Abstract
There have been virtually no studies of ‘who acquires infection from whom’ in wildlife populations, but patterns of transmission within and between different classes of host are likely to be reflected in the spatiotemporal distribution of infection among those host classes. Here, we use a modified form of K-function analysis to test for space–time interaction among bank voles and wood mice infectious with cowpox virus. There was no evidence for transmission between the two host species, supporting previous evidence that they act as separate reservoirs for cowpox. Among wood mice, results suggested that transmission took place primarily between individuals of the opposite sex, raising the possibility that cowpox is sexually transmitted in this species. Results for bank voles indicated that infected females might be a more important source of infection to either sex than are males. The suggestion of different modes of transmission in the two species is itself consistent with the apparent absence of transmission between species.
Keywords: Apodemus, Clethrionomys, host heterogeneity, infection, K-function
1. Introduction
Epidemiological studies of human diseases, particularly of sexually transmitted diseases, increasingly recognize that the rate and pattern of potentially infectious contacts vary between individuals (Garnett & Anderson 1993; Edmunds et al. 1997). Where membership of a population category (such as an age class) is a useful predictor of an individual's contact behaviour, the ‘who acquires infection from whom’ (WAIFW) matrix, containing contact rates between different host categories, may be a useful tool (Anderson & May 1991). Transmission rates for an ectoparasite-vectored wildlife disease have recently been inferred from parasite infestation levels (Perkins et al. 2003), but difficulties in defining and detecting potentially infective contacts have largely prevented the use of WAIFW approaches among directly transmitted wildlife diseases.
However, when the hosts of a directly transmitted infection occupy consistent home ranges, potentially infectious contacts are likely to be concentrated within that home range. The risk of infection posed by an infectious individual will, therefore, be reflected in a transient clustering of further infections around the individual in question. Such ‘space–time interaction’ is increasingly used in the study of human and livestock diseases to identify contagious processes and to estimate the scale at which infectious hosts pose a risk to others (Diggle et al. 1995; Carpenter 2001). Further, the risk posed by infectious hosts of one category to susceptible individuals of another category will be reflected in a transient clustering of infections among the second category around infected hosts of the first category. From this, we can infer WAIFW information directly, without knowledge of animals' contact patterns or the method of disease transmission. Indeed, the WAIFW matrix, coupled with knowledge of the animal's biology, may provide information about likely transmission modes.
We test these ideas using wild sympatric populations of bank voles (Clethrionomys glareolus Schreber) and wood mice (Apodemus sylvaticus L.) in which cowpox virus is endemic. These species, together with field voles (Microtus agrestis L.), are believed to be the reservoir hosts for cowpox in Britain (Chantrey 1999). Infected reservoir hosts show no visible signs of disease, but infection may influence their survival (Telfer et al. 2002) and fecundity (Feore et al. 1997; Telfer et al. 2005). Bank voles and wood mice infected with cowpox virus remain infectious for about four weeks, and produce antibody after about two weeks (Chantrey 1999). Recent analysis of the spatiotemporal pattern of infection (Carslake et al. 2005) showed that infectious bank voles and wood mice cause an elevated risk of infection to others of their own species for four weeks after their own infection (consistent with the infectious period) and within a range comparable to the home range diameter. This, together with evidence from other orthopoxviruses (Baxby & Bennett 1994), suggests that cowpox is transmitted by direct contact between hosts.
Space–time interaction may be thought of as correlation between the location and the timing of infections. It is generally considered a measure of the additional infection risk to others associated with an infectious animal (Carpenter 2001). Carslake et al. (2005) developed a test, based on the K-function for space–time interaction (Diggle et al. 1995), to test for space–time interaction among single species data collected on a rodent trapping grid. It differs from the standard technique in allowing for discontinuous spatial and temporal locations (traps and trapping sessions), and also considers the effect of any space–time interaction among the population at risk (i.e. transient clustering of the host population itself). This and other tests for space–time interaction have so far been applied only within a single class of host. Here, we extend the method to test for space–time interaction in one class of host with respect to the distribution of another class. Specifically, we investigate the evidence for transmission between the species and within and between the sexes. In order to do this, for each host class, or pair of classes, we test two hypotheses:
Hypothesis 1. Cases of cowpox infection will show positive space–time interaction at a scale corresponding to the home range diameter of the host class (or the mean home range diameter of two host classes) and the infectious period of cowpox.
