Abstract
Symbiotic associations often enhance hosts' physiological capabilities, allowing them to expand into restricted terrains, thus leading to biological diversification. Stable maintenance of partners is essential for the overall biological system to succeed. The viviparous tsetse fly (Diptera: Glossinidae) offers an exceptional system to examine factors that influence the maintenance of multiple symbiotic organisms within a single eukaryotic host. This insect harbours three different symbionts representing diverse associations, coevolutionary histories and transmission modes. The enterics, obligate mutualist Wigglesworthia and beneficial Sodalis, are maternally transmitted to the intrauterine larvae, while parasitic Wolbachia infects the developing oocyte. In this study, the population dynamics of these three symbionts were examined through host development and during potentially disruptive events, including host immune challenge, the presence of third parties (such as African trypanosomes) and environmental perturbations (such as fluctuating humidity levels). While mutualistic partners exhibited well-regulated density profiles over different host developmental stages, parasitic Wolbachia infections varied in individual hosts. Host immune status and the presence of trypanosome infections did not impact the steady-state density levels observed for mutualistic microbes in either sex, while these factors resulted in an increase in Wolbachia density in males. Interestingly, perturbation of the maternal environment resulted in the deposition of progeny harbouring greater overall symbiont loads. The regulation of symbiont density, arising from coadaptive processes, may be an important mechanism driving inter-specific relations to ensure their competitive survival and to promote specialization of beneficial associations.
Keywords: tsetse, symbiosis, Wigglesworthia, Sodalis, Wolbachia, coevolution
1. Introduction
Extraordinary metabolic and adaptive abilities allow bacteria to occupy a vast range of niches, including permanent habitation within other organisms (endosymbiosis). These relationships can be multifaceted, varying in their impact on host biology (spanning a wide breadth from parasitism to mutualism), prevalence and coevolutionary history. Hosts frequently exploit beneficial symbioses to augment their functional capabilities and to facilitate their expansion into novel niches. Among the eukaryotes that have succeeded in limited and often inhospitable ecological terrains are members of the Class Insecta, due in part to fitness advantages granted by symbiosis.
Obligate mutualists enable the survival of many insects on nutritionally deficient diets such as blood, plant phloem or wood (Buchner 1965). In turn, this restricted nutritional flexibility, strict intracellular localization within host tissues, and vertical transmission mode over a long evolutionary time span have impacted the genome size and functional repertoire of these captivated microbes. One of the more dramatic testimonies of these tailoring processes is exemplified by the intracellular obligate mutualists, where metabolic capabilities no longer necessary for symbiont survival have been purged, while functions indispensable for host fitness and selective maintenance of the symbiotic interaction have been preserved (reviewed in Wernegreen (2002)). In addition to these intracellular obligates, many insects also harbour secondary symbionts which exhibit a wider range of tissue tropism. In contrast to the obligate mutualists, which have a primarily nutrient provisioning role in host biology, a broader variety of functional contributions have been attributed to secondary symbionts, including tolerance to heat stress (Montllor et al. 2002), resistance to parasitoid infections (Oliver et al. 2003), broadening of host plant range (Tsuchida et al. 2004) and a role in host morphogenesis (Rakoff-Nahoum et al. 2004). In addition, parasitic microbes, such as Wolbachia and Spiroplasma spp., increase their symbiotic prevalence by inflicting various host reproductive alterations (reviewed in Bandi et al. (2001)).
The tsetse fly (Diptera: Glossinidae), which relies on multiple microbial species for fitness, provides a unique model to investigate and compare the population dynamics of symbionts that display varying levels of integration with host biology. The obligate mutualist, Wigglesworthia glossinidia (Proteobacteria: Enterobacteriaceae), is localized within epithelial cells (bacteriocytes) in the adult gut and shares an ancient concordant history of over 50 million years with its tsetse host species (Chen et al. 1999). The Wigglesworthia genome, approximately 700 kb in size, displays a drastically streamlined functional capacity. Despite this reduction, Wigglesworthia encodes a plethora of vitamin biosynthetic products that may promote host reproduction. This function may provide a selective pressure for the retention of the symbiotic system (Akman et al. 2002). In fact, in the absence of Wigglesworthia, tsetse flies are rendered sterile, although vitamin supplementation of the blood meal diet can rescue host sterility (Nogge 1976, 1982). In contrast, Sodalis glossinidius (Proteobacteria: Enterobacteriaceae), does not display a concordant evolutionary history with tsetse (Aksoy 1995), suggestive of a more recent symbiotic initiation. This secondary symbiont, with intra- and extra-cellular localization, is primarily found in the midgut, although it can also be detected in the haemolymph and other tissues depending on the tsetse species (Cheng & Aksoy 1999). While its 4.2 Mb genome size is close to those of related free-living enterics, analysis of its proteome indicates an unusually low coding density (49.2%), and a high number of pseudogenes, both of which are indicative of an ongoing genome erosion phenomenon and adaptation to the host's restricted ecology (Toh et al. 2006). The functional role of Sodalis symbiosis in tsetse is relatively unknown, although its specific elimination has been reported to result in a reduction in host longevity (Dale & Welburn 2001). Lastly, some tsetse populations harbour a third microbe related to Wolbachia pipientis (Proteobacteria: Rickettsiaceae). The tissue distribution and prevalence of Wolbachia in tsetse species ranges from intracellular infection of only germ-line tissue to persistence in various somatic tissues (Dobson et al. 1999; Cheng et al. 2000). Facultative Wolbachia, identified in numerous arthropods (Werren et al. 1995; Jeyaprakash & Hoy 2000), are generally considered parasitic. A wide range of phenotypic effects, such as host reproductive alterations, premature death and behavioural modifications have been ascribed to Wolbachia infections (reviewed in O'Neill et al. (1998)), but its role in tsetse remains to be elucidated.
