Abstract
Increased reproduction is frequently associated with a reduction in longevity in a variety of organisms. Traditional explanations of this ‘cost of reproduction’ suggest that trade-offs between reproduction and longevity should be obligate. However, it is possible to uncouple the two traits in model organisms. Recently, it has been suggested that reproduction and longevity are linked by molecular signals produced by specific reproductive tissues. For example, in Caenorhabditis elegans, lifespan is extended in worms that lack a proliferating germ line, but which possess somatic gonad tissue, suggesting that these tissues are the sources of signals that mediate lifespan. In this study, we tested for evidence of such gonadal signals in Drosophila melanogaster. We ablated the germ line using two maternal effect mutations: germ cell-less and tudor. Both mutations result in flies that lack a proliferating germ line but that possess a somatic gonad. In contrast to the findings from C. elegans, we found that germ line ablated females had reduced longevity relative to controls and that the removal of the germ line led to an over-proliferation of the somatic stem cells in the germarium. Our results contrast with the widely held view that it is downstream reproductive processes such as the production and/or laying of eggs that are costly to females. In males, germ line ablation caused either no difference, or a slight extension, in longevity relative to controls. Our results indicate that early acting, upstream reproductive enabling processes are likely to be important in determining reproductive costs. In addition, we suggest that the specific roles and putative patterns of molecular signalling in the germ line and somatic tissues are not conserved between flies and worms.
Keywords: lifespan, ageing, germ line, Drosophila melanogaster
1. Introduction
Life histories consist of age-specific schedules of fecundity and mortality (Fisher 1930; Hamilton 1966; Charlesworth 1973; Charlesworth 1980) and they show great diversity in the living world. One major generalization that has emerged from comparative studies is that the two key life history traits tend to vary in opposite directions from each other. Among both mammals and birds, long lifespans are associated with low fecundity (Read & Harvey 1989; Holmes et al. 2001). Experimental manipulations and natural genetic variation often have opposite effects on fecundity and lifespan (Calow 1979; Rose 1984; Bell & Koufopanou 1985; Partridge & Harvey 1985; Reznick 1985; Partridge et al. 1987). For example, in Drosophila melanogaster females, high early fecundity causes an increase in mortality rate later in life, an effect which can be eliminated by sterilizing females by X-irradiation or by introducing the ovoD mutation (Sgró & Partridge 1999), suggesting that longevity effects are mediated via changes in reproductive output. The frequent occurrence of the cost of reproduction (Williams 1966) suggests that its mechanisms may be conserved during evolution. However, although the cost of reproduction has long been recognized, its molecular basis has remained unresolved (Partridge et al. 2005).
The leading candidate mechanism for the cost of reproduction is the allocation of limited resources between reproduction and somatic maintenance (Levins 1968; Charlesworth 1973; Kirkwood 1977; Law 1979; Sibly & Calow 1986; van Noordwijk & de Jong 1986; Stearns 1992). Costly processes, such as reproduction, compete for resources with other processes such as repair or growth. Because resources are limited, traits competing for the same resource cannot all be maximized, leading to negative relationships between them within individuals. An alternative explanation for the cost of reproduction is that reproductive processes generate damage, which necessarily impairs somatic processes, causing a reduction in longevity (Fowler & Partridge 1989; Tatar & Carey 1995; Sgró & Partridge 1999; Barnes & Partridge 2003). Both explanations lead to an obligate trade-off between reproductive and somatic processes.