Hypothesis 2. Cowpox cases will show greater space–time interaction than randomly chosen individuals from the population at risk, within the hypothesized scale.
2. Material and methods
(a) Data collection
Data were collected for almost 7 years from a 1 ha study area within 8 ha of mixed woodland in north west England (Manor Wood: grid reference SJ 294816). One hundred pairs of Longworth live-capture rodent traps were set in a square grid pattern at intervals of 10 m. Trapping sessions usually consisted of three consecutive days at intervals of four weeks. Each capture event was therefore located in two spatial dimensions (10 m intervals) and one temporal dimension (four-week intervals). On first capture, each animal was given an electronic tag to allow individual identification if recaptured. At each session in which an animal was captured, a blood sample was taken from the tip of the tail and tested for the presence of antibodies to cowpox virus using an immunofluorescence assay (Bennett et al. 1997). To avoid bias, the analysis was restricted to those captures for which the animal at that time had the potential from its capture history to be classed as a cowpox case if it was antibody positive. Animals with previous positive tests were considered immune to infection and excluded from further analysis. An animal was also only included if it had tested negative not more than eight weeks previously, or if its weight indicated that it was less than 10 weeks old (see Telfer et al. (2002) for details of age categories). This excluded infections whose timing could not be determined with a reasonable degree of precision. Qualifying captures counted as cowpox cases if antibody positive, and non-cases if negative.
(b) Estimation of home range diameter
Using the capture locations, average home range diameters were estimated for each species, and for each sex within each species. An asymptotic function was fitted to the relationship between the range of an individual's captures over an eight-week period and the number of these captures. This was used to estimate the home range that would be recorded from an infinite number of captures (see Carslake et al. (2005) for full details). This method gives only an approximate estimate of home range size. However, home ranges were needed only to predict, a priori, the scale at which transmission took place, and the cumulative method by which transmission risk was calculated (see §2c) is not expected to be sensitive to moderate changes in scale.
(c) Calculation of test statistics
Carslake et al. (2005) describe a technique, adapted from the K-function for space–time interaction (Diggle et al. 1995), in which transmission within a class of host is analysed by examination of the spatial and temporal proximity of pairs of hosts from within that class. Here, we extend that technique to analyse transmission between different classes of host by examining the proximity of host pairs consisting of animals from two different host classes. This involves minor modifications to the definition of temporal proximity, the calculation of edge effects, and the means by which the null hypotheses are simulated. We therefore describe the between-class method in full here, and refer the reader to Carslake et al. (2005) for a description of the original within-class technique, which is also used in the present study.
The method estimates space–time interaction at a range of spatial and temporal scales, but the number of possible scales is limited by the discontinuous nature of capture locations and times. Time scales (t) were therefore 0–60 weeks, in increments of 4. Spatial scales (s) consisted of all possible distances, up to 45 m, between points on the trapping grid. Edge corrections become increasingly influential at larger scales than this.
To calculate between-class test statistics, all possible pairs, ij, were considered, consisting of the ith cowpox case from the first host class and the jth cowpox case from the second. Unlike the situation for within-class tests, it is possible to specify that infections among class 2 must take place either after, or at the same time as, the index infection in class 1. This gives us some power to distinguish between, for example, the risk posed by males to females, and the risk posed by females to males. The space–time clustering statistic, K(s, t), for each combination of s and t was calculated as
where elements are defined as follows:
- ∑∑
- indicates the sum for all pairs of cases ij;
- dij
- the spatial distance between case i and case j;
- tij
- the temporal separation between case i and case j, such that tij=tj−ti;
- Is
- an indicator function equal to 1 if dij≤s, and 0 otherwise;
- It
- an indicator function equal to 1 if 0≤tij≤t, and 0 otherwise;
- wij
- the spatial weighting (details below);
- Rs
- the number of points on the study grid (100 in this study);
- Rt
- the number of time points sampled (90 in this study);
- n1 and n2
- the number of cases in classes 1 and 2, respectively; n1n2 is thus the number of pairs of cases ij.