Tsetse reproductive biology is unusual—an adult female produces a single egg at a time that hatches and develops in utero (viviparity). After a period of maturation and sequential molting within the mother, a fully mature third instar larva is deposited and pupates shortly thereafter in the soil. As one of the few insect k-strategists, each tsetse female can deposit five to six offspring during her two- to three-month lifespan (Leak 1999). During intrauterine development, the enteric symbionts Sodalis and Wigglesworthia are transmitted through the mother's milk gland secretions to the larval progeny, where they establish new infections (Ma & Denlinger 1974; Cheng & Aksoy 1999). Wolbachia, however, infects germ-line cells and utilizes a transovarial transmission route (O'Neill et al. 1993). Given the unique reproductive biology of tsetse, all three symbionts, however, are in essence maternally acquired by the progeny. In addition to the multiple bacterial symbionts, tsetse flies can also acquire the protozoan parasite African trypanosomes from the mammalian hosts they feed on. Trypanosomes replicate and can establish chronic infections in the fly midgut in close proximity to the gut symbionts, and be transmitted to tsetse's vertebrate hosts through an infected bite.
We utilized the Glossina morsitans morsitans system to examine the population dynamics of multiple microbial symbionts through development in the same host individuals. The three symbionts analysed have different associative properties, transmission modes and coevolutionary histories with their tsetse host. The evolutionary success of enteric symbionts requires a highly faithful maternal transmission route to counteract the bottleneck process confronted at each generation. We investigated the influence of extrinsic factors that could impact symbiotic homeostasis, such as alterations in host immune status, presence of competitive parties, and humidity fluctuations in the maternal environment. We predicted that beneficial symbiotic associations would exhibit population dynamics profiles in concert with host physiological processes through development and critical life events. We also expected the perturbations in the maternal environment to have adverse effects on the transmission and maintenance of symbiosis in subsequent generations, leading to fitness loss of the biological system.
2. Material and methods
(a) Tsetse and trypanosome cultures
The Glossina morsitans morsitans colony maintained in the insectary at Yale University was established from puparia collected in Zimbabwe approximately twenty years ago. Flies were maintained at 24±1 °C with 50–55% relative humidity and received defibrinated bovine blood every other day by using an artificial membrane system (Moloo 1971). Females were mated at days 3–5 post emergence, males were removed after 48 h, and approximately 30 fertile females were maintained in each breeding cage, unless otherwise specified. Procyclic stage Trypanosoma brucei rhodesiense (strain YTAT 1.1) were maintained at 28 °C in SDM-79 medium supplemented with 10% heat-inactivated fetal bovine serum (Brun et al. 1979).
(b) Sample preparation and DNA isolation
Total DNA was extracted, using the Holmes–Bonner method (Holmes & Bonner 1973), from larvae, teneral (newly enclosed; unfed) two-week-old virgin and four-week-old virgin adults. DNA was similarly extracted from early (24–48 h post deposition) and late (28–30 days post deposition) pupae and 2–8-week-old mated females. Intrauterine larva were surgically removed from mothers and identified as L1, L2 and L3 based on morphological characteristics (Leak 1999). Flies were processed for DNA isolation 48 h following their last blood meal.
(c) Measuring symbiont density
Real-time quantitative PCR (QT-PCR) was performed with an icycler iQ real-time PCR detection system (Bio-Rad, Hercules, CA). Symbiont specific thiamine biosynthesis protein (thiC) (GenBank Accession no. AB063522), exochitinase (chi) (GenBank Accession no. BSPY11391) and outer surface protein (wsp) (GenBank Accession no. AF020079) were used for Wigglesworthia, Sodalis and Wolbachia quantification, respectively. While duplications of Wolbachia wsp have been noted (Wu et al. 2004), a single amplicon was obtained using WolF′ and WolR′ primers (R. V. M. Rio 2003, unpublished data). The tsetse chitinase gene (Gmchi, GenBank Accession no. AF337908), which is also single copy, was used to normalize for DNA extraction efficiency (Yan et al. 2002). Specific fluorescent probes designed to the central portion of the PCR product for the chi and Gmchi amplicons were utilized for quantification of Sodalis's genome and host cell copy numbers, respectively. The fluorometric intensity of SYBR Green I Dye (a fluorescently labelled double-stranded DNA binding dye) was used for quantification of the thiC and wsp genes.
Symbiont density is defined as the number of symbiont genomes relative to host cell number. DNA from each experimental sample was analysed to quantify the density of the three symbionts. Internal standard curves were generated for each primer combination using the four above-mentioned amplicons, each cloned into pGEM-T vector (Promega). Density estimates were obtained by comparison to a standard curve using Bio-Rad icycler iQ Multi-Color Real Time PCR data analysis software. All assays were carried out in duplicate, and replicates were averaged for each sample. Negative controls were included in all amplification reactions. The PCR-amplification parameters, reagents, primer design criteria and sequences used for quantification, are summarized in the electronic supplementary material.
(d) Genome copy number determination per Wigglesworthia cell
Bacteriomes were dissected, gently homogenized and DNA was isolated from a known number of Wigglesworthia cells (quantified by haemocytometer measurements). The copy number of thiC was determined by QT-PCR in serially diluted samples, and numbers of genomes per cell were extrapolated in three independent experiments (see electronic supplementary material).
(e) Impact of environmental stress on offspring symbiont density
Newly mated G. m. morsitans females were reared in single cages under fluctuating humidity levels (39–63%) for two weeks, then returned to normal conditions (50–55%). Offspring were collected in the order of deposition (i.e. 1st, 2nd, 3rd and 4th). Similarly, the offspring of females maintained under normal rearing conditions were sequentially collected. Following pupation, symbiont densities in the progeny were determined in the early (24–48 h post deposition) and late (28–30 days post deposition) pupal periods.
