was exactly the same as described for the UV treatment except that there were no stimulus shoals presented in the outer compartments. Here, in two sub-trials test fish were only given the choice between two sets of filter combinations (first, UV(+) and UV(−); second, ND1 and ND2; and vice versa) without any stimulus shoal behind the filters. All fish used in the two control experiments were sampled from the same population as those used in the choice experiment and treated similarly.
(d) Statistical analysis
All analyses were performed using SPSS v. 11.0 for Windows. Before statistical analyses, we tested variables for normality using the Kolmogorov–Smirnov test. The mean differences in all body characteristics between shoals in the UV test deviated from normality (p<0.05) and were, therefore, square root transformed in order to meet the normality assumptions for parametric statistical tests. The mean difference in SL between shoals in the brightness test were [1/x+1]-transformed for the same reason. We analysed the total amount of time test fish spent in front of the UV(+) and UV(−) shoals in both sub-trials using either a paired t-test or a Wilcoxon signed-rank test. p-values are two-tailed throughout.
(e) Ethical note
Animal care and experimental procedures were in accordance with the legal requirements of Germany. No additional license was required for this study.
3. Results
(a) Shoal choice and UV vision
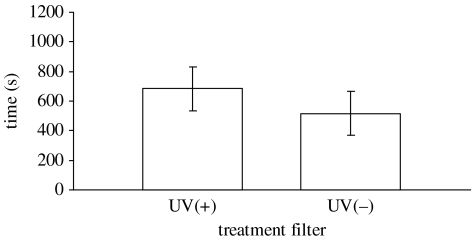
When given the choice between two shoals that could be seen either through a UV-transmitting or UV-blocking optical filter, test fish spent significantly more time near the shoal behind the UV-transmitting filter (mean±s.d.=682.6±150.25 and 517.4±150.25 s, respectively; paired t-test: t=2.749, n=25, p=0.011; figure 1).
Figure 1.
Mean (±s.d.) time in seconds spent by 25 test fish near the shoals seen through either the UV-transmitting (UV+) or the UV-blocking (UV−) filter during the 20 min choice time.
There were inevitably some differences between the simultaneously presented shoals but because of the paired experimental design the influence on the outcome of the UV test is conservative. Although shoals were matched as much as possible as to individuals' body size, the simultaneously presented shoals differed significantly as to mean SL (mean difference±s.d.=1.04±0.859 cm; range, 0–3.5), mean body mass (mean difference±s.d.=0.0537±0.0539 g; range, 0.002–0.184) and the mean CF (mean difference±s.d.=0.116±0.09 g cm−3; range, 0.015–0.366) of the fish in the shoal (t-tests, all t>8.7; all p<0.001). Additionally, the fish of the right shoals were by chance significantly larger (mean difference±s.d.=−0.56±1.24 cm; range, −3.5–1.75; paired t-test: t=−2.258, n=25, p=0.033) and heavier (mean difference±s.d.=−0.031±0.07 g; range, −0.1835–0.1528; paired t-test: t=−2.228, n=25, p=0.035) than those on the left side. No significant differences between the shoals on the right and left side were found as to the mean CF (mean difference±s.d.=−0.0117±0.152; range, −0.366–0.271; paired t-test: t=−0.384, n=25, p=0.704). Test fish showed no significant preference for either the bigger or smaller shoal regarding the mean SL (paired t-test: t=−0.222, n=24, p=0.826), the mean body mass (paired t-test: t=−0.615, n=25, p=0.545) or the mean CF (paired t-test: t=1.869, n=25, p=0.074) of the presented shoals.