In general, interventions that affect longevity, such as single gene mutations or dietary restriction, result in correlated changes in fecundity consistent with the existence of a trade-off (Partridge et al. 2005). The elimination of egg production (at least by irradiation or by using the ovoD mutation) can also reduce age-related mortality (Sgró & Partridge 1999). However, numerous apparent exceptions exist, indicating that trade-offs may not be obligate. Mutations exist in which longevity is extended with no apparent cost to fecundity. In Caenorhabditis elegans, individuals with specific age-1 (Johnson et al. 2003) and daf-2 (Kenyon et al. 1993; Gems et al. 1998) alleles show an extension of longevity, but unchanged reproductive ability. Other daf-2 alleles which had been reported to reduce fecundity, have subsequently been shown to exert their effects on longevity and fecundity at entirely different times during the worm's life. Knocking out daf-2 in the adult using RNAi extends longevity without affecting fecundity, the latter only being affected by pre-adult knockouts (Dillin et al. 2002). Likewise in flies, hypomorphic mutations in the ecdysone receptor (Simon et al. 2003), heterozygotes for the cotransporter gene mutation indy (Rogina et al. 2000), head specific knockouts of the forkhead transcription factor dFOXO (Hwangbo et al. 2004) and the application of drugs that activate protein deacetylases (SirTuin Activating Compounds—STACs; Wood et al. 2004) all result in an extension of longevity but no reduction, and occasionally an increase, in fecundity. Furthermore, chico1 heterozygotes continue to be long lived even in combination with ovoD1, a dominant sterile mutation (Clancy et al. 2001). If chico extends longevity by diverting resources away from reproduction, then wholly sterile flies should not be affected by chico, because somatic processes will be invested in maximally anyway. In mice, IGF1 receptor heterozygotes and fat-specific insulin receptor knockout (FIRKO) strains are also reported to show extended longevity with no reduction in fecundity (Blüher et al. 2003; Holzenberger et al. 2003). Long-lived Ames and Snell mice do show reduced fecundity, but restoring fecundity in adults by prolactin treatment does not eliminate the longevity effects (Brown-Borg et al. 1996; Flurkey et al. 2002). All of these examples indicate that increasing longevity does not have an obligate negative effect on fecundity.
Likewise, reducing fecundity does not necessarily result in increased longevity. Flies that have been sterilized by X-ray irradiation or ovoD1 mutation show a normal response to dietary restriction, an intervention that has been thought to affect ageing partly through its effect on fecundity (Mair et al. 2004). In C. elegans, inducing sterility in adults by the ablation of the gonad precursor cells in juveniles has no effect on longevity. However, in an intriguing result, ablating the precursors of the germ line cells (and therefore, leaving the somatic gonad intact), results in an adult with significantly extended longevity (Hsin & Kenyon 1999). Ablation of the germ line must be prior to germ line stem cell proliferation for lifespan extension to occur (Arantes-Oliveira et al. 2002). In addition, genes in the insulin signalling pathway, which are involved in ageing in a wide range of taxa (Gems et al. 1998; Clancy et al. 2001; Gems & Partridge 2001; Tatar et al. 2001; Blüher et al. 2003), must also be fully functioning for lifespan to be extended (Hsin & Kenyon 1999). The longevity phenotype has been interpreted as being the result of a putative molecular signal produced by the germ line (which interacts with a signal from the somatic gonad), which communicates reproductive status to known ageing pathways in the soma (Hsin & Kenyon 1999; Riddle 1999; Leroi 2001; Lin et al. 2001; Arantes-Oliveira et al. 2002). The identity of the putative signals remains unknown. The finding that it is not sterility per se that alters longevity, but rather the presence or absence of specific reproductive signals, has been presented by some as a challenge to resource based explanations of life-history trade-offs (e.g. Leroi 2001).
The widespread observation of ‘costs of reproduction’ in nature suggests the possibility that mechanisms regulating them are conserved. Here, we tested the hypothesis that the worm model is conserved in flies. Specifically, we wanted to see if the germ line signals reproductive rate to the soma in Drosophila, hence decreasing lifespan when it is present and extending lifespan when it is absent. In the first part of the study, we tested the effect on longevity of the absence of a proliferating germ line in D. melanogaster males and females compared to genetically matched controls. We generated flies lacking a germ line using two maternal effect mutants: germ cell-less (gcl: Jongens et al. 1992; Jongens et al. 1994) and tudor (tud, Boswell & Mahowald 1985). Both are developmental genes that determine a germ line fate for posterior regions of the egg. In their absence pole cells fail to form and the germ line is absent, but the somatic gonad remains intact. Because both tud and gcl act in germ line determination, no germ cells are able to proliferate in either mutant. Both genes are also maternal effect, and therefore, genetically identical matched controls for the mutant flies can be generated with ease (see §2). A simple crossing scheme allowed us to generate large sample sizes for both mutations, and this did not require the introduction of multiple genetic backgrounds or exposure to heat shock (e.g. as is necessary to produce agametic flies carrying the oskar mutation). gcl and tud were, therefore, ideal for this study.
To ensure the absence of the germ line, and to look for abnormalities in the associated proliferating somatic gonad tissue we analysed the structure of the gonad in female offspring of tud/tud or gcl/gcl mothers. To test whether gonad development was normal prior to germ cell proliferation, we counted ovarioles. We then visualized germ line and proliferating somatic gonad tissue using antibody and chemical stains specific to each tissue type, and quantified differences between germ line-less and control flies where possible.