(f) Impact of host immune induction and third party competition on symbiont density
(i) Host immune induction
Teneral flies received a single blood meal containing Escherichia coli K12 (106 cells ml−1) and were subsequently maintained on a normal diet as previously described (Hao et al. 2001). Symbiont genome densities were analysed 48 h and two weeks following bacterial challenge.
(ii) Third party competition
Teneral flies received a single blood meal containing mammalian stage trypanosomes (105 cells ml−1) and were subsequently maintained on a normal diet (Hao et al. 2001). Two weeks post acquisition of the infective blood meal, a PCR amplification assay was used to distinguish flies with trypanosome midgut infections (infected) from those that had successfully cleared their parasites (recovered). For this assay, primers (TBR1 and TBR2) specific for the T. brucei subspecies were used (Moser et al. 1989). Two weeks, which is sufficient time to determine trypanosome infection status in flies (Gibson & Bailey 2003), was used as the time point for examining symbiont densities as a function of third party competition.
(g) Statistical analysis
Data were analysed using SAS system v. 8.02 for Windows (Littell et al. 1996). Student's t-tests, one-way analysis of variance (ANOVA) and post hoc pairwise comparison of the mean were performed to determine whether densities significantly differed between time points. Where densities appeared skewed, the data were log transformed to satisfy normality. F-tests were applied to assess homogeneity of variances. When necessary, final ANOVA models were modified to account for unequal variances across groups.
To examine the impact of environmental stress on timing of offspring deposition, a general linear model for repeated measures was developed. This model employed an unstructured covariance matrix that allowed the duration between offspring depositions to be correlated across pupae (first through fourth). Likewise, as for the offspring symbiont density analyses, interaction between group (control versus experimental) and pupal stage was also investigated and included in the final model as necessary.
3. Results
(a) Symbiont genome density through host development
We used a real-time quantitative PCR assay to capture and compare genome densities of the three different symbionts harboured within the same individual host throughout development (i.e. spanning from first instar larva (L1) to 30 days into adulthood). Symbiont prevalence among the individuals analysed was 100%, in that every fly examined was infected with Wigglesworthia, Sodalis and Wolbachia.
Multiple genome copies per bacterium have previously been described for obligate insect symbionts with the abundance varying among different host developmental stages (Komaki & Ishikawa 2000). We determined the genome copy number per Wigglesworthia, and our results also indicate the presence of multiple genomes per bacterial cell, varying from 3 to 23 copies in the two-week-old female bacteriome (see electronic supplementary material). Thus, the symbiont density data presented here reflect symbiont genome copy number relative to tsetse host cell.
In general, Sodalis was maintained at a density three orders of magnitude lower than Wigglesworthia and Wolbachia through development (figure 1a). Although Sodalis shares the same transmission route into host progeny as Wigglesworthia (through maternal milk gland secretions), it colonizes the intrauterine larva during L1 instar, while Wigglesworthia does so in the L2 instar stage (figure 1a,b). During larval development, the enteric symbionts proliferated in parallel to host cell replication. However, significant differences in both symbiont densities were observed between early and late pupal stages, resulting in a threefold increase in Sodalis (figure 1a) and 16- and 30-fold increases in Wigglesworthia in male and female adults, respectively (figure 1b). This proliferation apparently occurs late during pupal development, as analyses of both symbiont densities at mid-pupal development (day 15 of an approximately 30 day pupal development period) indicated minimal expansion (data not shown). Following eclosion, symbiont proliferation continued for at least 48 h (figure 1a,b). During the adult lifespan monitored, Sodalis densities increased about twofold relative to tenerals (figure 1a). Although host sex did not appear to influence Sodalis's density, male and virgin female adult hosts exhibited different Wigglesworthia population dynamics (figure 1b). In males, the peak densities of Wigglesworthia were detected in tenerals, corresponding to a fourfold increase from the late pupal stage, while in virgin Wigglesworthia females continued to proliferate during the whole age spectrum analysed (figure 1b). No significant differences in Wolbachia densities were observed during larval, pupal and adult development in both sexes or among individuals of the same age class. In this case, the variability was too wide to allow for mean determination (figure 2a). Although sample sizes for Wolbachia density estimates were increased due to the high variability observed, this did not result in conformity. Hence, the median, rather than the mean, was used as a representative measure of Wolbachia density through host development. The median Wolbachia density (approximately 10 genome copies per host cell) was similar among tsetse larval, pupal and adult life stages. Furthermore, by examining Wolbachia density of the offspring from individual mothers, we also determined that this variability does not arise due to a given mother depositing offspring harbouring consistently high or low symbiont loads. Instead, the Wolbachia densities in the multiple progeny analysed from each mother fluctuated randomly independent of their order of deposition (figure 2b).
Figure 1.
Mutualistic symbiont density through host development and mating status. (a) Sodalis density. Sample sizes are indicated adjacent to each time point. (b) Wigglesworthia density, Wigglesworthia was not detectable (n.d.) in the majority of L1 samples. Male and virgin female densities are represented with solid and hatched lines, respectively (n≥5 samples at each time point for each sex). Each time point refers to a mean, error bars represent 1 standard error of the mean (s.e.m.). *, ** indicate significant differences between corresponding time points (p<0.05, 0.005; respectively). (c) Sodalis and Wigglesworthia densities in mated females. Sodalis (black bars) and Wigglesworthia (white bars) densities in 2, 4, 6 and 8 week old mated females. Each time point refers to a mean, error bars represent one 1 s.e.m. (n≥5 samples at each time point).
Figure 2.
Wolbachia density through host development and in different offspring. (a) Box-and-whiskers plots of Wolbachia density and distribution of variance through host development. Host development was separated into three discrete categories; larvae (n=30), pupae (n=109) and adult (n=58). The median (heavy line) ranged from the 25th to 75th percentile (boxed) and 10th and 90th percentiles (whiskers) are shown. (b) Wolbachia densities from 1st, 2nd, 3rd and 4th offspring of individual mothers (A–J) measured immediately following larval deposition.