Test fish spent, however, significantly more time near the shoal that showed the smallest difference in mean SL from test fish's SL (paired t-test: t=2.274, n=23, p=0.033). No such effect was found with respect to mean body mass nor mean CF (paired t-tests: t=0.845, n=25, p=0.406 and t=−0.599, n=25, p=0.555, respectively). This result suggests a size-assortative shoaling preference. We checked whether the strength of the UV(+) preference would be correlated with the difference in body size between the test fish and shoal fish. The UV(+) preference, expressed as [seconds on UV(+)−seconds on UV(−) side]/[seconds on both sides], was not significantly correlated with the difference in SL between the test fish and the preferred shoal (Spearman rank correlation: rs=0.137, n=23, p=0.533). We also found no significant correlation between the UV(+) preference and the ratio of the differences in body size to both simultaneously presented shoals ([1+mean SLpreferred shoal−SLtest fish]/[1+mean SLnon-preferred shoal−SLtest fish]; Spearman rank correlation: rs=0.124, n=24, p=0.562). The strength of the size-assortative shoaling preference, expressed as [seconds near similar shoal−seconds near dissimilar shoal]/[seconds near both shoals], was not significantly correlated with the degree of similarity (i.e. the difference in mean SL of the preferred shoal from that of the test fish) (Pearson rank correlation: rp=0.083, n=23, p=0.706). Finally, the UV(+) shoaling preference was not significantly correlated with the size-assortative shoaling preference (Pearson rank correlation: rp=0.226, n=23, p=0.3).
Test fish alternated 21 times (median; quartiles: 14 and 28.5; range 3–64) between the two simultaneously presented shoals. The number of changes did not significantly differ between fish that preferred UV(+) and those that did not (Mann-Whitney U-test: N1=17, N2=8, U=57, p=0.873).
(b) Shoal choice and achromatic brightness
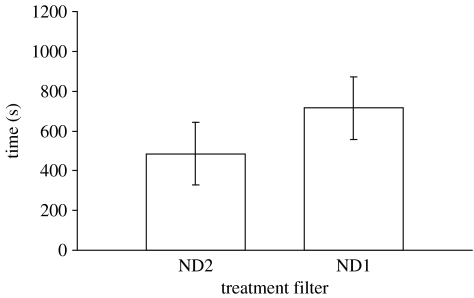
The choice of the test fish was also influenced by a difference in achromatic brightness but in a direction opposite to that in the UV test. Test fish spent significantly more time near the shoal viewed through the ND1 filter, which reduced quantal flux by ca 34% compared to the ND2 filter (mean±s.d.=715.18±157.64 and 484.82±157.64 s, respectively; paired t-test: t=−3.012, n=17, p=0.008; figure 2). This result shows a preference of test fish for the darker side.
Figure 2.
Mean (±s.d.) time in seconds spent by 17 test fish in front of each neutral density (ND) filter. The reduction in quantitative transmission from the ND2 to the ND1 filter was 34%.
In the brightness test, as well, the simultaneously presented shoals differed significantly as to mean SL (mean difference±s.d.=0.956±0.911 cm; range, 0–2.75), mean body mass (mean difference±s.d.=0.054±0.038 g; range, 0–0.122) and the mean CF (mean difference±s.d.=0.088±0.059 g cm−3; range, 0.002–0.22) of the fish in the shoal (t-tests: all t>5.8, all p<0.001). In contrast to the UV test, left and right shoals did not differ significantly with respect to mean SL in the brightness test (mean difference±s.d.=−0.25±1.32 cm; range, −2.5–2.75; paired t-test: t=−0.783, n=17, p=0.445), mean body mass (mean difference±s.d.=−0.018±0.065 g; range, −0.12–0.11; paired t-test: t=−1.141, n=17, p=0.271) and mean CF (mean difference±s.d.=−0.024±0.105 g, range:−0.22–0.18; paired t-test: t=−0.951, n=17, p=0.356). Test fish showed no significant assortative shoaling either with respect to mean SL, mean body mass or mean CF (paired t-tests: t=−1.272, n=14, p=0.224; t=−0.769, n=16, p=0.454 and t=0.577, n=17, p=0.572, respectively).
Test fish alternated 25 times (median; quartiles: 8.5 and 30; range 5–34) between the two shoals. The number of changes did not significantly differ between fish that preferred ND1 and those that did not (Mann-Whitney U-test: N1=13, N2=4, U=16, p=0.256).
(c) Habitat selection
Test fish did not exhibit a preference for either the UV(+) or UV(−) filter (Wilcoxon signed-rank test: n=29, Z=−1.47, p=0.141). Test fish alternated six times (median; quartiles: 5 and 10.5; range 1–20) between the two filters.
Similarly, no preference for either the ND1 or ND2 filter was found (paired t-test: t=−0.737, n=26, p=0.468). Test fish alternated six times (median; quartiles: 4 and 8.25; range 2–13) between the two neutral-density filters.