Purely spatial and temporal clustering statistics, K(s) and K(t), respectively, were calculated similarly:
The edge made up a considerable proportion of the trapping grid. A spatial weighting, wij, was therefore calculated as the number of grid points that would lie at distance dij from case i on an infinite grid, divided by the number of grid points present at distance dij from case i on the real grid (Carslake et al. 2005). A lesser ‘edge effect’ in the time dimension could not be corrected for. However, this edge effect is expected primarily to influence purely temporal clustering and is therefore of little consequence when space–time interaction, rather than K(t) itself, is the object of study.
K(s,t) is a measure of clustering in the parameter space defined by our two spatial dimensions and one temporal dimension. Such clustering can be the result of simple spatial or temporal clustering operating independently, patterns which are not considered a direct result of the transmission process. If spatial and temporal clustering patterns are independent, we expect K(s,t) to be equal to the product of K(s) and K(t). The degree of space–time interaction is therefore estimated by D0(s, t)
Positive values of D0(s, t) indicate a proportional increase in infection risk (e.g. a value of 1 indicates a 100% increase) associated with the presence of an infectious animal t weeks ago at distance s metres. Negative values indicate a decreased risk (the minimum of −1 representing complete elimination of the risk), and a value of zero conforms to the null hypothesis that the location and timing of infections are independent (and thus that the presence of an infectious animal has no effect on infection risk). Note that purely spatial or temporal patterns in infection influence the numerator (K(s, t)) and denominator (K(s) K(t)) of the above equation equally. This test is, therefore, not sensitive to systematic variation (‘non-stationarity’) in the underlying spatial and temporal patterns of infection.
(d) Randomization tests for space–time interaction
Simulations corresponding to different null hypotheses were carried out. Each was repeated 1000 times using the Poptools add-in (Hood 2002) on Microsoft Excel 2000. Test statistics were calculated similarly for each replicate and for the cases of infection, and a one-tailed estimate was made of the significance of any deviation from the null hypothesis.
For the within-class tests, two simulations were carried out as described by Carslake et al. (2005). One tested the null hypothesis that the location and timing of cases of infection were independent of each other, and the other that any non-independence in the location and timing of cases was due solely to such patterns present in the population at risk. An exact application of these simulations to the between-class tests was not possible, so an alternative approach was taken.
To test the first null hypothesis (no space–time interaction) between host classes, it was necessary to remove wij from the calculations and to treat the edge effect by another method. The trapping grid was considered to be joined in a circle in each dimension such that one edge was separated from the opposite edge by a distance equal to the sampling frequency (10 m in space and four weeks in time). This converts the trapping grid into a shape without edges. For each simulated dataset, the cases in one class were then shifted in each dimension by a random multiple of the sampling frequency, before re-calculating the test statistics. This has the effect of removing any space–time interaction between the classes while retaining all spatiotemporal pattern within the classes. It also has the undesired effect of removing any simple spatial or temporal clustering between the classes. The effect of this on the distribution of D0(s, t) is expected to be small and conservative (i.e. biased against statistical significance). The test statistic for each randomization, , was therefore derived from D0(s, t) (Carslake et al. 2005). Using subscripts ‘c’ and ‘r’ to indicate statistics calculated from the real cowpox cases and from the randomization, respectively, D(s, t) was calculated as
and summed over all scales within the hypothesized limits of four weeks and the mean home range diameter to give the test statistic .
The second simulation tested the null hypothesis that space–time interaction among the cowpox cases of host class 2 with respect to cases of host class 1 was no greater than that expected from any subsample of the class 2 population at risk (i.e. not necessarily infected). Random subsamples of size n2 were drawn from the class 2 population at risk and used as if they had been the cowpox cases. As for the first simulation, the test statistic was calculated using K(s) K(t) from the cowpox cases (with the standard edge correction) and D0(s, t) from each population subsample.
3. Results
(a) General
Details of the captures made in each host category are given in table 1. Among the animals available for infection, bank voles were significantly more likely than wood mice to be infected with cowpox (, p=0.000). There was no significant difference in cowpox incidence between the sexes in either bank voles (, p=0.869) or wood mice (, p=0.181). The predicted range at which animals of one host class posed a risk to susceptible animals of another class was calculated as the mean of the home range diameters for each class (table 1) and rounded up to the next value of s (table 2).
