Abstract
We report the development of a new quantitative method of assessing the effects of anthropogenic impacts on living beings; this method allows us to assess actual impacts and to travel backwards in time to assess impacts. In this method, we have crossed data on fluctuating asymmetry (FA, a measure of environmental or genetic stress), using Didelphis albiventris as a model, with geographical information systems data relating to environmental composition. Our results show that more impacted environments resulted in statistically higher levels of FA. Our method appears to be a useful and flexible conservation tool for assessing anthropogenic impacts.
Keywords: fluctuating asymmetry, marsupials, conservation, geographical information systems, new methodology
1. Introduction
Fluctuating asymmetry (FA) is defined as a non-directional variation in bilateral (left–right) characteristics, which results from a reduced ability to control development during periods of environmental or genetic stress (Møller & Swaddle 1997). Currently, FA is being used as a measurement in many areas of animal behaviour research (Hovorka & Robertson 2000), and has recently been applied to conservation problems (Lens et al. 1999; Anciães & Marini 2000). Human-induced perturbations in natural environments have increased the need for sensitive biological indicators to measure the impact of such perturbation on living beings (Leary & Allendorf 1989; Parsons 1992). There are many biological impacts that influence FA, such as environmental quality, stress during development, hybridization between species, inbreeding and fitness (Palmer & Strobeck 1986). For example, Lens et al. (2002) found that Taiata thrushes (Turdus helleri) with higher levels of asymmetry showed lower probabilities of survival in comparison to more symmetric individuals, and this effect was more pronounced in impacted environments. However, such biological impacts and their consequential production of FA do not always affect animal fitness (Stinge et al. 2005). As already mentioned, FA can be used as a measurement of environmental stress; however, to prove that anthropogenic activities are having a negative effect on animals, some method of assessing environmental impacts needs to be applied. Previous studies using FA utilized qualitative data or unrefined measurement; for example, researchers have estimated environmental impact or used simple measurements such as size of the area (e.g. Møller 1996; Wauters et al. 1996; Lens et al. 1999; Anciães & Marini 2000; Gallant & Teather 2001), degree of pollution in water (e.g. Bonada & Willians 2002; Servia et al. 2003; Bonada et al 2005; Marques et al. 2005) or contamination of the environment (e.g. Oleksyk et al. 2004; Sonne et al. 2005). All these studies have shown that greater environmental fragmentation, pollution or contamination result in higher levels of FA. Obviously, some methods can be criticized for their lack of objectivity (quantification) or for over-simplifying the problem. Furthermore, when considering conservation problems we need a system that can detect such problems as early as possible. In terms of conserving a species it is better to act before the species enters a situation of population decline: a number of studies have shown that it is possible to detect FA before this occurs (Leary & Allendorf 1989; Tsubaki 1998; Alford et al. 1999; Lens et al. 2002; Bonada et al. 2005). In addition, to convince governments that certain anthropogenic activities are causing conservation problems, we need quantitative data.
In 1984, the NASA Landsat Program launched the first satellite equipped with a thematic mapper (TM) sensor, Landsat 5, capable of producing high quality images that could be analysed to reveal the effects of anthropogenic impacts on the environment. In 1999, Landsat 7 ETM+ satellite was launched, with an enhanced TM sensor. Before 1984, Landsat satellites used simpler sensors (e.g. Landsat 1 launched in 1972 used an MSS sensor, a multi-spectral scanner), and aerial photographs were also available. These images, after interpretation, can be analysed using geographical information systems (GIS), such as ArcGIS 8 (ESRI 2002), to reveal the types and composition of habitats at specific geographical points (Jensen 1996). For example, in China ecological monitoring of giant panda (Ailuropoda melanoleuca) reserves has been done using this technique (Liu et al. 2001). Remote sensing software, such as ERDAS Imagine (ERDAS 1997), can automatically classify satellite images, via unsupervised or supervised classification algorithms, into types of vegetation cover and soil use, and thereby detect levels of anthropogenic impact (Hirsch 2003; Landau et al. in press).