4. Discussion
The present study is the first, to our knowledge, to examine shoaling behaviour in fish with respect to different environmental UV conditions. The results of the shoal choice experiment demonstrate that three-spined sticklebacks prefer to join shoals seen in an UV-rich environment (UV(+): 300–800 nm) compared to an UV-lacking one (UV(−): 400–800 nm). Thus, besides numerous other factors such as group size (Griffiths & Magurran 1997), familiarity (Barber & Ruxton 2000; Frommen & Bakker 2004) or conspecificity (Ward et al. 2002), environmental UV also matters in shoaling decisions.
The results of the habitat control experiment clearly show that the preferences for UV(+) of test fish are based on shoaling rather than habitat preferences. This is in contrast with a previous study by Boulcott et al. (2005), who examined the influence of UV-light on mate choice in sticklebacks. In control experiments, they did not find shoaling preferences with respect to different lighting conditions. It is unclear whether the contrasting results are a consequence of a difference in experimental set-up or population differences. Habitat preferences were excluded with a control experiment but were also unexpected because of: (i) the results of the achromatic brightness test, where fish preferred the darker side. If habitat selection took place, here one would expect a preference for the brighter side which is more similar to the UV(+) lighting conditions in the first experiment, and (ii) Boulcott et al. (2005) did not find a filter preference either.
Oddity theory predicts that, in a homogeneous group, phenotypically conspicuous individuals are more likely to be detected by predators (Theodorakis 1989). Hence, for individuals that reflect in UV light, shoaling with groups that bear UV-reflections should be beneficial. On the other hand, if potential predators are blind or less sensitive to UV, UV signalling could serve as a private communication channel. For example, in northern swordtails (Xiphophorus), males with UV ornamentation were more attractive to females but this UV ornamentation did not enhance the conspicuousness to one of their major predators (Cummings et al. 2003).
The UV(+) and UV(−) filters differed in total quantal flux and, therefore, one could raise the objection that the UV preference found was more probably based on brightness differences caused by the two optical filters used rather than on a difference in hue. However, the result of the achromatic brightness control experiment with neutral-density filters, which only differed in quantal flux but not in wavelength transmission, rebuts this objection: here, test fish showed a significant preference for the darker side (ND1). This finding strengthens the conclusion that the result of the UV experiment was caused by a difference in perceived hue rather than brightness. Whether fish also prefer to shoal with individuals on the darker side under UV(−) conditions is unknown. In female mate choice experiments, females showed no significant preference for ND1 or ND2 males either under UV(+) or UV(−) conditions (I. P. Rick 2003, unpublished data). The opposing effects of UV reflectance and brightness suggest the existence of a trade-off between these two factors in shoaling decisions.
By chance, there was a significant difference in mean SL and mean body mass between the simultaneously presented shoals on the left and right side, which could have influenced the UV-attractiveness of shoals and hence the UV-preference of test fish. But test fish showed no significant preference either for the shoal with the smaller or bigger fish. Instead, we found a size-assortative shoaling preference in test fish. This means that test fish spent significantly more time near the shoal which showed the smallest difference in SL to that of the test fish. Such a size-assortative shoaling preference is well-known in fishes (Ranta et al. 1992; Krause 1994; Hoare et al. 2000). However, this size-assortative shoaling preference was not significantly correlated with the UV(+) preference. The two preferences seem to be independent of each other, which makes it unlikely that the preference for UV(+) shoals was based on a preference for similarity in body size. Furthermore, due to the paired experimental design in which each shoal was presented to the test fish under UV(+) and UV(−) conditions for equal durations, any effect of shoal attractiveness other than UV reflectance (e.g. due to body size) should level out between sub-trials.
It is conceivable that UV radiation is needed to assess body characteristics of potential shoal mates. But if so we also would expect a size-assortative shoaling behaviour in the neutral-density experiment, where UV radiation was present on both sides. However, no such preference was found there.
In conclusion, we established three different shoaling preferences in reproductively non-active sticklebacks. We found, for the first time, to our knowledge, that shoaling decisions in fish are influenced by UV radiation. We further measured a size-assortative shoaling preference that was independent of the UV(+) shoaling preference. The UV(+) shoaling preference probably has to be traded off against a shoaling preference for reduced luminance. The UV(+) shoaling preference raises a number of other potentially interesting questions. For example, how could UV reflectance in sticklebacks function as a private communication channel and is it used in coordinated manoeuvres within a shoal? Does shoaling with UV(+) shoals reduce an individual's risk of being attacked by predators? Does there exist variation in UV-reflectance and also in preferences between populations from different habitats?