Table 1.
Details of the animals captured, excluded as unavailable for infection, and classified as cowpox cases. Total cases include young animals seropositive at first capture (‘young cases’) and those with gaps in their capture record of up to eight weeks. The sex category ‘all’ includes some animals that were not sexed. Also shown are the estimated home range diameters (HRD).
| species | sex | individual animals | total captures | excluded captures | total cases | young cases | gap cases | HRD (m) |
|---|---|---|---|---|---|---|---|---|
| bank vole | male | 645 | 1872 | 797 | 70 | 10 | 9 | 34.63 |
| bank vole | female | 611 | 1886 | 704 | 79 | 23 | 5 | 20.93 |
| bank vole | all | 1402 | 3923 | 1643 | 152 | 33 | 14 | 27.66 |
| wood mouse | male | 775 | 2649 | 779 | 54 | 5 | 5 | 37.03 |
| wood mouse | female | 579 | 1961 | 433 | 33 | 7 | 5 | 28.07 |
| wood mouse | all | 1365 | 4622 | 1222 | 87 | 12 | 10 | 33.11 |
Table 2.
The predicted spatial scale of cowpox infection risk for transmission between different host classes, calculated as the mean home range diameter. Within that spatial scale and a temporal scale of four weeks (one infectious period), the test statistic was calculated using either the conventional edge correction or the circular edge correction (superscript ‘†’). Simulations were made under the null hypothesis of space–time independence (subscript ‘i’) and under the null hypothesis that cowpox cases were merely a subsample of the population at risk (subscript ‘p’). Critical values are 95 percentiles of the simulated distributions. Monte Carlo p values were calculated.
| infection | spatial scale (m) | observed (critical) | observed (critical) | |||
|---|---|---|---|---|---|---|
| from | to | pi | pp | |||
| bank voles | bank voles | 28.28 | 247.7 (83.6) | 247.7 (116.7) | 0.000 | 0.000 |
| bank voles | wood mice | 31.62 | 39.0† (156.6†) | −0.17 (112.5) | 0.326† | 0.377 |
| wood mice | wood mice | 36.06 | 647.9 (339.9) | 647.9 (555.5) | 0.001 | 0.032 |
| wood mice | bank voles | 31.62 | 1.0† (146.8†) | −54.3 (1.5) | 0.485† | 0.194 |
| ♀ bank voles | ♀ bank voles | 22.36 | 383.4 (117.7) | 383.4 (163.0) | 0.000 | 0.000 |
| ♀ bank voles | ♂ bank voles | 28.28 | 93.6† (147.9†) | 127.0 (142.7) | 0.144† | 0.079 |
| ♂ bank voles | ♂ bank voles | 36.06 | 82.4 (326.3) | 82.4 (441.5) | 0.312 | 0.457 |
| ♂ bank voles | ♀ bank voles | 28.28 | 94.9† (178.0†) | 110.8 (130.1) | 0.164† | 0.071 |
| ♀ wood mice | ♀ wood mice | 28.28 | 152.9 (439.8) | 152.9 (660.8) | 0.239 | 0.317 |
| ♀ wood mice | ♂ wood mice | 36.06 | 509.9† (859.9†) | 623.3 (702.0) | 0.135† | 0.065 |
| ♂ wood mice | ♂ wood mice | 40.00 | 561.6 (684.3) | 561.6 (814.2) | 0.076 | 0.127 |
| ♂ wood mice | ♀ wood mice | 36.06 | 363.1† (759.3†) | 727.1 (706.3) | 0.203† | 0.048 |
(b) Space–time interaction within and between species
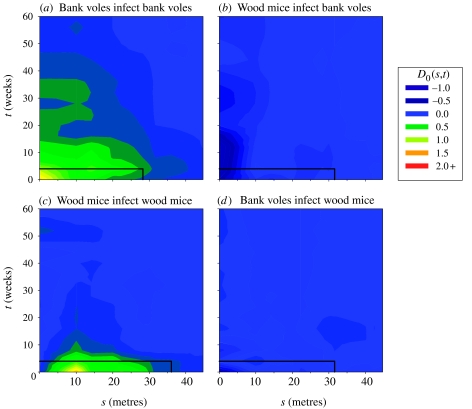
Within both host species, cowpox cases showed space–time interaction within the predicted scales of one home range diameter and four weeks that was significantly in excess of that expected under the null hypothesis of space–time independence, and significantly greater than any space–time interaction shown by the population at risk (table 2). Plots of D0(s, t) reveal the increased risk to be contained largely within the hypothesized spatial and temporal scales for each species (figure 1a,c).