Marsupials are an important group of mammals found in the Americas and Australia that occupy a wide variety of ecological niches and have lifestyles that range from generalists to specialists. We chose the white-eared opossum (Didelphis albiventris) as the model species for our study because it occurs in large numbers and in a wide variety of environments (i.e. it has many characteristics of an indicator species), and is well represented in scientific collections. To conduct analyses of satellite images of environments where our study species was collected it is necessary to have home-range data. For the genera Didelphis we found data about the home range of the following species: D. marsupialis (home range sizes of 4.7±−1.8 ha; Sunquist et al. 1987; home range=5.6 ha; Vaughan & Hawkins 1999), D. aurita (home range=0.2−3.0 ha; Cárceres & Monteiro-Filho 2002) and D. albiventris home range =3.8 ha (Alessio et al. 2004). Due to the lack of the data on D. albiventris (based on only three individuals), we instead used the home range of D. marsupialis as this species is similar in terms of size and habitat (home range=4.7±1.8 ha; Sunquist et al. 1987).
The objective of the present study was to develop a new methodology, applicable in conservation, which could relate FA and anthropogenic impacts, using GIS and remote sensing data.
2. Material and methods
We measured a total of 98 skulls of D. albiventris from the Scientific Collections of the Laboratory of Mammalian Biology at the Federal University of Minas Gerais (UFMG) and the Natural Science Museum of Pontifícia Universidade Católica Minas, both situated in Belo Horizonte, Minas Gerais, Brazil. A total of 75 individuals had their collection place covered with satellite images and/or aerial photographs at a total of 35 different locations in Brazil. More than one animal was collected from many locations but in different areas, and at different times. All the animals were collected in the core of this species distribution (Eisenberg & Redford 1999). This is important because FA can be greater at the limits of a species' distribution (Kark et al. 2004). For the final analyses, the collected sample comprised 75 animals, but 15 of these were excluded because it was not possible to calculate the FA5 index (Palmer 1994), which is considered the most appropriate for large sample sizes with small measurement differences. The FAS index is calculated as Σ(R−L)2/n, where R is right-hand side measurement, L is left-hand side measurement and n is sample size (table 1). For analyses of FA a total of at least 30 animals is necessary, including all the sites sampled. Therefore, the sample cannot be small (Palmer 1994).
Table 1.
Numbers of individuals measured with satellite images and/or aerial photographs interpreted.
| data collected | Didelphis albiventris |
|---|---|
| number of measured skulls | 98 |
| number of individuals with satellite images or aerial photographs available | 78 |
| number of analysed localities | 35 |
| number of individuals with images or aerial photographs analysed and fluctuating asymmetry (sample size) | 75 |
| number of individuals for whom it was not possible to calculate FA5 index | 15 |
| total number of skulls analysed in this study | 60 |
For FA measurements, we used the digital caliper Starret 727, with an accuracy of 0.01 mm. A pilot study, where many measurements were taken, was carried out to establish which measurements should be used in this study. We chose measurements that allowed a high degree of confidence because of their size and location on the skull. We also used previously established limits to avoid measurement errors. Four measurement points were located: (i) length of nasal bone; (ii) length of superior canine tooth; (iii) width of mandible condile; and (iv) distance between mentual foramen and post-incisive diastem.
In this study, we employed a double-blind methodology to minimize the risks of false positives. Thus, the researcher making the FA measurements did not know where the skulls had been collected or the results from the researcher analysing the satellite images (who also received no information about the FA measurements). This method is recommended for all FA analyses and provides more reliable data (Palmer 1994). For this reason, two researchers took the measurements of the right- and left-hand sides of the skull of each individual at different times and dates. Thus, all measurements were made in two stages. In the first, some skulls were separated and arranged into one box, selected randomly and the measurements of one side recorded in a pre-determined order. In the second, the skulls were taken again randomly for the measurements of the opposite side to be recorded. To avoid false-positive results, the column containing the first measurements was hidden during the second stage of measurement. Furthermore, the caliper was zeroed after each measurement. The pre-determined order given on the data-sheet was as follows: (i) the side of skull to be measured (right or left), in a random order and previously established; (ii) the measurements to be taken, also in random order and previously established; and (iii) a field to fill in with the skull number. If the skull was broken we did not take measurements. After all measurements had been taken following the protocol described, further information was collected about each animal: (i) location of collection (locality, municipality, State, geographical coordinates); (ii) gender; and (iii) date of collection. With this information we constructed a table and all points of collection were plotted on a map, using the ArcGIS 8.2 program (ESRI 2002), at the Mastozoology Laboratory at UFMG.
