Abstract
In terrestrial environments, the exchange of respiratory gases exacts a water cost: obtaining oxygen or carbon dioxide requires losing water. Insect eggs should be especially sensitive to this tradeoff—because they are unable to forage for water, have high surface area-to-volume ratios, and experience large temperature-driven changes in oxygen demand. Previous work from our laboratory, on eggs of a common hawkmoth, Manduca sexta, has shown that, during development, metabolic rate and water loss rates rise in parallel. These correlative data suggest that eggshell conductance increases to accommodate increasing metabolic demand. Here, we test this idea experimentally by subjecting eggs of M. sexta to 15, 21 (normoxia) and 35% oxygen for 24 h, while measuring rates of metabolism (as carbon dioxide emission) and water loss. Hypoxia depressed egg metabolic rates, but led to pronounced, rapid increases in water loss. By contrast, hyperoxia had no significant effect on metabolism or water loss. These data demonstrate that insect eggs actively participate in balancing oxygen gain and water loss, and that they use tissue oxygen status, or some correlate of it, as a cue for increasing eggshell conductance. Rapid control over conductance may allow eggs to conserve water during an initial period of low metabolic demand, thereby deferring water costs of respiratory gas exchange until late in development.
Keywords: egg, embryo, eggshell, diffusive transport, Manduca sexta, metabolism
1. Introduction
For terrestrial organisms, the exchange of respiratory gases imposes unavoidable water costs. Insects obtaining oxygen from the tracheal system, for example, lose water from the spiracles (Lehmann 2001) and plants obtaining carbon dioxide for photosynthesis lose water through stomata (Hetherington & Woodward 2003). Organisms can alter the water costs they pay, by short-term physiological change or by acclimation, and populations or species may adapt in ways that minimize respiratory water loss (Addo-Bediako et al. 2001; Chown & Davis 2003). Regardless, the physiology of gas exchange is constrained: large fluxes of oxygen and carbon dioxide are associated with potentially large rates of water loss and, conversely, water conservation restricts fluxes of oxygen and carbon dioxide.
Among life stages, eggs are particularly vulnerable to oxygen–water tradeoffs. While juveniles and adults may search out water, eggs usually are immobilized in their oviposition microsite. Juveniles and adults also possess rapid-acting systems for regulating oxygen flux and water balance (e.g. Lehmann 2001; Woods & Harrison 2001), but early embryos have neither the sensory organs for monitoring water and oxygen status nor the physiological systems for exerting control over them. Even after such systems are functioning, the embryo often is surrounded by eggshell layers whose properties may be difficult to alter over short time-scales (e.g. the calcareous eggshell of birds). Nonetheless, eggshells of several taxa do exhibit higher conductances as development progresses: insects (Woods et al. 2005), cephalopod molluscs (Cronin & Seymour 2000), amphibians (Seymour & Bradford 1987; Seymour et al. 1991; Mills et al. 2001), birds (Carey 1979; Booth & Seymour 1987; Booth & Rahn 1990; Kern et al. 1992) and alligators (Kern & Ferguson 1997). Increasing conductance presumably accommodates increasing demand for oxygen late in development. For terrestrial eggs in the preceding list, low early eggshell conductance would conserve water at a time of low oxygen demand.
Insect eggs—because they are comparatively small—should experience oxygen–water tradeoffs acutely. First, they have very high surface-area-to-volume ratios, about 50 times higher than hens' eggs (Hinton 1981). Relatively enormous surface areas facilitate oxygen acquisition but also water loss. Second, the small size of insect eggs suggests that they are always in thermal equilibrium with local microsite temperatures, which may vary markedly (Willmer 1986). Because temperature strongly affects egg metabolic rates (Woods & Hill 2004), eggs may experience many-fold differences in oxygen demand over periods of a few minutes or hours.