Acknowledgments
We are grateful to Joachim Frommen, Timo Thünken and Kirsten Klappert for discussion. We gratefully acknowledge the permission of Jürgen Wittler for catching sticklebacks at the field site. We thank Lutz Fromhage, Joachim Frommen, and three anonymous reviewers for their constructive comments, and Leif Engqvist for statistical advice. This work was partly supported by the Deutsche Forschungsgemeinschaft (Ba 2885/1–2).
References
- Barber I, Ruxton G.D. The importance of stable schooling: do familiar sticklebacks stick together? Proc. R. Soc. B. 2000;267:151–155. doi: 10.1098/rspb.2000.0980. 10.1098/rspb.2000.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber I, Hoare D, Krause J. Effects of parasites on fish behaviour: a review and evolutionary perspective. Rev. Fish Biol. Fish. 2000;10:131–165. 10.1023/A:1016658224470 [Google Scholar]
- Bennett A.T.D, Cuthill I.C. Ultraviolet vision in birds—what is its function? Vision Res. 1994;34:1471–1478. doi: 10.1016/0042-6989(94)90149-x. 10.1016/0042-6989(94)90149-X [DOI] [PubMed] [Google Scholar]
- Bennett A.T.D, Cuthill I.C, Partridge J.C, Maier E.J. Ultraviolet vision and mate choice in zebra finches. Nature. 1996;380:433–435. 10.1038/380433a0 [Google Scholar]
- Bennett A.T.D, Cuthill I.C, Partridge J.C, Lunau K. Ultraviolet plumage colors predict mate preferences in starlings. Proc. Natl Acad. Sci. USA. 1997;94:8618–8621. doi: 10.1073/pnas.94.16.8618. 10.1073/pnas.94.16.8618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram B.C.R. Living in groups: predators and prey. In: Krebs J.R, Davies N.B, editors. Behavioural Ecology. Blackwell; Oxford, UK: 1978. pp. 64–96. [Google Scholar]
- Bolger T, Conolly P.L. The selection of suitable indices for the measurement and analysis of fish condition. J. Fish Biol. 1989;34:171–182. [Google Scholar]
- Boulcott P.D, Walton K, Braithwaite V.A. The role of ultraviolet wavelengths in the mate-choice decisions of female three-spined sticklebacks. J. Exp. Biol. 2005;208:1453–1458. doi: 10.1242/jeb.01569. 10.1242/jeb.01569 [DOI] [PubMed] [Google Scholar]
- Calvert W.H, Hedrick L.E, Brower L.P. Mortality of the monarch butterfly, Danaus plexippus: avian predation at five over-wintering sites in Mexico. Science. 1979;204:847–851. doi: 10.1126/science.204.4395.847. [DOI] [PubMed] [Google Scholar]
- Church S.T, Bennett A.T.D, Cuthill I.C, Partridge J.C. Ultraviolet cues affect the foraging behaviour of blue tits. Proc. R. Soc. B. 1998;265:1509–1514. 10.1098/rspb.1998.0465 [Google Scholar]
- Cummings M.E, Rosenthal G.G, Ryan M.J. A private ultraviolet channel in visual communication. Proc. R. Soc. B. 2003;270:897–904. doi: 10.1098/rspb.2003.2334. 10.1098/rspb.2003.2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleishman L.J, Loew E.R, Leal M. Ultraviolet vision in Lizards. Nature. 1993;365:397–397. 10.1038/365397a0 [Google Scholar]
- Foster S.A, Treherne J.E. Evidence for the dilution effect in the selfish herd from fish predation on a marine insect. Nature. 1981;293:466–467. 10.1038/293466a0 [Google Scholar]
- Frommen J.G, Bakker T.C.M. Adult three-spined sticklebacks prefer to shoal with familiar kin. Behaviour. 2004;141:1401–1409. 10.1163/1568539042948196 [Google Scholar]
- Griffiths S.W, Magurran A.E. Schooling preferences for familiar fish vary with group size in a wild guppy population. Proc. R. Soc. B. 1997;264:547–551. 10.1098/rspb.1997.0078 [Google Scholar]