To estimate the degree of anthropogenic impact we elaborated maps showing the types of vegetation cover and soil use. To draw these maps we used:
Landsat 5 TM satellite images from 1984, scale 1 : 100.000, spatial resolution 30 m, assigned by iNPE (National Institute of Space Research);
aerial photographs standard CEMIG from 1989, scale 1 : 10.000, spatial resolution 1 m, assigned by CEMIG (Energy Company of Minas Gerais);
Landsat 5 TM satellite image from 1997/1998, scale 1 : 100.000, spatial resolution 30 m, assigned by Department of the Environment and Conservation (DMC) of IEF/MG (Forest Institute of Minas Gerais); and CI (Conservation International);
Landsat 7 ETM+ (Enhanced Thematic Mapper) satellite image from 2000/2001, scale 1 : 100.000, spatial resolution 30 m, assigned by EMBRAPA (Brazilian Agency for Agriculture and Cattle Research).
The animal's date of collection was classified according to the date of satellite images and aerial photographs obtained. Animals with dates of collection between 1976 and 1984 were associated with the Landsat 5 TM images from 1984, animals collected between 1985 and 1993 were associated with aerial photographs from 1989, and animals collected between 1994 and 2002 were associated with Landsat 5 TM and 7 ETM+ images from 1997 and 2001.
The rate of degradation of habitat was calculated from data published by the S.O.S Mata Atlântica Foundation. In 1912, there was 97% of Atlantic forest coverage in Minas Gerais, in 1947 this was reduced to 37%, in 1953 to 25%, and 1963 to 20% (Fundação SOS Mata Atlântica & INPE 1993; Fundação SOS Mata Atlântica, INPE & ISA 1998). Recent data indicate that Minas Gerais has 14.57% of its original Atlantic forest remaining (Fundação SOS Mata Atlântica & INPE 2002). However, maps (scale 1 : 250.000) of Rio Doce Basin show that the average rate of deforestation in the remnants of Atlantic forest increased from 6.23% in 1985–1990 to 9.21% in 1990–1995 (Fundação SOS Mata Atlântica & INPE 1993; Fundação SOS Mata Atlântica, INPE & ISA 1998). Thus, the grouping of areas into time bands in this study means that, if an animal was collected in 1976 we used a 1984 satellite image, which would probably reveal a more impacted environment than in 1976. Although this obviously introduces some error into our analyses, this error is likely to be small (of the order of four to eight per cent) given the rates of deforestation during our sample periods.
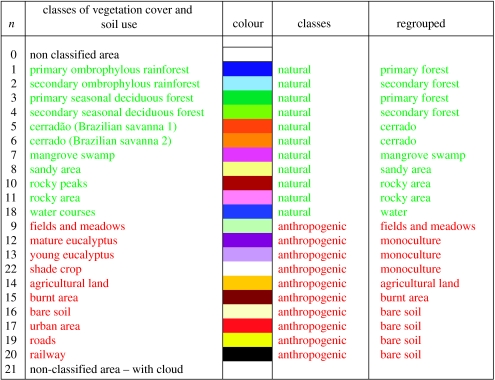
A total of twenty-one classes of vegetation cover and soil use were identified, of which 11 were natural classes (e.g. primary forest) and 10 anthropogenic classes (e.g. urban areas). These classes were obtained from Landsat 5, Landsat 7 images and interpretation and classification of aerial photographs. In this process, we utilized GIS and remote sensing softwares, such as ArcGIS 8 and ERDAS Imagine (ERDAS 1997), based on supervised classification techniques and maximum likelihood algorithms with a transformed divergence distance equal to or greater than 1900, as developed by Jensen (1996) and tested by Hirsch (2003). The general accuracy accepted in the error matrix of classification was 90% or greater, considering that in the literature more than 85% is generally acceptable (Jensen 1996).
Next the classes of vegetation cover and soil use were regrouped to natural similarity to reduce the number of classes (table 2).
Table 2.
Relation of class of vegetal cover and soil use.