Here, we use short-term experimental manipulation of oxygen availability to examine oxygen–water tradeoffs in eggs of a common hawkmoth, Manduca sexta L. Manduca sexta live in diverse climates across North and South America (Rothschild & Jordan 1903), including the southwestern deserts of the US and more mesic habitats in the eastern US. Females provide no maternal care and oviposit primarily on leaves of different hosts in the family Solanaceae. Eggs may therefore experience substantial temporal variation in temperature and vapour pressure. Woods & Hill (2004) showed that even at moderate temperatures (32–37 °C) egg metabolism is oxygen limited and internal PO2 can be very low, despite short diffusion distances (less than a few hundred micrometres) between ambient air and embryonic tissue (Lamer & Dorn 2001). Recently, Woods et al. (2005) showed that metabolic rates (measured as CO2 emission) of eggs of M. sexta rise almost fivefold from laying to hatching. Rates of water loss rise in parallel—indicating that eggshell conductance increases over development, most likely to accommodate rising embryonic demand for oxygen.
What mechanism determines the timing and rapidity of such changes? Two alternatives are (i) that increasing conductance results from a built-in developmental program unrelated to tissue oxygen status, or (ii) that embryos or even eggshell layers themselves sense internal oxygen status (or some biochemical correlate of O2 status), and in response exert direct control over eggshell conductance. We test these alternatives by exposing eggs to hypoxia (15% O2), normoxia (21% O2), or hyperoxia (35% O2) for 24 h and simultaneously measuring rates of metabolism (as CO2 emission) and water loss using a flow-through system.
2. Material and methods
(a) Colony and egg collection
Eggs originated from a laboratory colony of M. sexta. All stages were reared on a 14 : 10 h L : D photoperiod. Eggs, larvae and pupae were kept at 27 °C and adults at 24 °C. Adult moths were fed a 30% honey solution and provided with a tobacco plant for oviposition. Adults were allowed to oviposit for approximately 3.5 h. Newly laid eggs were then collected and kept at 27 °C for 48–50 h before testing. At 27 °C, the average time from oviposition to hatching is approximately 90 h (Woods & Hill 2004).
(b) Carbon dioxide emission and water loss
Batches of 40 eggs (weighed on a microbalance to ±1 μg, Sartorius MC5, Goettingen, Germany) were placed into water-jacketed stainless-steel chambers and sealed with threaded steel screws containing built-in circular rings. The chambers were interfaced with a computer-controlled gas multiplexer (TR-RM8, Sable Systems, Las Vegas, NV, USA). See Woods & Hill (2004) for details. Chamber temperature was controlled by a recirculating water bath.
Dry, CO2-free air from pre-mixed cylinders of gas (15, 21 and 35% O2 in N2; Airgas Southwest, LaPorte TX, USA) was directed through the chambers at 50 ml min−1 (standard temperature and pressure, dry (STPD)) and then past a calibrated thin-film capacitance relative humidity metre (RH-100, Sable Systems; calibration done using dry air and a stream of air humidified to 2142 Pa by bubbling through a flask of water at 18.6 °C). The air stream was then scrubbed of water vapour in a small Drierite column and directed through a carbon dioxide analyser (CA-2A, Sable Systems) that had been calibrated with pure N2 and 505 ppm CO2 in N2. Analogue signals from the instruments were converted to digital by an A/D converter (UI2, Sable Systems) and recorded to a computer using Expedata software (v. 0.2.48, Sable Systems). Temperature was recorded using a T-type thermocouple (connected to a thermocouple metre, TC-1000, Sable Systems) that was sealed into one of the chambers.
Batches of eggs were exposed for 24 h to 15, 21, or 35% O2 and CO2 emission and water loss were measured at 3 h intervals. Because O2 levels originated from different cylinders, each experimental batch of eggs was paired with a baseline chamber containing no eggs. Measurements alternated between blank and egg-filled chambers and individual chambers were sampled for 30 min each. Mass-specific CO2 emission and water loss rates were calculated using flow rates and batch masses. Data were analysed using linear mixed-effects models (Pinheiro & Bates 2000) implemented in S-Plus (v. 6.1, Insightful Corporation, Seattle, WA).
3. Results
Average mass of eggs used in both experiments was 1.36±0.05 mg, and average final hatching rate was 83.8%. No eggs hatched during experiments.