Figure 1.
Cumulative plots of space–time interaction, D0(s, t), among cowpox cases (a, c) within and (b, d) between the two host species. Solid black lines represent the hypothesized scale of transmission, determined spatially as the mean home range diameter and temporally as the infectious period.
However, cowpox cases in neither species showed significant space–time interaction with respect to cases among the other species (figure 1b,d; table 2). The degree of space–time interaction shown by cases in each species with respect to cases in the other species was not significantly greater than that shown by the population at risk (table 2).
(c) Wood mice; space–time interaction within and between the sexes
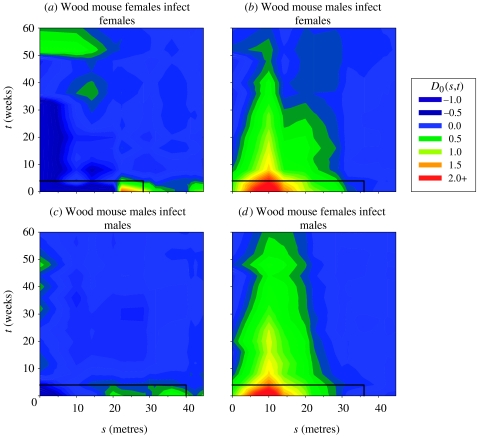
There was a strong suggestion that transmission among wood mice takes place primarily between the sexes. Peak D0(s, t) values of about 2 suggest that an individual's risk of having cowpox triples when an infected animal of the opposite sex is captured up to 10 m away in the same session. When patterns among the population at risk were taken into account, infected male wood mice posed a significant risk of infection to females within the hypothesized scale, while the result for females infecting males narrowly missed significance (table 2). However, neither of the between-sex results was significant when the population at risk was not taken into account. This suggests that the distribution of the population at risk did not contribute to the positive space–time interaction among cowpox cases. Rather, it contributed negatively, increasing the inferred strength of the space–time interaction due to transmission. These marginally significant results should be treated with caution. However, patterns of space–time interaction between the sexes (figure 2b,d) were very similar to the (highly significant) results for the species as a whole (figure 1c), whereas those within the sexes were not. This further supports our interpretation that most cowpox transmission in wood mice takes place between the sexes. It should be noted that data for t=0 are common to figure 2b,d, and do not allow us to determine the direction of the between-sex infection risk at this time scale. However, for both orientations of between-sex transmission, there was supporting evidence at t=4 weeks, at which scale the two directions of infection are independent.
Figure 2.
Cumulative plots of space–time interaction, D0(s, t), among cowpox cases (a, c) within and (b, d) between the sexes among wood mice. Solid black lines represent the hypothesized scale of transmission, determined spatially as the mean home range diameter and temporally as the infectious period.
Within each sex, there was no indication of space–time interaction at small spatial scales within 20 m, although both sexes showed slightly elevated values at the shortest time scale and a spatial scale corresponding to the limit of the home range (figure 2a,c). Space–time interaction within the hypothesized scales was not significantly greater than that expected under space–time independence, or greater than that of the population at risk (table 2).
(d) Bank voles; space–time interaction within and between the sexes
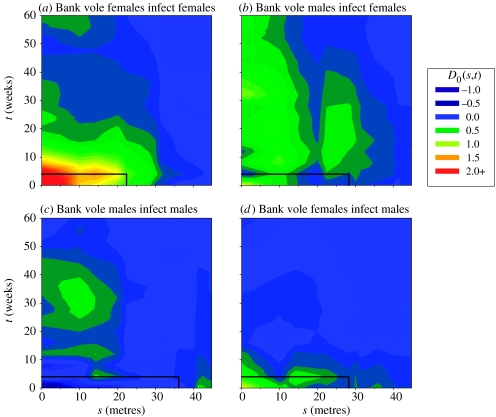
Space–time interaction among female bank vole cowpox cases was largely contained within the expected spatial and temporal scales of one infectious period and one home range diameter (figure 3a), and was highly significant within these scales (table 2). D0(s, t) values of up to 3 indicate that a female bank vole is up to four times more likely to be infected if an infectious female is caught nearby. The pattern was very similar to that among bank voles as a whole (figure 1a) but of greater magnitude, suggesting that female–female transmission was responsible for most of the space–time interaction observed among bank voles.