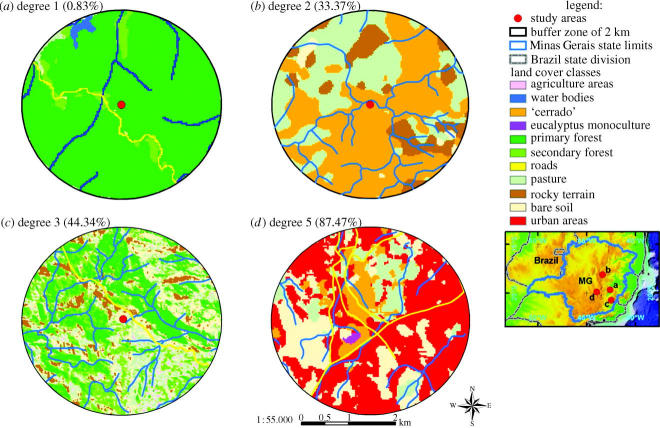
In fact, we did not analyse the whole reserve where the animals were collected; instead we analysed a buffer zone of 2 km around each collection point, as this area would relate to the species' home range (see §1). A buffer zone consists of a circular area around the central point where the individuals were collected. Each environment analysed was classified into one of five categories in accordance with its degree of anthropogenic impact (categories: 1, 0–20%; 2, 21–40%; 3, 41–60%; 4, 61–80% and 5, 81–100%).
To analyse the data, we first used the Anderson–Darling test of normality to verify the normal distribution of the data (Zar 1999) and thereby check for antisymmetry in the sample (Palmer 1994). We also used a T-test to reject directional asymmetry in the sample (Palmer 1994). After rejecting these two kinds of adaptative asymmetry, we measured the accuracy between the two sets (the two observers) of FA results using a Student t-test (Zar 1999).
The FA data were converted to the index score FA5 (Palmer 1994). The FA5 data were log-transformed to meet the requirements of parametric statistics.
We then analysed these data using a one-way analysis of variance (ANOVA) with categories of anthropogenic impact as the factor, and we used a post hoc Tukey test to determine the difference between each anthropogenic category.
To correlate the FA index and the anthropogenic percentage at each location, and the FA index of each animal and the percentage of each class of vegetation cover and soil use, we used the Spearman rank correlation.
We also calculated, using ArcGIS software, the total continuous area of natural vegetation cover connected to each collection point (buffer-zone) and then correlated this, using a Spearman rank correlation, with FA5.
3. Results
The results showed a significant effect of anthropogenic impact on FA (F=3.32; d.f.=4,55; p<0.05). A post hoc Tukey test showed that the level of FA was significantly lower in category 1 in comparison with all other categories, and no further statistical differences were found. The individuals in category 1 showed one-third of the FA relative to those in other categories (0.01440±0.00675 versus 0.0448±0.0158). The numbers of specimens in each anthropogenic category were: 1, 12; 2, 11; 3, 9; 4, 6 and 5, 25. We calculated, using GIS software, the total continuous area of natural vegetation cover connected to each collection point (buffer-zone) and then correlated this, using a Spearman rank correlation, with FA5 (figure 1). The result showed a positive trend (p<0.1) but not a significant result. However, there was a significant negative correlation between size of continuous area and percentage of anthropogenic impact (rs=−0.796; n=60; p<0.001).
Figure 1.
Examples of buffer zones and degree of anthropogenic impact. Carotgraphic: Hirsch 2003; ESRI 2002; IBGE 1998. (Geoprocessing work: André Hirsch, Department of Zoology, Biological Science Institute, Federal University of Minas Gerais. Belo horizonte, Abril de 2005.)
4. Discussion
These results show, as predicted, that white-eared opossums living in anthropogenically impacted environments show increased FA, and that FA data with GIS analyses provide a new and quantitative method for analysing the effects of anthropogenic impacts. This new methodology can be applied to any location in the world, to any group of living creatures and may be applied retrospectively (until 1972).
Despite the fact that the white-eared opossum, commonly known as gambá, is renowned for its ability to invade new habitats and therefore is a generalist (Eisenberg & Redford 1999), it was affected negatively by anthropogenic impact. The surprising result was that an anthropogenic impact of only 20% caused a tripling in the level of FA. Normally, we would expect a generalist that is known to have invaded man-made environments (e.g. gardens in cities) to have a greater tolerance to modified environments. A low threshold was a surprise because white-eared opossums evolved in environments subject to large natural impacts, such as fire and severe storms (Collinge 1996). Fire is one of the most common and frequent natural impacts that occurs in the Brazilian savanna (Klink & Machado 2005). To date, no one has measured the effects of natural catastrophes on FA, and in our present study we do not know if the levels of FA we detected caused health or welfare problems for the animals. However, even if the level of FA in our sample did not create health problems for our animals, the fact that we were able to detect it supports the hypothesis that it could be used as an early warning system in animal conservation programs (Leary & Allendorf 1989; Tsubaki 1998; Alford et al. 1999; Lens et al. 2002; Bonada et al. 2005). For example, Lens et al. (2002) found that FA levels in Taiata thrushes increased prior to negative population changes, which had subsequent deleterious impacts on local population survival.