(b) The impact of host mating on symbiont density
Symbiont densities from two-, four-, six- and eight-week-old mated females were examined to determine whether host reproductive status influences symbiont dynamics. Wigglesworthia density in mated females was similar to virgin levels (figure 1b,c), while Sodalis density varied. The two-week-old fertile flies showed a twofold increase of Sodalis relative to similarly aged virgins (figure 1a,c), while the four-week-old group declined, only to return to higher levels by eight weeks. No significant differences were observed in Wolbachia densities as a function of age and mating status (data not shown). Hence, mating did not appear to be an influential physiological factor in the regulation of symbiont genome densities, although Wigglesworthia was more stable than Sodalis. Since Wigglesworthia products are thought to be indispensable for host fecundity, it remains to be seen whether they are subject to transcriptional upregulation in mated female flies.
(c) Impact of host immunity challenge and third party competition on symbiont density
Because the host immune system plays a pivotal role in controlling foreign invaders, we evaluated how stimulating tsetse immune responses affected its resident microbial flora. Symbiont densities were examined following host challenge with E. coli and trypanosomes. Both of these organisms have been shown to increase the expression of several tsetse immunity genes, resulting in the synthesis of antimicrobial peptides as well as reactive oxygen and nitrogen intermediates (Hao et al. 2001, 2003; Boulanger et al. 2002). Furthermore, a proportion of the tsetse flies challenged with trypanosomes had established persistent midgut parasite infections and served to explain the effects of additional trophic complexity on symbiont populations.
Sodalis density measured from two-week-old adults with midgut trypanosome infections did not differ from flies that had successfully cleared their parasite infections (figure 3a, TI versus TR). Similar non-disruptive results on Sodalis were observed 48 h (data not shown) and two weeks following per os E. coli challenge (figure 3a). Wigglesworthia densities in these same individuals also did not differ in comparison to unchallenged controls (figure 3b). These results suggest that both symbionts were unaffected by either the antimicrobial specific host immune response or the potential competitive interactions with the parasitic trypanosomes replicating in the midgut. Although Wolbachia densities measured from the same individuals challenged with E. coli were comparable to the controls, rampant Wolbachia replication was observed in males exposed to trypanosomes regardless of their infection status (ANOVA, p<0.0001, figure 3c). In addition, both infected and recovered 6-week-old males still harboured significantly higher Wolbachia densities (ANOVA, p=0.0015, figure 3d), suggesting either a sex-specific opportunist role for this symbiont, or loss of host control in males upon specific challenge. In contrast, Wolbachia densities in females were not affected by either trypanosome or E. coli challenge.
Figure 3.
Impact of host immunity and third party competition on symbiont densities. (a) Sodalis density measured from two-week-old adults (males and females combined). (b) Wigglesworthia density measured from two-week-old males and females. (c) Wolbachia density in two-week-old males and females from groups; C (control), TI (trypanosome infected), TR (trypanosome recovered, tsetse which were challenged but able to clear the infection) and Ec (per os E. coli challenge). Sample sizes are indicated. (d) Mean Wolbachia density in six-week-old males and females corresponding to; C (control), TI (trypanosome infected) and TR (trypanosome recovered, tsetse which were challenged but able to clear the infection). n≥5 samples for each category. A designates treatments that are significantly different from others (p<0.005). Means are represented and error bars signify 1 s.e.m.
(d) Impact of environmental stress on offspring symbiont density
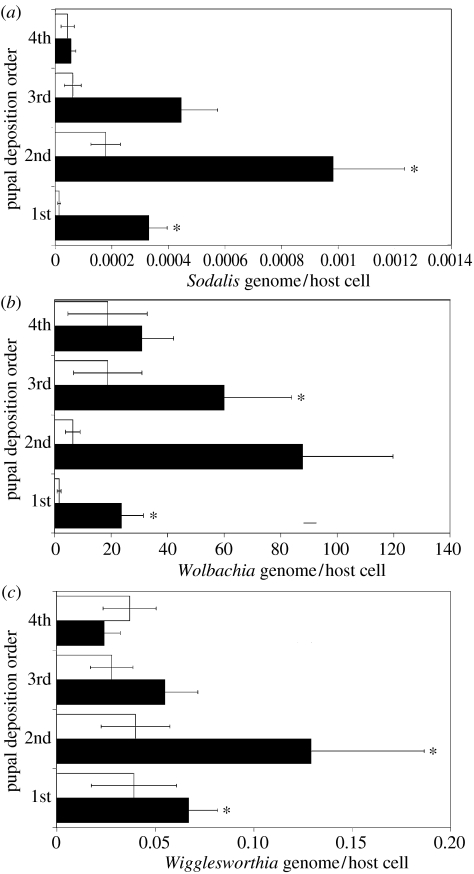
The delays between each larval deposition cycle and the offspring symbiont loads, were determined from mothers exposed to varying humidity levels (environmentally stressed) during their first gestational period. Delayed life history effects were expected solely in offspring from the first gonotrophic cycle because mothers were directly exposed to humidity fluctuations only during this period. A general linear model for repeated measures did not support differences in the duration between larval deposition cycles among control and stressed mothers. The difference (in days) in the duration between larval deposition cycles between the two groups was found to be 0.65 days (95% confidence interval: 0.109–3.866, p=0.682). However, the impact of maternal stress extended beyond the time of environmental stress. Fluctuations in humidity resulted in an increase in the density of all three symbionts in subsequent progeny (figure 4). Sodalis and Wigglesworthia density significantly differed between the first and second offspring deposited by environmentally stressed and control females, and similar differences in Wolbachia density were also observed in the first and third offspring. These environmental effects subsided with time, as symbiont densities in the fourth offspring were comparable to controls. It is worth noting that in the fourth offspring from the environmentally stressed mothers, deposited about one month following humidity fluctuations, Wolbachia densities still remained high (approximately seven times greater than those from control group), but the association is not statistically significant (table 1). The restoration of homeostasis also occurred with progression of progeny age, as older individuals analysed from experimental and control groups as late pupae contained similar symbiont densities (data not shown).