Figure 3.
Cumulative plots of space–time interaction, D0(s, t), among cowpox cases (a, c) within and (b, d) between the sexes among bank voles. Solid black lines represent the hypothesized scale of transmission, determined spatially as the mean home range diameter and temporally as the infectious period.
There was some suggestion that infectious bank voles posed a risk to those of the opposite sex (figure 3b,d). Much of the space–time interaction accounting for the near-significance of these results (table 2) was, however, at the timescale t=0, at which male–female and female–male transmission cannot be distinguished. The possible risk posed by females to males (figure 3d) was similar in pattern (though weaker in magnitude) to that posed to other females (figure 3a), with some evidence of space–time interaction at t=4 weeks. This space–time interaction at t=4 weeks implies transmission from female to male, since the female was infected before the male.
Cowpox cases among male bank voles, however (figure 3c), showed no significant space–time interaction within the predicted scale, either relative to space–time independence or relative to the population at risk (table 2).
4. Discussion
(a) Space–time interaction within and between species
Like all studies of this type, we make the assumption throughout that observed patterns of space–time interaction are a consequence of the transmission process. Results within each host species (figure 1a,c) have been discussed elsewhere (Carslake et al. 2005). Briefly, they indicate that an infectious animal poses a risk of cowpox transmission to other animals of the same species within the expected spatial and temporal scales of one infectious period and one home range diameter. The absence of space–time interaction between the two host species at these scales (figure 1b,d) strongly suggests that transmission between them is very rare, or entirely absent. This use of spatiotemporal patterns to reconstruct transmission events provides independent support for the results of Begon et al. (1999), who used whole-population transmission coefficients for a shorter time series of the same study populations.
The persistence of a pathogen in a host population can depend on host population size in relation to a deterministic or stochastic threshold (Anderson & May 1991). If cowpox were freely transmitted between the two host species, they could be considered as a single, large population of hosts, further from any threshold than either species would be alone. This, though, appears not to be the case. The apparent absence of transmission between the two host species also effectively rules out cowpox, as a directly transmitted virus, from being an agent of apparent competition between these species (Hudson & Greenman 1998).
Host–pathogen dynamics may also depend critically, though, on some low level of between-species transmission that would not be detected as statistically significant by the present method, but could be biologically significant. Grenfell & Harwood (1997) note that, to a parasite, classes of hosts between which transmission is rare may be considered analogous to habitat patches in a classic metapopulation. Stochastic extinctions of the virus in one host population may be ‘rescued’ by occasional infections from the other. To test for important but rare between-species transmission would require a different analytical or experimental approach from that used here, perhaps involving discrimination between different pathogen strains.
(b) Wood mice; space–time interaction within and between the sexes
The results suggest that most wood mice becoming infected with cowpox virus acquired the infection from a wood mouse of the opposite sex. During the breeding season, mature female wood mice hold territories that are largely exclusive of other females. Males occupy larger overlapping home ranges (Flowerdew 1991), which overlap a number of female territories at random (Wolton 1985). If contact rates between two individuals depend on the degree of shared home range, we might therefore expect contact rates between the sexes to be greater than within either sex, even if behaviour is independent of sex. However, correcting for patterns among the population at risk increased the significance of the positive space–time interaction between the sexes (table 2). This may have been due to the population at risk showing slightly negative space–time interaction at some small scales (Carslake 2003), as well as statistical noise due to the small sample sizes for between-sex tests (table 1). From the rejection of the second null hypothesis (table 2), we conclude that the spatial distribution of the sexes does not explain the predominance of between-sex transmission, and must consider the cause to be behavioural.