The results of this research are not important just because they show the link between the degree of anthropogenic impact and FA, but also because we have done this using a new methodology. We believe this new methodology represents a major advance in comparison to previous methods (e.g. Møller 1996; Wauters et al.1996; Lens et al. 1999; Anciães & Marini 2000; Gallant & Teather 2001; Marques et al. 2005; Sonne et al. 2005) because it is totally quantitative and, if applied in a double-blind manner, not subject to human biases. Probably every country in the world has museums or universities with collections of animals and plants, plus many countries have vast collections of exotic flora and fauna (e.g. Great Britain). Besides, it is also possible to capture common species in the field and measure their degree of FA (e.g. Lens et al. 1999; Anciães & Marini 2000; Lens et al. 2002). Satellite images of the whole earth are available from a number of agencies (e.g. NASA and ESA) and can be bought, or are sometimes donated for research use. Courses of GIS techniques are now taught throughout the world and the software is usually available (via licenses) in universities that have biology or geography departments. Thus our approach can be applied to a range of other species and in our geographical regions to increase our understanding of FA in disturbed areas.
In this study, we have proved that this new methodology has much merit but we have not really begun to explore its full potential. The first images that can easily be analysed to provide information about vegetation cover and soil use were produced in 1984 by Landsat 5 TM. This means that we can now travel backwards in time to look at how impacts in an environment were affecting the flora or fauna at that time (provided that sufficient specimens exist in museums). For example, imagine a reserve was created in 1984 and has been rigorously protected against deforestation ever since, and has had considerable reforestation. We could measure the impacts of this on the flora and fauna over time using the methodology presented in this paper. This could inform us about how much time flora and fauna need to recuperate after anthropogenic impacts.
The methodology presented here can be applied rapidly to areas suffering anthropogenic or other impacts, and provides an early warning of conservation problems. Given the highly quantitative nature of this methodology, we believe the implications of research utilizing it will be important to agencies concerned with the conservation of biodiversity.
Acknowledgements
We wish to express our thanks to the following institutions for donating the images used in this study: CEMIG, IEF, INPE, INTERSAT and Conservation International. The Museum of Natural Sciences PUC Minas and the Mastozoology Laboratory of UFMG kindly allowed us access to their specimens. The content of this article was greatly improved by the referees' comments and to them we are most grateful.
References
- Alessio F.M, Mendes Pontes A.R, Silva V.L. Área de uso de Didelphis albiventris em um remanescente de Mata Atlântica no Nordeste do Brasil. Anais do XXV Congresso Brasileiro de Zoologia. 2004 pp.246. [Google Scholar]
- Alford R, Bradfield K, Richards S.J. Measuring and analysing developmental instability as a tool for monitoring frog populations. In: Campbell A, editor. Declines and disappearances of Australian frogs. Environment Australia; Canberra: 1999. pp. 34–43. [Google Scholar]
- Anciães M, Marini M.Â. The effects of fragmentation on fluctuating asymmetry in passerine birds of Brazilian tropical forests. J. Appl. Ecol. 2000;37:1013–1028. 10.1046/j.1365-2664.2000.00554.x [Google Scholar]