Figure 4.
Symbiont density in the offspring of environmentally stressed and control mothers. (a) Sodalis density. (b) Wigglesworthia density. (c) Wolbachia density in the first four offspring of environmentally stressed and control mothers. Means are represented and error bars signify 1 s.e.m. (n≥10 samples for each group). * indicates significant difference between corresponding offspring (p<0.05). Open bars: control, 24–48 h; filled bars: experimental, 24–48 h.
Table 1.
ANOVA estimation of the geometric mean density ratios of first through fourth offspring symbiont loads deposited by environmentally stressed versus control tsetse.
| offspring deposition number | Sodalis | Wigglesworthia | Wolbachia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| geometric mean ratioa | 95% CI | p-value | geometric mean ratioa | 95% CI | p-value | geometric mean ratioa | 95% CI | p-value | ||||
| lower | upper | lower | upper | lower | upper | |||||||
| 1 | 38.092 | 10.870 | 133.847 | <0.0001 | 4.330 | 1.559 | 12.025 | 0.0057 | 30.435 | 3.505 | 264.225 | 0.0022 |
| 2 | 4.665 | 1.425 | 15.270 | 0.0115 | 4.651 | 1.525 | 14.188 | 0.0078 | 6.505 | 0.865 | 48.901 | 0.0685 |
| 3 | 2.739 | 0.705 | 28.789 | 0.1438 | 2.135 | 0.700 | 6.513 | 0.1784 | 12.752 | 1.423 | 114.263 | 0.0233 |
| 4 | 0.763 | 0.175 | 3.329 | 0.7166 | 1.130 | 0.370 | 3.446 | 0.8275 | 7.037 | 0.624 | 79.376 | 0.1133 |
Ratio of geometric mean densities between stressed versus control mothers; 95% CI, 95% confidence interval.
4. Discussion
Our results illustrate that host colonization by symbiotic flora is an active and continuous process, with the composition of these communities varying spatially and temporally throughout development. Our findings demonstrate plasticity of symbiont density in response to a multitude of host (i.e. development, sex, immunity) and environmental factors (i.e. humidity, third parties). Symbiont responses to these influential factors exhibited distinct dynamics probably reflective of the age of the association, the functional repertoires of the symbionts and varying levels of integration with host biology.
Consistent densities relative to host cell numbers observed among similarly aged tsetse individuals for Sodalis and Wigglesworthia suggest an adaptive regulation through development for these beneficial interactions that are established through maternal milk gland secretions. The teneral adult stage (24 h after eclosion and prior to the first blood meal) provided the one exception, during which increased proliferation was documented for both symbionts. The teneral state has also been associated with greater susceptibility to trypanosome infections (Welburn & Maudlin 1992), possibly because the immature state of tsetse immunity allows for increased parasite survival. The lack of a robust immune status may also account for the increased proliferation noted during late pupal and early adult development for Wigglesworthia and Sodalis. This increase in the density of both symbionts may also be either a host or symbiont mediated response, resulting from the transition to a life stage independent of the maternal environment. More specifically, symbiont flora contributions may not be as significant during in utero development, as resources can be directly allocated from the mother. By maintaining lower symbiont loads, the metabolic cost of infections during these immature stages of development may be reduced.
In contrast to the beneficial symbionts, wide variability was observed in different individuals of similar ages for Wolbachia density through development. The variations in Wolbachia densities suggest the lack of optimal host–symbiont dynamics for this organism, perhaps consequential to its recent invasion, potentially parasitic nature, or oocyte transmission mode. These findings are similar to other insect species, where high variability in Wolbachia loads among similarly aged individuals were also reported (McGraw et al. 2001; Noda et al. 2001; Ikeda et al. 2003). Potential causes of variability may include unequal vertical transmission into progeny as we detected in the sequential offspring from the same mother, or stochastic variation in Wolbachia replication rates (Veneti et al. 2005), both of which might result from the lack of sufficient co-adaptation (McGraw et al. 2001). Interestingly, median Wolbachia density, relative to host cell number, was similar throughout tsetse development suggesting global stability. Host selection for reductions in parasite densities can normally give rise to a conflict in optimal densities in parasitic associations. However, if the parasite infections confer a reproductive advantage to the host, as seen with CI expression in Wolbachia, hosts may be expected to exhibit a relaxed density control over these infections. It will now be interesting to elucidate whether the variations in Wolbachia density we observe in tsetse individuals of similar age may lead to differences in the intensity of the potential physiological modifications they may manifest, such as male killing or cytoplasmic incompatibility, as has been reported in other host systems (Hurst et al. 2000; McGraw et al. 2001; Clark & Karr 2002; Veneti et al. 2003).
An additional level of specificity towards the host physiological state was observed with the obligate mutualist Wigglesworthia, where higher densities in females suggest a role in hosts' reproductive fitness. Nogge (1976, 1982) demonstrated that the sterility observed in females in the absence of the obligate mutualist could be rescued by provisioning the fly's diet with vitamin supplements, supporting a putative nutritional role for Wigglesworthia in fecundity. In support of this role, more prominent bacteriomes have been described in females, in comparison to males, presumably related to the increased metabolic processes necessary for support of progeny (Nogge & Ritz 1982). Higher Wigglesworthia densities during the female adult stages may allow for both an increase in the production of metabolites such as vitamins required for fertility, and also greater symbiont transmission efficiency to progeny. It will be interesting to determine whether the increase in Wigglesworthia genome copy we noted represents an increase in cell numbers or genome copies per cell given that these bacteria have multiple chromosomes.
