Abstract
The reaction of the body to prolonged stress has many harmful effects. Classical theory assumes that stress responses have evolved due to their short-term selective advantages (‘flight or fight’), and despite their adverse long-term effects. In contrast, we demonstrate that the adverse effects of stress responses may have a selective advantage. Using an analytical model we show that a gene that causes the early death of a relatively unfit individual can increase in frequency in a structured population even if it has no positive effect on that individual. This result offers a new perspective on the relations between stress factors, stress responses and stress-related diseases.
Keywords: stress, evolution, altruism, stress-related disease
1. Introduction
Stress-related diseases are an important cause of death for many animals, including humans (Selye 1946; Herbert & Cohen 1993; Moberg 2000). In addition to the harmful effects of the factors causing the stress in the first place, responses to stress often have their own detrimental effects (Irwin 1994; Sapolsky 1996; Black 2002). Chronic stress is common in nature, for example in subordinate individuals within social species (Blanchard et al. 1993; Coe 1993). Furthermore, increased mortality associated with stress often occurs within reproductive ages, especially in the form of susceptibility to infectious diseases (Jacob & Ostfeld 1977; Kaplan et al. 1982; Biondi & Zannino 1997). The way an organism responds to stressful conditions is, therefore, likely to be subject to natural selection.
The harmful effects of stress responses are usually regarded as an inevitable long-term outcome of responses that evolved for their short-term adaptive benefits (e.g. ‘fight or flight’ responses; McEwen & Lasley 2002). However, many aspects of stress-induced immune suppression, such as lymphocyte apoptosis (Wyllie 1980; Cohen & Duke 1984), remain puzzling, as the benefits associated with them are unclear. Here we study a model that suggests an alternative evolutionary explanation, namely that evolution may in fact favour genes that code for adverse responses to chronic stress.
2. The model
Consider a population of long-lived individuals, where each individual takes up a given space. Let us assume that individuals vary considerably in their fitness, and an inferior individual has a very low chance to reproduce. A prolonged state of stress may be a good indicator of that individual's low fitness. If by dying the stressed individual frees up space (or any other shared resource), and if the individuals that benefit from that are likely to be its kin, then its death may actually help copies of its genes to spread. The effect is enhanced by such an individual relieving its family group not only of its own burden, but also the burden of its unfit offspring.
In order to quantify this intuition we use the following mathematical model. Consider an infinite haploid population, where each genome is composed of two loci, with alleles A/a and B/b, and recombination rate r between them. The population is divided into an infinite number of groups (‘demes’) of n breeding adults, with mating occurring randomly within demes. The relative fertility of a couple is determined by the number of parents (0, 1, or 2) carrying the allele b, so that each mate carrying this allele reduces the fertility of the couple by a multiplicative factor (1−θ). B mutates to b at a low rate μ. The allele A codes for stress susceptibility, and its only effect is to increase the mortality of Ab relative to all other genotypes. Any young individual leaves its deme with probability α, in which case it settles in any deme in the population with the same probability (figure 1). Individuals do not help each other directly, but when an adult dies it is replaced by one of the young individuals present in the deme (either a resident or a migrant).
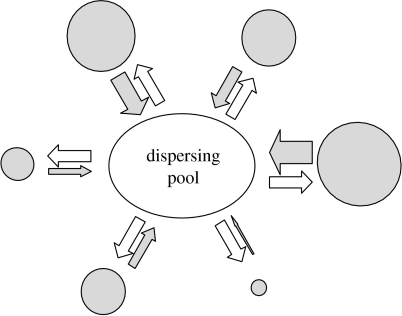
Figure 1.
Diagram of the population structure in the model. The population is divided into demes differing in their average fertility. Demes with higher average fertility are plotted as larger discs. All young individuals have the same probability α to disperse and move to the general ‘dispersing pool’. Demes with higher fertility produce more emigrants (thick shaded arrows), and the fraction of immigrant young within them is lower.