Cowpox in wood mice occurs in annual outbreaks during the breeding season that are more regular and short-lived than patterns observed in bank voles (Carslake et al. 2005). While some authors have suggested that wood mice may form heterosexual bonds during the breeding season, others consider them to mate promiscuously (Flowerdew 1991). Wolton (1985) found that while wood mice often shared nests in the winter, there was little evidence for nest sharing in the breeding season, and no tendency for heterosexual pairs to share a nest site. The seasonality of cowpox infection and the predominance of between-sex transmission therefore raise the possibility that cowpox is sexually transmissible among wood mice.
(c) Bank voles; space–time interaction within and between the sexes
Among bank voles, there was strong evidence only for female–female transmission (table 2), yet cowpox was almost equally prevalent in the two sexes (table 1). How do the males get infected? The existence of infected males might be accounted for by the suggestion of female–male transmission (figure 3b, table 2), but if female–male transmission accounts for space–time interaction between the sexes at the shared t=0 timescale (figure 3b,d), little evidence remains that males can infect other bank voles of either sex. It is not clear what behavioural or physiological difference between the sexes might lead to females having a greater transmission capacity than males, though one possibility is that transmission is associated with the maintenance of exclusive territories, which are held only by females (Alibhai & Gipps 1991).
The home ranges of male bank voles are generally larger than those of females (Alibhai & Gipps 1991), and home range size is often underestimated when calculated from trapping data (Kikkawa 1964). The use of spatiotemporal patterns to infer transmission of infection assumes that infection occurs within limited spatial and temporal scales. This has previously been demonstrated for these host species (Carslake et al. 2005). However, the method has greater power to detect transmission the smaller the scale at which it occurs, since the space–time interaction is not ‘diluted’ over a larger scale. It is therefore possible that males do transmit cowpox to other bank voles, but at a much larger spatial scale than females, and the results for bank voles should be treated with caution.
While we tentatively conclude that bank voles are at risk of cowpox transmission mainly from females (figure 3), transmission among wood mice appears to be primarily from animals of the opposite sex (figure 2). This contrast between the two host species suggests that cowpox is transmitted by a different behavioural interaction within each species, a suggestion entirely consistent with the apparent absence of between-species transmission (figure 1).
There has been little attempt to apply space–time interaction methods to wildlife diseases (Carslake et al. 2005), and to the best of our knowledge, this is the first time that between-class tests have been applied in a wildlife context. We have shown that spatiotemporal patterns of infection can yield useful information in our search for an understanding of the dynamics of wildlife diseases. It is hoped that the future development of tests such as these, together with the collection of the large datasets they require, will help to realize the full potential of spatiotemporal analysis as a tool in wildlife disease studies.
Acknowledgments
We thank Kevin Bown, Rachel Cavanagh, Julian Chantrey, Trevor Jones, Chris McCracken and many others for their help in the field and laboratory. Peter Diggle, Nigel French and Helen Clough all gave helpful discussions of space–time interaction statistics. Manor Wood was used by permission of Leverhulme estates. Work was funded by the Natural Environment Research Council and licensed under home office project licence PPL 40/1813.
References
- Alibhai S.K, Gipps J.H.W. Bank vole, Clethrionomys glareolus. In: Corbet G.B, Harris S, editors. The handbook of British mammals. Blackwell science; Oxford, UK: 1991. pp. 192–203. [Google Scholar]
- Anderson R.M, May R.M. Oxford university press; Oxford, UK: 1991. Infectious diseases of humans: dynamics and control. [Google Scholar]
- Baxby D, Bennett M. Cowpox virus. In: Webster R.G, Granoff A, editors. Encyclopaedia of virology. vol. 1. Academic Press; London: 1994. pp. 261–267. [Google Scholar]
- Begon M, Hazel S.M, Baxby D, Bown K, Cavanagh R, Chantrey J, Jones T, Bennett M. Transmission dynamics of a zoonotic pathogen within and between wildlife host species. Proc. R. Soc. B. 1999;266:1939–1945. doi: 10.1098/rspb.1999.0870. 10.1098/rspb.1999.0870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M, et al. Cowpox in British voles and mice. J. Comp. Pathol. 1997;116:35–44. doi: 10.1016/s0021-9975(97)80041-2. [DOI] [PubMed] [Google Scholar]
- Carpenter T.E. Methods to investigate spatial and temporal clustering in veterinary epidemiology. Prev. Vet. Med. 2001;48:303–320. doi: 10.1016/s0167-5877(00)00199-9. 10.1016/S0167-5877(00)00199-9 [DOI] [PubMed] [Google Scholar]
- Carslake, D. 2003 Spatial dynamics of cowpox virus infection in wild rodent populations. Ph.D thesis, The University of Liverpool, UK.