- Bonada N, Willians D.D. Exploration of the utility of fluctuating asymmetry as an indicator of river condition using larvae of the caddisfly Hydropsyche morose (Trichoptera: Hydropsychidae) Hydrobiologia. 2002;481:147–156. 10.1023/A:1021297503935 [Google Scholar]
- Bonada N, Vives S, Rieradevall M, Prat N. Relationship between pollution and fluctuating asymmetry in the pollution-tolerant caddisfly Hydropsyche exocellata (Trichoptera Insecta) Arch. Hydrobiol. 2005;162:167–185. 10.1127/0003-9136/2005/0162-0167 [Google Scholar]
- Cáceres N.C, Monteiro-Filho E.L.A. Food habits, home range and activity of Didelphis aurita (Mammalia, Marsupialia) in a forest fragment of Southern Brazil. Stud. Neotrop. Environ. 2002;36:85–92. 10.1076/snfe.36.2.85.2138 [Google Scholar]
- Collinge S.K. Ecological consequences of habitat fragmentation: implications for landscape architecture and planning. Landsc. Urban Plan. 1996;36:59–77. 10.1016/S0169-2046(96)00341-6 [Google Scholar]
- Eisenberg J.F, Redford K.H. Mammals of the neotropics—the central neotrpics. vol. 3. The University of Chicago Press; Chicago, IL: 1999. [Google Scholar]
- ERDAS 1997 ERDAS Imagine v. 8.4 Field Guide, 4th edn. Atlanta, GA: ERDAS. See http://gis.leica-geosystems.com/ (Accessed 20/02/2003)
- ESRI 2002 ArcView GIS v. 8.2. Environmental Systems Research Institute, Redlands/CA. See http://www.esri.com/data/index.html (Accessed at 20/02/2003)
- Fundação SOS Mata Altantica, INPE Atlas da Evolução dos Remanescentes Florestais e Ecossistemas Associados do Domínio da Mata Altânica no Período 1985–1990São Paulo1993Fundação SOS Mata Atlântica & Instituto Nacional de Pesquisas Espaciais; Brazil [Google Scholar]
- Fundação SOS Mata Atlântica, INPE & ISA . Fundação SOS Mata Atlântica; São Paulo: 1998. Atlas da evolução dos remanescentes florestais e ecossistemas associados no domínio da mata atlântica no período de 1995 a 2000. 54 pp. + Mapas temáticos. [Google Scholar]
- Fundação SOS Mata Atlântica & INPE . Fundação SOS Mata Atlântica; São Paulo: 2002. Atlas dos remanescentes florestais da Mata Atlântica no período 1995 a 2000. 46 pp. [Google Scholar]
- Gallant N, Teather K. Differences in size, pigmentation, and fluctuating asymmetry in stressed and nonstressed northern leopard frogs (Rana pipiens) Ecoscience. 2001;8:430–436. [Google Scholar]
- Hirsch A. Habitat fragmentation and priority areas for primate conservation in the Rio Doce Basin Minas Gerais. Neotrop. Primates. 2003;11:195–196. [Google Scholar]
- Hovorka M.D, Robertson R.J. Food stress, nestling growth, and fluctuating asymmetry. Can. J. Zool. 2000;78:28–35. 10.1139/cjz-78-1-28 [Google Scholar]
- IBGE 1998 Dados Gerais do Brasil, Informações Estatísticas e Geocientíficas. Rio de Janeiro, Brazil: Fundação Instituto Brasileiro de Geografia e Estatística (IBGE).
- Jensen J.R. 2nd edn. Prentice Hall; New Jersey: 1996. Introductory digital image processing: a remote sensing perspective. [Google Scholar]
- Kark S, Lens L, Van Dongen S, Schmit E. Asymmetry patterns across the distribution range: does the species matter? Biol. J. Linn. Soc. 2004;81:313–324. 10.1111/j.1095-8312.2004.00296.x [Google Scholar]
- Klink C.A, Machado R.B. A Conservação do Cerrado. Megadiversidade. 2005;1:147–155. [Google Scholar]
- Landau, A., Hirsch, A., Musinsky, J. In press. Vegetation cover and land use in the Atlantic forest of southern Bahia, Brazil, based on satellite imagery: a comparison among municipalities. In The Atlantic Coastal Forest of Brazil North of the Rio Doce (ed. W. Thomas). New York: Memoirs of the New York Botanical Garden.