The four types of adult individuals in our model are denoted ‘1’ (AB), ‘2’ (aB), ‘3’ (ab) and ‘4’ (Ab). pi,k,l denotes the proportion of demes with i members of type 1 (genotype AB), k members of type 3 (genotype ab), l members of type 4 (Ab) and j=n−i−k−l members of type 2 (aB). We shall denote demes with that composition by (i,k,l). The mortalities of the different genotypes are m1, m2, m3 and m4, where m1=m2=m3=mu and m4≥mu. The expected number of offspring for the different types of mating pairs in this model would be as follows: 1 for types 11, 12 and 22; 1−θ for types 13, 14, 23 and 24; (1−θ)2 for types 33, 34 and 44. The expected number of offspring of a certain type among the offspring of an (i,k,l) deme can be calculated by considering all the pair combinations that can yield such offspring, and multiplying the number of pairs of each type within the deme by the fertility of such pair and by the proportion of the desired type of offspring among all its possible offspring. For example, an offspring of type 1 can be produced by two parents of type 1 whether or not recombination occurs, but provided mutation does not occur. In an (i,k,l) deme, this would happen on average i(i−1)(1−μ) times in a mating season. For a type 1 offspring to be produced by parents of types 2 and 4, recombination should occur but mutation should not, resulting in the term jl(1−θ)(1−μ)r. Overall, the frequency of type 1 offspring among all the offspring of an (i,k,l) deme is given by
| 2.1 |
where ωi,k,l is the cumulative fertility of the (i,k,l) deme, namely
Similarly, the frequency of offspring of type 3 among the offspring of an (i,k,l) deme would be
| 2.2 |
the frequency of offspring of type 4 among the offspring of an (i,k,l) deme would be
| 2.3 |
and the frequency of type 2 would be
| 2.4 |
The frequency of genotype g∈{1,2,3,4} among all migrants is
where
is the average fertility of a deme in the population.
Within an infinitesimal time period Δt, a deme of type (i,k,l) can be converted to any of 12 ‘neighbouring’ deme types in the four-dimensional space of genotype frequencies: (i−1,k+1,l), (i−1,k,l), (i,k+1,l), (i,k−1,l), (i+1,k−1,l), (i+1,k,l), (i−1,k,l+1), (i,k−1,l+1), (i,k,l+1), (i+1,k,l−1), (i,k+1,l−1) and (i,k,l−1) upon the death of a single deme member and its replacement by a young individual. With probability
the new member is a migrant (that can arrive from any deme in the population), and otherwise it is an offspring of that deme. The probability of a change of the form ‘death of an individual of type x and acceptance of an individual of type y’ (x,y=1,2,3,4) during a small time interval Δt would thus be
| 2.5 |
where is the frequency of type x among the adults of deme type (i,k,l), namely i/n, j/n, k/n, or l/n. The overall change in the frequency of (i,k,l) demes during Δt would be
| 2.6 |
In the model described so far, susceptibility to stress was defined as increased mortality if the individual's fertility f fell below a certain absolute threshold (f≤1−θ). To demonstrate the difference between such alleles of susceptibility to ‘absolute stress’ versus susceptibility to ‘relative stress’, we studied a model in which the fertility of an individual is determined both by its genotype and by the environment, with periods of environmental stress taking the form of 10% decrease in the fertility of all individuals. For the model described so far only individuals of types 3 and 4 experienced absolute stress in a ‘normal’ environment (and only type 4 individuals were susceptible to it), whereas in the stressful environment all individuals experienced absolute stress (and both types 1 and 4 were susceptible to it). Susceptibility to relative stress was defined as increased mortality if the individual's relative fertility was lower than its deme's average. Denoting by fg the fertility of an individual with genotype g (in the given environment) and by the average fertility of a deme of type (i,k,l) in the same environment, the mortality of an individual of genotype g in a deme of type (i,k,l) is then given by
with ms≥mu. All other equations remain unchanged.
3. Results
The parameter range for which allele A increases when rare and eventually reaches fixation can be found by iterating the system of equations obtained from equation (2.6) above when substituting all valid deme types for (i,k,l). In the absolute stress model with a constant environment, the allele A either increased to fixation or was eliminated. No stable polymorphism was observed, and the outcome did not depend on starting conditions. For various combinations of the recombination rate r, migration rate α and deme size n, the population was initialized with a majority of pure aB demes, and frequency 0.01 of demes containing a single AB individual and n−1 aB individuals. We iterated the equations from this state, and searching over different values of θ, found the critical value of θ above which allele A increases to fixation. This critical value is plotted in figure 2 for several parameter combinations. The range that allows invasion of the allele A narrows with increasing recombination, migration (figure 2a) and deme size (figure 2b). The parameter range for which the allele A spreads might be wider if migration were not global, so that demes with stress-susceptible individuals were likely to be spatially adjacent. The relative stress model did not differ significantly from the absolute stress one when the environment was constant and differences in fertility were due solely to genetic differences. But the results were dramatically different when periods of environmental stress resulted in decreased fertility across the population. Figure 3 compares the dynamics of the two models in that case, starting from identical conditions, where half of the population carries the allele A and half carries the allele a. In the relative stress model, the allele A increases steadily to fixation (figure 3a). The increase in the frequency of allele A is accompanied by an increase in the average population fitness due to more efficient selection against the deleterious allele b. The frequency of the A allele in the absolute stress model, on the other hand, decreases following each occurrence of environmental stress (figure 3b), and the allele does not spread in the population.