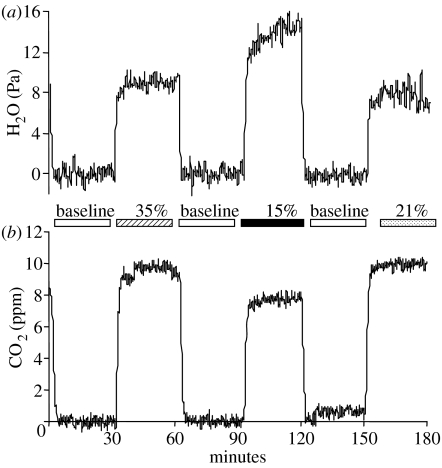
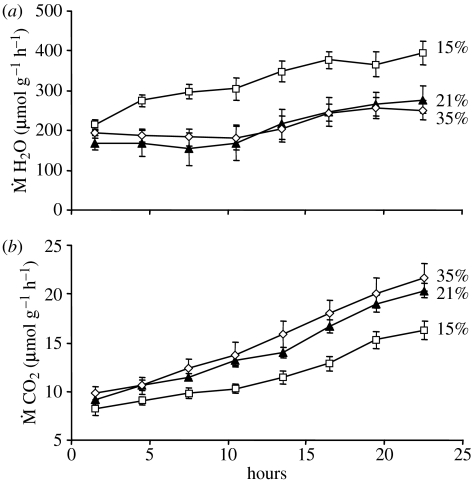
Figure 1 shows a typical 3 h trace obtained during one cycle through all chambers. Baselines were stable, and the signal-to-noise ratios high. In the first measurement period, CO2 emission and water loss did not differ significantly among oxygen treatments (table 1). Over time, both CO2 emission and water loss increased significantly (table 1), consistent with work by Woods & Hill (2004), showing that metabolic rates increase during the last half of development. However, the trajectory over time depended on ambient oxygen availability. While hyperoxia appeared to have little or no effect on metabolism or water loss, hypoxia affected both, but in different ways: it depressed CO2 emission but elevated water loss (figure 2). Statistically, this result is reflected as significant oxygen×time interaction (table 1). In additional analyses (not shown) of a reduced dataset (containing just batches of eggs exposed to 21 and 35% oxygen), the interaction term was not significant, suggesting that most of the significance in the full dataset arises from differences between batches exposed to 15% and those exposed to higher oxygen levels.
Figure 1.
Example traces of (a) water and (b) carbon dioxide from a 3 h cycle through six metabolic chambers. Each experimental chamber was paired with its own blank chamber (blank preceding experimental in each pair). During an experiment, the entire set of chambers was sampled eight times over a 24 h period.
Table 1.
Summary of significance values from linear mixed-effects modelling of carbon dioxide and water emission during 24 h developmental tests.
| source | num. d.f. | den. d.f. | CO2 emission | water emission | ||
|---|---|---|---|---|---|---|
| F-value | p-value | F-value | p-value | |||
| intercept | 1 | 124 | 96.8 | <0.001 | 133.5 | <0.001 |
| oxygen | 1 | 16 | 1.1 | 0.32 | 1.1 | 0.31 |
| time | 1 | 124 | 614.4 | <0.001 | 89.0 | <0.001 |
| oxygen×time | 1 | 124 | 11.3 | 0.001 | 7.0 | 0.009 |
Figure 2.
(a) Water loss and (b) carbon dioxide emission by batches of eggs (Manduca sexta) during 24 h of exposure to either 15, 21, or 35% oxygen. At each oxygen level, six batches of eggs were followed (18 total batches in the experiment). Bars are standard errors.
4. Discussion
Experimental hypoxia depressed egg metabolic rates but led to pronounced, rapid increases in water loss. By contrast, hyperoxia had no significant effect on either metabolism or water loss. Eggs thus appear capable of increasing, but not decreasing, eggshell conductance in response to oxygen status. These data support the second hypothesis raised in §1: that embryos or eggshell layers sense internal oxygen status (or a correlate of it) and in response alter eggshell conductance. The alternative—that increasing conductance results from a built-in developmental programme unrelated to tissue oxygen status—is not entirely excluded. For example, such a long-term programme could be superimposed on the short-term control observed here. However, the most parsimonious explanation of developmentally increasing conductance is that it stems also from active responses to low oxygen levels.