Wigglesworthia and Sodalis densities remained stable during stimulation of the host immune system. These findings suggest that these organisms are either able to evade the effects of their host's immune arsenal due to their intracellular localization, or display greater acquired resistance as a result of their long coevolutionary histories (Hao et al. 2001, 2003; Boulanger et al. 2002). In support of the latter hypothesis, Sodalis does exhibit a greater resistance towards the bacteriocidal actions of tsetse's antimicrobial peptides Diptericin and Attacin (Hao et al. 2001; Hu & Aksoy 2005), possibly reflecting cellular adaptations acquired as a result of constant exposure to host physiology. In G. m. morsitans males, Wolbachia has a broader tissue distribution, being detected in the head, thorax and abdomen. In contrast, this bacterium in females is strictly restricted to germ-line tissue in this species of flies (Dobson et al. 1999). This lack of density regulation of Wolbachia in males is further accentuated following trypanosome exposure, regardless of the eventual infection status of the host. It remains to be seen whether density regulation in females is host- or symbiont-mediated. The cost of any fitness damage in females, through increases in density and extended tissue tropisms, would have greater detrimental impact on Wolbachia fitness than does harming males.
Environmental factors, including temperature, food quality and host rearing density, alter symbiont densities and the intensity of their effects on directly impacted individuals (Chen & Purcell 1997; Clancy & Hoffmann 1998). Furthermore, our results demonstrate that perturbations in the maternal environment have dramatic effects on the transmission and maintenance of symbiosis in subsequent generations. However, restoration of mutualist density levels following these perturbations was observed with time (i.e. symbiont densities returned to levels comparable to the controls in progeny deposited later in maternal life as well as with progeny age). Temperature fluctuations have been shown to alter Wolbachia and Sodalis abundance (Chen & Purcell 1997; Welburn & Maudlin 1991), suggesting that these symbionts are able to respond to fluctuations in the host environment. Indeed, the recently sequenced genome of Sodalis indicates the presence of various two-component regulatory systems that may mediate such recognition (Toh et al. 2006). This is in contrast to the Wigglesworthia proteome, which lacks any such regulatory systems. How Wigglesworthia density is regulated in the host remains to be determined, and possibly may involve host-specific factors.
As a k-strategist, tsetse deposit few offspring. Hence, fidelity of mutualistic symbiont transmission in tsetse is of utmost importance for the evolutionary success of the system. The bottlenecks encountered during maternal transmission of Wigglesworthia and Sodalis populations necessitate accommodations in host reproductive biology to ensure successful colonization of progeny. Given that traits involved in the specialization of symbiotic associations are likely under strong selective pressure (Thompson 1982), there may be signalling events tightly regulating the invasion of larval gut tissues and subsequent maintenance of the infections in progeny. It is interesting that Sodalis's genome has retained three putative type-III secretion systems (TTSS), reminiscent of the apparatus that related pathogenic microbes utilize to gain access to their eukaryotic host cells. At least two of these TTSSs are transcriptionally expressed during the early larval development stage in tsetse, and might be involved in the transmission and/or symbiotic establishment processes in the progeny (Toh et al. 2006). The annotated genome sequences of tsetse symbionts can now be used to examine the expression profiles of genes, such as those involved in invasion, quorum sensing and biofilm formation processes, which may be central for the described temporal dynamics, symbiotic acquisition, progression and maintenance processes. Our experimental results described here with controlled laboratory lines strongly suggest a correlation between the length of coevolutionary history and symbiont density dynamics, where fine-tuned regulation is consequential of adaptive processes. The coordination of density may be an influential factor for reducing conflict between partners, driving inter-specific associations and further promoting specialization of established symbioses.
Acknowledgements
We thank members of the Aksoy lab for helpful discussions and particularly thank Irene Kasumba, Sarah Perkin and Douglas Smith for their meticulous attention during experimental trials and Brian Weiss for editorial assistance. We gratefully acknowledge Joerg Graf, Jennifer Wernegreen, and the two anonymous reviewers for helpful comments and suggestions. This work was funded by NIH/NIAID grant AI-34033 and NSF/MCB 0237305 to S.A., R.V.M.R. is the recipient of a NIH Training grant T32AI07404, a CDC fellowship for training in vector-borne infectious diseases T01/CCT122306-01 and a Sigma Xi Grant-in-Aid award.
Footnotes
Present address: Department of Molecular and Cellular Biology, University of Connecticut, 75 North Eagleville Road Unit 3125, Storrs, CT 06269-3125, USA.
Present address: Department of Statistical Sciences, Southern Methodist University, Dallas, TX 75275-0332, USA.
Present address: Institute for Health Care Research and Improvement, Baylor Health Care System, Dallas, TX 75206, USA.