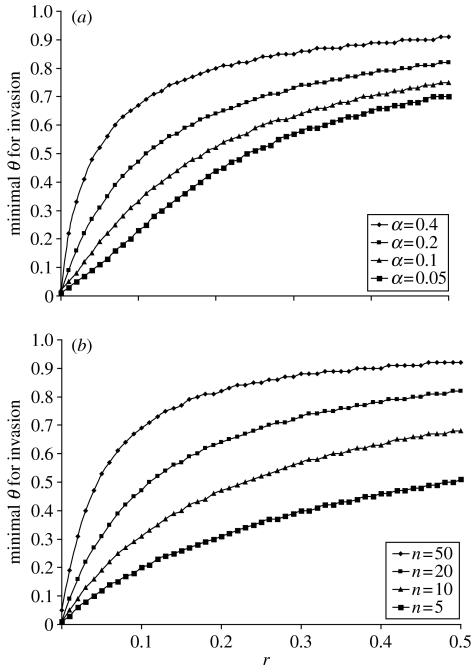
Figure 2.
Invasion of the allele for stress susceptibility, causing increased mortality when relative fertility is low, occurs for any value of theta higher than the plotted critical value. (a) The critical value of θ is plotted as a function of r for different values of α, with n=20. (b) The critical value of θ is plotted as a function of r for different values of n, with α=0.2. We studied the population dynamics for different values of r, n, α and θ. The critical value of θ increases as a function of r, α (a) and deme size n (b). That is, invasion requires that variation in fertility within the population (θ) be high enough, and that the population be sufficiently structured (α and n small enough). Invasion occurs more easily when the locus affecting mortality is linked more tightly to the locus inducing low fertility (lower r). μ=0.001, and the expected longevity is 10 mating seasons for stress-susceptible individuals when under stress, and 50 mating seasons for all other individuals.
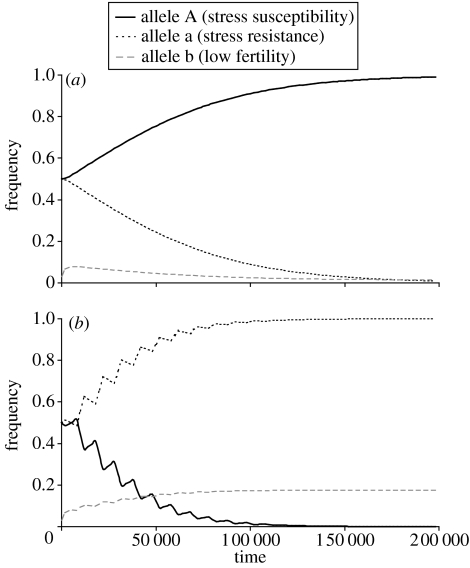
Figure 3.
Relative versus absolute stress. The frequencies of the different alleles are plotted as a function of time from the introduction of the allele A. Every 10 000 mating seasons, 10 seasons of ‘environmental stress’ occurred, decreasing the fertility of the whole population by 10%. (a) Susceptibility to relative stress. An allele for stress-related mortality based on relative stress, which causes the ‘A’ individuals to suffer increased mortality only when their fitness is lower than the deme's average. This allele increases to fixation, and the increase is accompanied by a decrease in the frequency of the allele b, and, therefore, an increase in the average population fitness. (b) Susceptibility to absolute stress. The rare events of environmental stress are sufficient to eliminate the allele for stress-related mortality based on absolute stress. The expected longevity is 10 mating seasons for stress-susceptible individuals when under stress, and 50 mating seasons for all other individuals, with n=5, θ=0.5, α=0.1, r=0.05, μ=0.01. The results do not depend on initial conditions.