How is conductance altered? Using a mathematical model of gas flux together with data on metabolism and water loss over development, Woods et al. (2005) identified two layers, the wax and crystalline chorionic layers, as candidate physical loci controlling oxygen and water traffic across the eggshell. One possibility is that these layers respond directly to local oxygen levels—e.g. oxygen may bind to proteins in the crystalline layer and alter its conductance. Alternatively, embryos themselves may sense tissue oxygen status and secrete materials or signals that alter eggshell properties. In this scenario, the wax layer seems a more likely target of control, as it lies nearer the embryo. A third candidate sensor is the serosa, an extraembryonic layer of cells fully formed approximately 12 h after oviposition (Lamer & Dorn 2001). The layer is strategically placed between embryo and eggshell—close enough to the embryo to detect its oxygen status and close enough to eggshell layers to affect their conductance.
Control over eggshell conductance may play several important roles in the developmental ecology of M. sexta. In nature, eggs are unlikely to experience ambient hypoxia, because the species occupies habitats primarily below about 2000 m elevation, and egg oviposition sites, on leaves, are well ventilated. However, two other factors—moderately high temperatures and increasing developmental demand for oxygen—can lead to very low PO2 in tissues beneath the eggshell (Woods & Hill 2004). Given, the environmental uncertainty that a freshly oviposited egg faces, and the possibility of rapid, temperature-driven increases in oxygen demand, control over eggshell conductance may provide a mechanism for deferring increased rates of water loss until, as late in development as possible.
This conclusion is derived from considering egg responses to internal oxygen status, but modification of eggshell conductance also affects fluxes of water (Woods et al. 2005). Therefore, future work should examine, whether modification of conductance depends on internal water status. At first sight, it would seem that an egg's response to desiccation should be to decrease eggshell conductance, or to delay its increase. However, low conductance may allow in so little oxygen that total development time is extended, thus increasing total water loss or leading to unacceptably high risk of predation. How eggs solve this dilemma is of particular interest. Theoretically, eggs should optimize conductance to simultaneously maximize oxygen flux and minimize total water loss (Alexander 1996; Woods & Bonnecaze, submitted).
Acknowledgments
This work was supported by the National Science Foundation (IBN-0213087 to H.A.W.), with supplemental REU funding support for B.Z. B.Z. was also supported by an Undergraduate Research Fellowship from the University of Texas at Austin. We thank two anonymous reviewers for clarifying comments.
References
- Addo-Bediako A, Chown S.L, Gaston K.J. Revisiting water loss in insects: a large-scale view. J. Insect Physiol. 2001;47:1377–1388. doi: 10.1016/s0022-1910(01)00128-7. 10.1016/S0022-1910(01)00128-7 [DOI] [PubMed] [Google Scholar]
- Alexander R.M. Princeton University Press; Princeton, NJ: 1996. Optima for animals. [Google Scholar]
- Booth D.T, Rahn H. Factors modifying rate of water loss from birds' eggs during incubation. Physiol. Zool. 1990;63:697–709. [Google Scholar]
- Booth D.T, Seymour R.S. Effect of eggshell thinning on water vapour conductance of malleefowl (Leipoa ocellata) eggs. Condor. 1987;89:453–459. [Google Scholar]