Supplementary Material
References
- Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. Genome sequence of the endocellular obligate symbiont of tsetse, Wigglesworthia glossinidia. Nat. Genet. 2002;32:402–407. doi: 10.1038/ng986. 10.1038/ng986 [DOI] [PubMed] [Google Scholar]
- Aksoy S. Molecular analysis of the endosymbionts of tsetse flies: 16S rDNA locus and over-expression of a chaperonin. Insect Mol. Biol. 1995;4:23–29. doi: 10.1111/j.1365-2583.1995.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Bandi C, Dunn A.M, Hurst G.D, Rigaud T. Inherited microorganisms, sex-specific virulence and reproductive parasites. Trends Parasitol. 2001;17:88–94. doi: 10.1016/s1471-4922(00)01812-2. 10.1016/S1471-4922(00)01812-2 [DOI] [PubMed] [Google Scholar]
- Boulanger N, Brun R, Ehret-Sabatier L, Kunz C, Bulet P. Immunopeptides in the defense reactions of Glossina morsitans to bacterial and Trypanosoma brucei brucei infections. Insect Biochem. Mol. Biol. 2002;32:369–375. doi: 10.1016/s0965-1748(02)00029-2. 10.1016/S0965-1748(02)00029-2 [DOI] [PubMed] [Google Scholar]
- Brun R, Jenni L, Tanner M, Schonenberger M, Schell K.F. Cultivation of vertebrate infective forms derived from metacyclic forms of pleomorphic Trypanosoma brucei stocks. Acta Trop. 1979;36:387–390. [PubMed] [Google Scholar]
- Buchner P. Wiley; New York: 1965. Endosymbiosis of animals with plant microorganisms. [Google Scholar]
- Chen D.Q, Purcell A.H. Occurrence and transmission of facultative endosymbionts in aphids. Curr. Microbiol. 1997;34:220–225. doi: 10.1007/s002849900172. 10.1007/s002849900172 [DOI] [PubMed] [Google Scholar]
- Chen X.A, Song L, Aksoy S. Concordant evolution of a symbiont with its host insect species: molecular phylogeny of genus Glossina and its bacteriome-associated endosymbiont Wigglesworthia glossinidia. J. Mol. Evol. 1999;48:49–58. doi: 10.1007/pl00006444. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Aksoy S. Tissue tropism, transmission, and expression of foreign genes in vivo in midgut symbionts of tsetse flies. Insect Mol. Biol. 1999;8:125–132. doi: 10.1046/j.1365-2583.1999.810125.x. 10.1046/j.1365-2583.1999.810125.x [DOI] [PubMed] [Google Scholar]
- Cheng Q, Ruel T, Zhou W, Moloo S, Majiwa P, O'Neill S, Aksoy S. Tissue distribution and prevalence of Wolbachia infections in tsetse flies, Glossina spp. Med. Vet. Entomol. 2000;14:44–50. doi: 10.1046/j.1365-2915.2000.00202.x. 10.1046/j.1365-2915.2000.00202.x [DOI] [PubMed] [Google Scholar]
- Clancy D.J, Hoffmann A.A. Environmental effects on cytoplasmic incompatibility and bacterial load in Wolbachia-infected Drosophila simulans. Entomol. Exp. Appl. 1998;86:13–24. 10.1023/A:1003043814761 [Google Scholar]
- Clark M.E, Karr T.L. Distribution of Wolbachia within Drosophila reproductive tissue: implications for the expression of cytoplasmic incompatibility. Int. Comp. Biol. 2002;42:332–339. doi: 10.1093/icb/42.2.332. [DOI] [PubMed] [Google Scholar]
- Dale C, Welburn S.C. The endosymbionts of tsetse flies: manipulating host–parasite interactions. Int. J. Parasitol. 2001;31:628–631. doi: 10.1016/s0020-7519(01)00151-5. [DOI] [PubMed] [Google Scholar]
- Dobson S, Bourtzis K, Braig H, Jones B, Zhou W, Rousset F, O'Neill S. Wolbachia infections are distributed throughout the insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 1999;29:153–160. doi: 10.1016/s0965-1748(98)00119-2. 10.1016/S0965-1748(98)00119-2 [DOI] [PubMed] [Google Scholar]
- Gibson W.C, Bailey M. The development of Trypanosoma brucei within the tsetse fly midgut observed using green fluorescent trypanosomes. Kinetoplastid. Biol. Dis. 2003;2:1–3. doi: 10.1186/1475-9292-2-1. 10.1186/1475-9292-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Kasumba I, Lehane M.J, Gibson W.C, Kwon J, Aksoy S. Tsetse immune responses and trypanosome transmission: implications for the development of tsetse-based strategies to reduce trypanosomiasis. Proc. Natl Acad. Sci. USA. 2001;98:12648–12653. doi: 10.1073/pnas.221363798. 10.1073/pnas.221363798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Kasumba I, Aksoy S. Proventriculus (cardia) plays a crucial role in immunity in tsetse fly (Diptera: Glossinidae) Insect Biochem. Mol. Biol. 2003;33:1155–1164. doi: 10.1016/j.ibmb.2003.07.001. 10.1016/j.ibmb.2003.07.001 [DOI] [PubMed] [Google Scholar]
- Holmes D.S, Bonner J.B. Preparation, molecular weight, base composition, and secondary structure of giant ribonucleic acid. Biochemistry. 1973;12:2330–2338. doi: 10.1021/bi00736a023. 10.1021/bi00736a023 [DOI] [PubMed] [Google Scholar]
- Hu Y, Aksoy S. An antimicrobial peptide with trypanocidal activity characterized from Glossina morsitans morsitans. Insect Biochem. Mol. Biol. 2005;35:105–115. doi: 10.1016/j.ibmb.2004.10.007. 10.1016/j.ibmb.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Hurst G.D.D, Johnson A.P, von der Schulenburg J.H.G, Fuyama Y. Male-killing Wolbachia in Drosophila: a temperature sensitive trait with a threshold bacterial density. Genetics. 2000;156:699–709. doi: 10.1093/genetics/156.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Ishikawa H, Sasaki T. Infection density of Wolbachia and level of cytoplasmic incompatibility in the Mediterranean flour moth, Ephestia kuehniella. J. Invertebr. Pathol. 2003;84:1–5. doi: 10.1016/s0022-2011(03)00106-x. 10.1016/S0022-2011(03)00106-X [DOI] [PubMed] [Google Scholar]
- Jeyaprakash A, Hoy M.A. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 2000;9:393–405. doi: 10.1046/j.1365-2583.2000.00203.x. 10.1046/j.1365-2583.2000.00203.x [DOI] [PubMed] [Google Scholar]