4. Discussion
We show that alleles whose only effect is to increase mortality under stressful conditions can succeed in a structured population. Our results further suggest that alleles causing increased mortality due to relative stress factors (e.g. alleles causing immune suppression in reaction to low social status) would more readily fix in the population than those causing increased mortality due to absolute stress that may act on the entire population (e.g. cold). This is in accord with the strong association of low social status with increased mortality, which has been observed in various species (Cohen et al. 1991; Krantz & McCeney 2002). Two examples of particular interest in that context are the demonstration of higher sensitivity to viral infection in mice that suffered social stress than in those with a similar level of stress hormones resulting from restraint stress (Sheridan et al. 2000), and the increased sensitivity to infections resulting from low social status, but not from experimentally induced social instability (including fighting) in primates (Cohen et al. 1997).
The present hypothesis yields several other predictions that can be tested empirically. First, susceptibility to stress (i.e. invasion of the allele A) is more likely to evolve when there are large differences in fertility between different genotypes within the population (i.e. invasion of the allele A depends on θ; see figure 2). This prediction is consistent with the higher occurrence of male mortality in many non-monogamous species, where male fitness is more variable than that of females (Richard 1985). The opposite is expected when it is female fecundity that is the more variable (Creel et al. 1997). A second prediction is that, everything else being equal, susceptibility to stress will be more strongly favoured in species with family grouping and limited dispersal, resulting in higher local relatedness (figure 2, dependence on α). That result also suggests that if different levels of local relatedness occur at different stages of life, the organism will be more susceptible to stress and disease when local relatedness is higher, for example when sharing nest space and food with siblings. Third, for the allele causing stress susceptibility in our model to spread in the population, its effect should be highly correlated with low fitness. Thus, we would expect a strong response to prolonged stress, but a relatively weak response to transient hardships. Likewise, we would expect stress-related mortality to decrease with correlates of high fitness. Such correlates may include social status (Sapolsky & Mott 1987; Hessing et al. 1994), as well as more direct evidence such as the frequency of mating for males (Smith et al. 1997).
The notion of ‘death’ in this model can be replaced by elimination from the breeding pool through reduction of the individual's fertility to zero, provided there is a resource shared by breeding adults (e.g. when there is strong sperm competition). Indeed, stress-induced reduction in fertility has been observed in many species, including humans and mice (Rose et al. 1975; Rivier et al. 1986).
Recently, stress-induced alternative splicing has been suggested as a mechanism that might be involved in translating the experience of stress in the individual's brain to physiological stress responses, with potential harmful effects on survival and fertility. Changes in gene expression patterns under stress, and stress-induced alternative splicing in particular, have been observed in different genes (Tomasin et al. 2001; McCob et al. 2003; Nijholt et al. 2004). Particularly interesting is the example of the alternative variant of Acetylcholine that is expressed preferentially under stress, involved in the regulation of stress response (Pick et al. 2004), and associated with trait and state anxiety (Sklan et al. 2004). Overexpression of that alternative splice variant in mice resulted in decreased fertility (Mor et al. 2001).
Finally, it is worth noting that our model can be regarded as a model for the evolution of extreme altruism. The individual dying from stress can be viewed as sacrificing itself in order to release common resources such as food or territory to its neighbours. Similarly to classical models of altruism (Eshel 1972; Wilson 1977), this may evolve only in structured populations. Unlike most previous models, the assumption of negative association between altruism and fitness allows the evolution of extreme altruism, where the cost to the altruist seems higher than the benefit to its neighbours. Introducing other forms of explicit altruism into the same model may affect its results. For example, in some social animals the benefits of directly helping one's kin would be greater than those of indirect help by dying and freeing-up space and other shared resources. However, the basic argument of our model applies more generally: forms of altruism in which less fit individuals are more altruistic may evolve more easily. Similar effects of fitness association have been suggested for the evolution of recombination (Hadany & Beker 2003).
Classical theory assumes that stress responses have evolved due to their short-term benefits, and despite their long-term detrimental effects (McEwen & Lasley 2002). Our results demonstrate that stress responses could have evolved partly due to their long-term detrimental effects, even if they provide no short-term benefit to the individual. The two explanations are not, however, mutually exclusive. Our analysis suggests that responses producing a slight advantage in the short term but having a high cost in the long term may readily evolve, in particular in the context of social competition.