- Hoare D.J, Ruxton G.D, Godin J.G.J, Krause J. The social organization of free-ranging fish shoals. Oikos. 2000;89:546–554. 10.1034/j.1600-0706.2000.890314.x [Google Scholar]
- Kenward R.E. Hawks and doves: attack success and selection in goshawk flights at wood pigeons. J. Anim. Ecol. 1987;47:449–460. [Google Scholar]
- Kodric-Brown A, Johnson S.C. Ultraviolet reflectance patterns of male guppies enhance their attractiveness to females. Anim. Behav. 2002;63:391–396. 10.1006/anbe.2001.1917 [Google Scholar]
- Krause J. The influence of hunger on shoal size choice by three-spined sticklebacks, Gasterosteus aculeatus. J. Fish Biol. 1993;43:775–780. 10.1111/j.1095-8649.1993.tb01154.x [Google Scholar]
- Krause J. The influence of food competition and predation risk on size-assortative shoaling in juvenile chub (Leuciscus cephalus) Ethology. 1994;96:105–116. [Google Scholar]
- Krause J, Hartmann N, Pritchard V.L. The influence of nutritional state on shoal choice in zebrafish, Danio rerio. Anim. Behav. 1999;57:771–775. doi: 10.1006/anbe.1998.1010. 10.1006/anbe.1998.1010 [DOI] [PubMed] [Google Scholar]
- Landeau L, Terborgh J. Oddity and the ‘confusion effect’ in predation. Anim. Behav. 1986;34:1372–1380. [Google Scholar]
- Losey G.S, Cronin T.W, Goldsmith T.H, Hyde D, Marshall N.J, McFarland W.N. The UV visual world of fishes: a review. J. Fish Biol. 1999;54:921–943. 10.1111/j.1095-8649.1999.tb00848.x [Google Scholar]
- Lythgoe J.N. Clarendon Press; Oxford, UK: 1979. The ecology of vision. [Google Scholar]
- Pitcher T.J, Magurran A.E, Winfield I. Fish in larger shoals find food faster. Behav. Ecol. Sociobiol. 1982;10:932–934. 10.1007/BF00300175 [Google Scholar]
- Pitcher T.J, Wyche C.J. Predator avoidance behaviour of sand-eel schools: why schools seldom split? In: Noakes D.L.G, Lindquist B.G, Helfman G.S, Ward J.A, editors. Predators and prey in fishes. Junk; The Hague: 1983. pp. 193–204. [Google Scholar]
- Ranta E, Lindström K, Peuhkuri N. Size matters when three-spined sticklebacks go to school. Anim. Behav. 1992;43:160–162. [Google Scholar]
- Rick I.P, Modarressie R, Bakker T.C.M. Male three-spined sticklebacks reflect in ultraviolet light. Behaviour. 2004;141:1531–1541. 10.1163/1568539042948222 [Google Scholar]
- Rick, I. P., Modarressie, R. & Bakker, T. C. M. In press. UV wavelengths affect female mate choice in three-spined sticklebacks. Anim. Behav.
- Rowe M.P, Baube C.L, Loew E.R, Phillips J.B. Optimal mechanisms for finding and selecting mates: how threespine stickleback (Gasterosteus aculeatus) should encode male throat colors. J. Comp. Physiol. A. 2004;190:241–256. doi: 10.1007/s00359-004-0493-8. 10.1007/s00359-004-0493-8 [DOI] [PubMed] [Google Scholar]
- Sliney D.H. How light reaches the eye and its components. Int. J. Toxicol. 2002;21:501–509. doi: 10.1080/10915810290169927. 10.1080/10915810290169927 [DOI] [PubMed] [Google Scholar]
- Street N.G, Hart P.J.B. Group size and patch location by the stoneloach, Noemacheilus barbatulus, a non-visually foraging predator. J. Fish Biol. 1985;217:785–792. [Google Scholar]
- Theodorakis C.W. Size segregation and the effect of oddity on predation risk in minnow schools. Anim. Behav. 1989;38:496–502. [Google Scholar]
- Ward A.J.W, Axford S, Krause J. Mixed-species shoaling in fish: the sensory mechanisms and costs of shoal choice. Behav. Ecol. Sociobiol. 2002;52:182–187. 10.1007/s00265-002-0505-z [Google Scholar]