- Carslake D, Bennett M, Bown K, Hazel S, Telfer S, Begon M. Space–time clustering of cowpox virus infection in wild rodent populations. J. Anim. Ecol. 2005;74:647–655. 10.1111/j.1365-2656.2005.00966.x [Google Scholar]
- Chantrey, J. 1999 The epidemiology of cowpox in its reservoir hosts. Ph.D. thesis, The University of Liverpool, UK.
- Diggle P.J, Chetwynd A.G, Haggkvist R, Morris S.E. Second-order analysis of space–time clustering. Stat. Methods Med. Res. 1995;4:124–136. doi: 10.1177/096228029500400203. [DOI] [PubMed] [Google Scholar]
- Edmunds W.J, O'Callaghan C.J, Nokes D.J. Who mixes with whom? A method to determine the contact patterns of adults that may lead to the spread of airborne infections. Proc. R. Soc. B. 1997;264:949–957. doi: 10.1098/rspb.1997.0131. 10.1098/rspb.1997.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feore S.M, Bennett M, Chantrey J, Jones T, Baxby D, Begon M. The effect of cowpox virus infection on fecundity in bank voles and wood mice. Proc. R. Soc. B. 1997;264:1457–1461. doi: 10.1098/rspb.1997.0202. 10.1098/rspb.1997.0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowerdew J.R. Wood mouse, Apodemus sylvaticus. In: Corbet G.B, Harris S, editors. The handbook of British mammals. Blackwell science; Oxford, UK: 1991. pp. 218–229. [Google Scholar]
- Garnett G.P, Anderson R.M. Contact tracing and the estimation of sexual mixing patterns—the epidemiology of gonococcal infections. Sex. Transm. Dis. 1993;20:181–191. doi: 10.1097/00007435-199307000-00001. [DOI] [PubMed] [Google Scholar]
- Grenfell B, Harwood J. (Meta)population dynamics of infectious diseases. Trends Ecol. Evol. 1997;12:395–399. doi: 10.1016/s0169-5347(97)01174-9. 10.1016/S0169-5347(97)01174-9 [DOI] [PubMed] [Google Scholar]
- Hood, G. 2002 Poptools—software for the analysis of ecological models, version 2.4. Available from: www.cse.csiro.au/CDG/poptools
- Hudson P, Greenman J. Competition mediated by parasites: biological and theoretical progress. Trends Ecol. Evol. 1998;13:387–390. doi: 10.1016/s0169-5347(98)01475-x. 10.1016/S0169-5347(98)01475-X [DOI] [PubMed] [Google Scholar]
- Kikkawa J. Movement, activity and distribution of the small rodents Clethrionomys glareolus and Apodemus sylvaticus in woodland. J. Anim. Ecol. 1964;33:259–299. [Google Scholar]
- Perkins S.E, Cattadori I.M, Tagliapietra V, Rizzoli A.P, Hudson P.J. Empirical evidence for key hosts in persistence of a tick-borne disease. Int. J. Parasitol. 2003;33:909–917. doi: 10.1016/s0020-7519(03)00128-0. 10.1016/S0020-7519(03)00128-0 [DOI] [PubMed] [Google Scholar]
- Telfer S, Bennett M, Bown K, Cavanagh R, Crespin L, Hazel S, Jones T, Begon M. The effects of cowpox virus on survival in natural rodent populations: increases and decreases. J. Anim. Ecol. 2002;71:558–568. 10.1046/j.1365-2656.2002.00623.x [Google Scholar]
- Telfer S, Bennett M, Bown K, Carslake D, Cavanagh R, Hazel S, Jones T, Begon M. Infection with cowpox virus decreases female maturation rates in wild populations of woodland rodents. Oikos. 2005;109:317–322. [Google Scholar]
- Wolton R.J. The ranging and nesting behavior of wood mice, Apodemus sylvaticus (Rodentia, Muridae), as revealed by radio-tracking. J. Zool. 1985;206:203–224. [Google Scholar]