Acknowledgments
We wish to thank Gal Chechik, Olivia Judson, Daniela Kaufer, Joanna Masel and Hermona Soreq for reading the manuscript and providing helpful comments. This study was supported in part by NIH grant GM28016 and by the Israel Science Foundation Bikura Fellowship.
References
- Biondi M, Zannino L.G. Psychological stress, neuroimmunomodulation, and susceptibility to infectious diseases in animals and man: a review. Psychother. Psychosom. 1997;1:3–26. doi: 10.1159/000289101. [DOI] [PubMed] [Google Scholar]
- Black P.H. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav. Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. 10.1016/S0889-1591(02)00021-1 [DOI] [PubMed] [Google Scholar]
- Blanchard D.C, Sakai R.R, McEwen B, Weiss S.M, Blanchard R.J. Behavioral, brain, and neuroendocrine correlates. Behav. Brain. Res. 1993;58:113. doi: 10.1016/0166-4328(93)90096-9. 10.1016/0166-4328(93)90096-9 [DOI] [PubMed] [Google Scholar]
- Coe C.L. Psychosocial factors and immunity in nonhuman primates: a review. J. Psychosom. Med. 1993;55:298. doi: 10.1097/00006842-199305000-00007. [DOI] [PubMed] [Google Scholar]
- Cohen J.J, Duke R.C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J. Immunol. 1984;132:38–42. [PubMed] [Google Scholar]
- Cohen S, Tyrrell D.A.J, Smith A.P. Psychological stress and susceptibility to the common cold. N. Engl. J. Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Cohen S, Line S, Manuck S.B, Rabin B.S, Heise E.R, Kaplan J.R. Chronic social stress, social status, and susceptibility to upper respiratory infections in nonhuman primates. J. Psychosom. Med. 1997;59:213. doi: 10.1097/00006842-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Creel S, Creel N.M, Mills M.G.L, Monfort S.L. Rank and reproduction in cooperatively breeding African wild dogs: behavioral and endocrine correlates. Behav. Ecol. 1997;8:298–306. [Google Scholar]
- Eshel I. On the neighbor effect and the evolution of altruistic traits. Theor. Popul. Biol. 1972;3:258–277. doi: 10.1016/0040-5809(72)90003-2. 10.1016/0040-5809(72)90003-2 [DOI] [PubMed] [Google Scholar]
- Hadany L, Beker T. On the evolutionary advantages of fitness-associated recombination. Genetics. 2003;165:2167–2179. doi: 10.1093/genetics/165.4.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert T.B, Cohen S. Stress and immunity in humans—a meta-analytic review. Psychosom. Med. 1993;55:364–379. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Hessing M.J.C, Scheepens C.J.M, Schouten W.G.P, Tielen M.J.M, Wiepkema P.R. Social rank and disease susceptibility in pigs. Vet. Immunol. Immunopathol. 1994;43:373–387. doi: 10.1016/0165-2427(94)90158-9. 10.1016/0165-2427(94)90158-9 [DOI] [PubMed] [Google Scholar]
- Irwin M. Stress-induced immune suppression—role of brain corticotropin releasing hormone and autonomic nervous system mechanisms. Adv. Neuroimmunol. 1994;4:29–47. doi: 10.1016/s0960-5428(06)80188-9. [DOI] [PubMed] [Google Scholar]
- Jacob S, Ostfeld A. An epidemiological review of the mortality of bereavement. J. Psychosom. Med. 1977;39:344. doi: 10.1097/00006842-197709000-00006. [DOI] [PubMed] [Google Scholar]
- Kaplan J.R, Manuck S.B, Clarkson T.B, Lusso F.M, Taub D.M. Social-status, environment, and atherosclerosis in Cynomolgus monkeys. Arteriosclerosis. 1982;2:359. doi: 10.1161/01.atv.2.5.359. [DOI] [PubMed] [Google Scholar]
- Krantz D.S, McCeney M.K. Effects of psychological and social factors on organic disease: a critical assessment of research on coronary heart disease. Annu. Rev. Psychol. 2002;53:341–369. doi: 10.1146/annurev.psych.53.100901.135208. 10.1146/annurev.psych.53.100901.135208 [DOI] [PubMed] [Google Scholar]