- Leary R.F, Allendorf F.W. Fluctuating asymmetry as an indicator of stress: implications for conservation biology. Trends Ecol. Evol. 1989;4:214–217. doi: 10.1016/0169-5347(89)90077-3. 10.1016/0169-5347(89)90077-3 [DOI] [PubMed] [Google Scholar]
- Lens L, Dongen van S, Wilder C.M, Brooks T.M, Matthysen E. Fluctuating asymmetry increases with habitat disturbance in seven bird species of a fragmented afrotropical forest. Proc. R. Soc. B. 1999;266:1241–1246. 10.1098/rspb.1999.0769 [Google Scholar]
- Lens L, Dongen van S, Matthysen E. Fluctuating asymmetry as an early warning system in the critically endangered Taita thrush. Conserv. Biol. 2002;16:479–487. 10.1046/j.1523-1739.2002.00516.x [Google Scholar]
- Liu J, Linderman M, Ouyang Z, An L, Yang J, Zhang H. Ecological degradation in protected areas: the case of Wolong nature reserve for Giant Pandas. Science. 2001;292:98–101. doi: 10.1126/science.1058104. 10.1126/science.1058104 [DOI] [PubMed] [Google Scholar]
- Marques J.F, Costa J.L, Cabral H.N. Variation in bilateral asymmetry of the Lusitanian toadfish along the Portuguese coast. J. Appl. Ichthyol. 2005;21:205–209. 10.1111/j.1439-0426.2005.00627.x [Google Scholar]
- Møller A.P. Parasitism and developmental instability of hosts: a review. Oikos. 1996;77:189–196. [Google Scholar]
- Møller A.P, Swaddle J.P. Oxford University Press; Oxford, UK: 1997. Asymmetry, developmental stability and evolution. [Google Scholar]
- Oleksyk T.K, Novak J.M, Purdue J.R, Gashchak S.P, Smith M.H. High levels of fluctuating asymmetry in populations of Apodemus flavicollis from the most contaminated areas in Chornobyl. J. Environ. Radioact. 2004;73:1–20. doi: 10.1016/j.jenvrad.2003.07.001. 10.1016/j.jenvrad.2003.07.001 [DOI] [PubMed] [Google Scholar]
- Palmer R.A. Fluctuating asymmetry analyses: a primer. In: Markoow T.A, editor. Developmental instability: its origins and evolutionary implications. Dordrecht; Kluwer: 1994. pp. 335–364. [Google Scholar]
- Palmer R.A, Strobeck C. Fluctuating asymmetry: measuarement, analysis and patterns. Annu. Rev. Ecol. Syst. 1986;17:391–421. 10.1146/annurev.es.17.110186.002135 [Google Scholar]
- Parsons P.A. Fluctuating asymmetry: a biological monitor of environmental and genomic stress. Heredity. 1992;68:361–364. doi: 10.1038/hdy.1992.51. [DOI] [PubMed] [Google Scholar]
- Servia M.J, Cobo F, González M.A. Multiple-trait analysis of fluctuating asymmetry levels in anthropogenically and naturally stressed sites: a case study using Chironomus riparius Meigen, 1804 Larvae. Environ. Monit. Assess. 2003;90:101–112. doi: 10.1023/b:emas.0000003569.22040.ac. 10.1023/B:EMAS.0000003569.22040.ac [DOI] [PubMed] [Google Scholar]
- Stinge L.C, Slagsvold T, Vollestad L.A. Individual fluctuating asymmetry in pied flycatchers (Ficedula hypoleuca) persists across moults, but is not heritable and not related to fitness. Evol. Ecol. Res. 2005;7:381–406. [Google Scholar]
- Sonne C, Riget F.F, Dietz R, Kirkegaard M, Born E.W, Letcher R, Muir D.C.G. Trends in fluctuating asymmetry in East Greenland polar bears (Ursus maritimus) from 1892 to 2002 in relation to organohalogen pollution. Sci. Total Environ. 2005;341:81–96. doi: 10.1016/j.scitotenv.2004.09.027. 10.1016/j.scitotenv.2004.09.027 [DOI] [PubMed] [Google Scholar]
- Sunquist M.E, Austad S.N, Sunquist F. Movement patterns and home range in common opossum (Didelphis marsupialis) J. Mammal. 1987;68:173–176. [Google Scholar]
- Tsubaki Y. Fluctuating asymmetry of the Oriental fruit fly (Dacus dorsalis) during the process of its extinction from the Okinawa Islands. Conserv. Biol. 1998;12:926–929. 10.1046/j.1523-1739.1998.97159.x [Google Scholar]
- Vaughan C.S, Hawkins L.F. Late dry season habitat use of common opossum Didelphis marsupialis (Marsupialia: Didelphidae) in neotropical lower montane agricultural areas. Revista de Biológia Tropical. 1999:47. [Google Scholar]
- Wauters L, Dhondt A.A, Heike K, Parkin D.T. Fluctuating asymmetry and body size as indicators of stress in red squirrel populations in woodland fragments. J. Appl. Ecol. 1996;33:735–740. [Google Scholar]
- Zar J.H. 4 edn. Prentice Hall; Saddle River, NJ: 1999. Biostatistical analysis; p. 663. [Google Scholar]