- Carey C. Increase in conductance to water vapour during incubation in eggs of two avian species. J. Exp. Zool. 1979;209:181–186. 10.1002/jez.1402090122 [Google Scholar]
- Chown S.L, Davis A.L.V. Discontinuous gas exchange and the significance of respiratory water loss in scarabaeine beetles. J. Exp. Biol. 2003;206:3547–3556. doi: 10.1242/jeb.00603. 10.1242/jeb.00603 [DOI] [PubMed] [Google Scholar]
- Cronin E.R, Seymour R.S. Respiration of the eggs of the giant cuttlefish Sepia apama. Mar. Biol. 2000;136:863–870. 10.1007/s002270000274 [Google Scholar]
- Hetherington A.M, Woodward F.I. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi: 10.1038/nature01843. 10.1038/nature01843 [DOI] [PubMed] [Google Scholar]
- Hinton H.E. Biology of insect eggs. vol. 1. Pergamon Press; Oxford: 1981. [Google Scholar]
- Kern M.D, Ferguson M.W.J. Gas permeability of American alligator eggs and its anatomical basis. Physiol. Zool. 1997;70:530–546. doi: 10.1086/515860. [DOI] [PubMed] [Google Scholar]
- Kern M.D, Cowie R.J, Yeager M. Water loss, conductance and structure of eggs of pied flycatchers during egg laying and incubation. Physiol. Zool. 1992;65:1162–1187. [Google Scholar]
- Lamer A, Dorn A. The serosa of Manduca sexta (Insecta, Lepidoptera): ontogeny, secretory activity, structural changes and functional considerations. Tissue Cell. 2001;33:580–595. doi: 10.1054/tice.2001.0213. 10.1054/tice.2001.0213 [DOI] [PubMed] [Google Scholar]
- Lehmann F.-O. Matching spiracle opening to metabolic need during flight in Drosophila. Science. 2001;294:1926–1929. doi: 10.1126/science.1064821. 10.1126/science.1064821 [DOI] [PubMed] [Google Scholar]
- Mills N.E, Barnhart M.C, Semlitsch R.D. Effects of hypoxia on egg capsule conductance in Ambystoma (Class Amphibia, Order Caudata) J. Exp. Biol. 2001;204:3747–3753. doi: 10.1242/jeb.204.21.3747. [DOI] [PubMed] [Google Scholar]
- Pinheiro J.C, Bates D.M. Springer; New York: 2000. Mixed-effects models in S and S-plus. [Google Scholar]
- Rothschild L.W.R, Jordan K. A revision of the lepidopterous family sphingidae. Novitates Zoologicae. 1903;9 suppl. [Google Scholar]
- Seymour R.S, Bradford D.F. Gas exchange through the jelly capsule of the terrestrial eggs of the frog, Pseudophryne bibroni. J. Comp. Physiol. B. 1987;157:477–481. 10.1007/BF00691832 [Google Scholar]
- Seymour R.S, Geiser F, Bradford D.F. Gas conductance of the jelly capsule of terrestrial frog eggs correlates with embryonic stage, not metabolic demand or ambient PO2. Physiol. Zool. 1991;64:673–687. [Google Scholar]
- Willmer P. Microclimatic effects on insects at the plant surface. In: Juniper B.E, Southwood T.R.E, editors. Insects and plant surfaces. Edward Arnold; London: 1986. pp. 65–80. [Google Scholar]
- Woods H.A, Harrison J.F. The beneficial acclimation hypothesis versus acclimation of specific traits: physiological change in water-stressed Manduca sexta caterpillars. Physiol. Biochem. Zool. 2001;74:32–44. doi: 10.1086/319302. 10.1086/319302 [DOI] [PubMed] [Google Scholar]
- Woods H.A, Hill R.I. Temperature-dependent oxygen limitation in insect eggs. J. Exp. Biol. 2004;207:2267–2276. doi: 10.1242/jeb.00991. 10.1242/jeb.00991 [DOI] [PubMed] [Google Scholar]
- Woods H.A, Bonnecaze R.T, Zrubek B. Oxygen and water flux across eggshells of Manduca sexta. J. Exp. Biol. 2005;208:1297–1308. doi: 10.1242/jeb.01525. 10.1242/jeb.01525 [DOI] [PubMed] [Google Scholar]
- Woods, H. A. & Bonnecaze, R. T. Submitted. Insect eggs at a transition between reaction and diffusion limitation: temperature, oxygen and water. J. Theor. Biol. [DOI] [PubMed]