- McCob D.P, Hara Y, Lai G.J, Mahmoud S.F, Flugge G. Subordination stress alters alternative splicing of the Slo gene in tree shrew adrenals. Horm. Behav. 2003;43:180–186. doi: 10.1016/s0018-506x(02)00010-7. 10.1016/S0018-506X(02)00010-7 [DOI] [PubMed] [Google Scholar]
- McEwen B, Lasley E.N. Joseph Henry Press; Washington, DC: 2002. The end of stress as we know it. [Google Scholar]
- Moberg G.P. Biological responses to stress: implications for animal welfare. In: Moberg G.P, Mench J.A, editors. The biology of animal stress: basic principles and implications for animal welfare. CABI publishers; New York: 2000. pp. 1–22. [Google Scholar]
- Mor I, et al. Modified testicular expression of stress-associated ‘readthrough’ acetylcholinesterase predicts male infertility. FASEB J. 2001;15:2039–2041. doi: 10.1096/fj.00-0814fje. [DOI] [PubMed] [Google Scholar]
- Nijholt I, et al. Stress-induced alternative splicing of acetylcholinesterase results in enhanced fear memory and long-term potentiation. Mol. Psychiatry. 2004;9:174–183. doi: 10.1038/sj.mp.4001446. 10.1038/sj.mp.4001446 [DOI] [PubMed] [Google Scholar]
- Pick M, Flores-Floers C, Soreq H. From brain to blood: alternative splicing evidence for the cholinergic basis of mammalian stress responses. Stress. 2004;1018:85–98. doi: 10.1196/annals.1296.010. [DOI] [PubMed] [Google Scholar]
- Richard A.F. Freeman Press; New York: 1985. Primates in nature. [Google Scholar]
- Rivier C, Rivier L, Vale W. Stress-induced inhibition of reproductive functions: role of endogenous corticotropin-releasing factor. Science. 1986;231:607–609. doi: 10.1126/science.3003907. [DOI] [PubMed] [Google Scholar]
- Rose R.M, Bernstein I.S, Gordon T.P. Consequences of social conflict on plasma testosterone levels in rhesus monkeys. Psychosom. Med. 1975;37:50–61. doi: 10.1097/00006842-197501000-00006. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M, Mott G.E. Social subordination in wild baboons is associated with suppressed high-density lipoprotein cholesterol concentrations—the possible roles of chronic social stress. Endocrinology. 1987;121:1605–1610. doi: 10.1210/endo-121-5-1605. [DOI] [PubMed] [Google Scholar]
- Selye H. The general adaptation syndrome and the diseases of adaptation. J. Clin. Endocrinol. 1946;6:117–230. doi: 10.1210/jcem-6-2-117. [DOI] [PubMed] [Google Scholar]
- Sheridan J.F, Stark J.L, Avitsur R, Padgett D.A. Social disruption, immunity, and susceptibility to viral infection: role of glucocorticoid insensitivity and NGF. Ann. N. Y. Acad. Sci. 2000;917:894–905. doi: 10.1111/j.1749-6632.2000.tb05455.x. [DOI] [PubMed] [Google Scholar]
- Sklan E.H, et al. Acetylcholinesterase/paraoxonase genotype and expression predict anxiety scores in health, risk factors, exercise training, and genetics study. Proc. Natl Acad. Sci. USA. 2004;101:5512–5517. doi: 10.1073/pnas.0307659101. 10.1073/pnas.0307659101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.D, Frankel S, Yarnell J. Sex and death: are they related? Findings from the Caerphilly cohort study. Br. Med. J. 1997;315:1641–1644. doi: 10.1136/bmj.315.7123.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasin R, Samir A.A, Vaccaro M.I, Pebusque M.J, Dagorn J.C, Iovanna J.L, Dusetti N.J. Molecular and functional characterization of the stress-induced protein (SIP) gene and its two transcripts generated by alternative splicing—SIP induced by stress and promotes cell death. J. Biol. Chem. 2001;276:44 185–44 192. doi: 10.1074/jbc.M105647200. 10.1074/jbc.M105647200 [DOI] [PubMed] [Google Scholar]
- Wilson D.S. Structured demes and the evolution of group-advantageous traits. Am. Nat. 1977;111:157–185. 10.1086/283146 [Google Scholar]
- Wyllie A.H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. 10.1038/284555a0 [DOI] [PubMed] [Google Scholar]