- Komaki K, Ishikawa H. Genomic copy number of intracellular bacterial symbionts of aphids varies in response to developmental stage and morph of their host. Insect Biochem. Mol. Biol. 2000;30:253–258. doi: 10.1016/s0965-1748(99)00125-3. 10.1016/S0965-1748(99)00125-3 [DOI] [PubMed] [Google Scholar]
- Leak S.G.A. Tsetse biology and ecology, their role in the epidemiology and control of trypanosomes. CABI publishing; New York: 1999. [Google Scholar]
- Littell R.C, Milliken G.A, Stroup W.W, Wolfinger R.D. SAS Institute, Inc; Cary, NC: 1996. SAS system for mixed models. [Google Scholar]
- Ma W.C, Denlinger D.L. Secretory discharge and microflora of milk gland in tsetse flies. Nature. 1974;247:301–303. 10.1038/247301a0 [Google Scholar]
- McGraw E, Merritt D.J, Droller J.N, O'Neill S.L. Wolbachia-mediated sperm modification is dependent on host genotype in Drosophila. Proc. R. Soc. B. 2001;268:2565–2570. doi: 10.1098/rspb.2001.1839. 10.1098/rspb.2001.1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloo S.K. An artificial feeding technique for Glossina. Parasitol. 1971;63:507–515. doi: 10.1017/s0031182000080021. [DOI] [PubMed] [Google Scholar]
- Montllor C.B, Maxmen A, Purcell A.H. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum, under heat stress. Ecol. Entomol. 2002;27:189–195. 10.1046/j.1365-2311.2002.00393.x [Google Scholar]
- Moser D.R, Cook G.A, Ochs D.E, Bailey C.P, McKane M.R, Donelson J.E. Detection of Trypanosoma congolense and Trypanosoma brucei subspecies by DNA amplification using the polymerase-catalyzed chain reaction. Parasitology. 1989;99:57–66. doi: 10.1017/s0031182000061023. [DOI] [PubMed] [Google Scholar]
- Noda H, Koizumi Y, Zhang Q, Deng K. Infection density of Wolbachia and incompatibility level in two planthopper species, Laodelphax striatellus and Sogatella furcifera. Insect Biochem. Mol. Biol. 2001;31:727–737. doi: 10.1016/s0965-1748(00)00180-6. 10.1016/S0965-1748(00)00180-6 [DOI] [PubMed] [Google Scholar]
- Nogge G. Sterility in tsetse flies (Glossina morsitans Westwood) caused by loss of symbionts. Experentia. 1976;32:995–996. doi: 10.1007/BF01933932. 10.1007/BF01933932 [DOI] [PubMed] [Google Scholar]
- Nogge G. Significance of symbionts for the maintenance of an optional nutritional state for successful reproduction in hematophagous arthropods. Parasitology. 1982;82:299–304. [Google Scholar]
- Nogge G, Ritz R. Number of symbionts and its regulation in tsetse flies, Glossina spp. Entomol. Exp. Appl. 1982;31:249–254. [Google Scholar]
- O'Neill S.L, Hoffmann A, Werren J.H. Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press; Oxford, UK: 1998. [Google Scholar]
- O'Neill S.L, Gooding R.H, Aksoy S. Phylogenetically distant symbiotic microorganisms reside in Glossina midgut and ovary tissues. Med. Vet. Entomol. 1993;7:377–383. doi: 10.1111/j.1365-2915.1993.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Oliver K.M, Russell J.A, Moran N.A, Hunter M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl Acad. Sci. USA. 2003;100:1803–1807. doi: 10.1073/pnas.0335320100. 10.1073/pnas.0335320100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. 10.1016/j.cell.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Thompson J.N. Interaction and co-evolution. Wiley; New York: 1982. [Google Scholar]
- Toh, H., Weiss, L. B., Perkin, S., Yamashita, A., Oshima, K., Hattori, M. & Aksoy, S. 2006 Massive genome erosion and functional adaptations provide insights into the symbiotic lifestyle of Sodalis glossinidius in the tsetse host. Genome Res 16 In press. [DOI] [PMC free article] [PubMed]
- Tsuchida T, Koga R, Fukatsu T. Host plant specialization governed by facultative symbiont. Science. 2004;303:1989. doi: 10.1126/science.1094611. 10.1126/science.1094611 [DOI] [PubMed] [Google Scholar]
- Veneti Z, Clark M.E, Zabalou S, Savakis C, Karr T.L, Bourtzis K. Cytoplasmic incompatibility and sperm cyst infection in different Drosophila–Wolbachia associations. Genetics. 2003;164:545–552. doi: 10.1093/genetics/164.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneti Z, Clark M.E, Karr T.L, Savakis C, Bourtzis K. Heads or tails: host–parasite interactions in the Drosophila–Wolbachia system. Appl. Environ. Microbiol. 2005;70:5366–5372. doi: 10.1128/AEM.70.9.5366-5372.2004. 10.1128/AEM.70.9.5366-5372.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn S.C, Maudlin I. Rickettsia-like organisms, puparial temperature and susceptibility to trypanosome infection in Glossina morsitans. Parasitology. 1991;102:201–206. doi: 10.1017/s0031182000062491. [DOI] [PubMed] [Google Scholar]
- Welburn S.C, Maudlin I. The nature of the teneral state in Glossina and its role in the acquisition of trypanosome infection in tsetse. Ann. Trop. Med. Parasitol. 1992;86:529–536. doi: 10.1080/00034983.1992.11812703. [DOI] [PubMed] [Google Scholar]
- Wernegreen J.J. Genome evolution in the bacterial endosymbionts of insects. Nat. Rev. Genet. 2002;3:850–861. doi: 10.1038/nrg931. 10.1038/nrg931 [DOI] [PubMed] [Google Scholar]
- Werren J.H, Windsor D, Guo L.R. Distribution of Wolbachia among neotropical arthropods. Proc. R. Soc. B. 1995;262:197–204. [Google Scholar]
- Wu M, et al. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLOS Biol. 2004;2:0327–0341. doi: 10.1371/journal.pbio.0020069. 10.1371/journal.pbio.0020327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Cheng Q, Narashimhan S, Li C.B, Aksoy S. Cloning and functional expression of a fat body-specific chitinase cDNA from the tsetse fly, Glossina morsitans morsitans. Insect Biochem. Mol. Biol. 2002;32:979–989. doi: 10.1016/s0965-1748(02)00034-6. 10.1016/S0965-1748(02)00034-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.