2. Material and methods
(a) Fly stocks and husbandry
The wild-type (WT) stock was collected in Dahomey (now Benin) in 1970 and has been maintained since then in large population cages with overlapping generations on a 12 : 12 h light : dark cycle at 25 °C. tud1 bw1/CyO flies were donated by Prof. Marianna Wolfner (Cornell University) and backcrossed into the Dahomey backgound for at least four generations. tud acts in the determination of pole plasm, from which the pole cells are formed (Boswell & Mahowald 1985). gcl bw1/CyO flies were donated by Dr Tom Jongens (University of Pennsylvania) and had not been backcrossed to Dahomey at the time of these experiments. The two mutations were, therefore, in different genetic backgrounds. gcl is expressed in the pole plasm, and is important in the formation of pole cells (from which the germ line is derived; Jongens et al. 1992). The specific roles of both mutations are unknown. All stocks were maintained and experiments run on standard sugar/yeast (SY) medium (100 g autolysed yeast powder, 100 g sugar, 20 g agar, 30 ml nipagin (100 g l−1), 3 ml propionic acid, 1 l distilled water).
(b) Longevity of gcl and tud virgin males and females
We compared the longevity of virgin male and female flies lacking a germ line to flies with a fully intact gonad. Germ line-less flies were the male and female offspring of homozygous tud or gcl mothers that had been mated to Dahomey males. Control flies were the male and female offspring of heterozygous tud or gcl mothers, also mated to Dahomey males. Because gcl and tud are maternal effect mutants, it is possible to make genetically identical experimental and control flies. To use gcl as an example, the offspring of gcl/gcl females mated to WT males are gcl/+ and lack germ cells, while half of the offspring of gcl/+ females and WT males are also gcl/+ but possess germ cells. tud and gcl are, therefore, ideal for this work because we could produce experimental flies that differed from controls in their gonad structure but which were otherwise genetically identical. This is not true of the male offspring, which differ in the origin of their X chromosomes.
To obtain flies for the experiments, adult virgin gcl and tud females were collected on ice and split into homo- and heterozygotes. These were housed with virgin Dahomey males in a 1 : 1 ratio for 48 h before being transferred to a grape juice agar medium (50 g agar, 600 ml grape juice (Continental Wine Experts Limited; Cawston, Norwich), 42 ml nipagin (100 g l−1), 1 l distilled water) for oviposition. Eggs were left to hatch and 1st instar larvae were collected in vials containing 7 ml SY medium at a standard density of 50 per vial, to minimize environmentally derived differences in body size. Emerging adults were collected as virgins using ice.
For the tud flies, germ line-less (n=70 females, n=64 males) and control (n=99 females, n=157 males) flies were housed in separate single sex vials containing 7 ml SY medium at standard density (10 per vial) and scored for death daily. All flies were transferred to a new vial twice weekly. Any association between longevity and the pleiotropic effect of the tud mutation on abdominal segment number was assessed by counting the number of abdominal segments present in the experimental tud flies after death and then testing for a relationship between abdominal segment number and longevity.
A similar longevity protocol was followed for the gcl flies. However, gcl only has about 75% penetrance (Robertson et al. 1999 and A. I. Barnes 2003, personal observation), hence 25% of the offspring of gcl/gcl mothers do possess a germ line. To identify and remove individuals possessing a germ line from the experimental female group, we first housed all female gcl flies individually in vials for five days post-eclosion, and scored these vials for the presence of eggs. Female offspring of gcl/gcl mothers that produced eggs were then used to establish a second control group. The gcl female experimental treatments were therefore: germ line-less virgins (n=249), controls with gcl homozygote mothers (n=75), controls with gcl heterozygotye mothers (n=197).
We did not test for a germ line in the male offspring of gcl/gcl mothers in our experiment, because this would have involved dissecting and scoring testis morphology post-mortem, which is not reliable in old males. However, there are no reported sex differences in gcl penetrance (Robertson et al. 1999), therefore, the ratio of non-penetrant individuals is likely to be similar in both sexes. The gcl male experimental treatments were therefore: ‘germ line-less’ males (n=183), of which ca 25% actually had a germ line, and control males (n=75). The effect of fertile males in the germ line-less group of males made the test for longevity extension in the absence of a germ line more conservative.
(c) Analysis of the reproductive structures of female germ line-less flies
(i) Ovariole number in gcl females
To test whether ovariole number was significantly affected by the absence of a germ line, we compared ovariole counts for gcl and control females. Virgin females were obtained from the crosses described above. Ovaries were then removed from females less than 4 days old in PBS under a dissecting microscope (Leica MZ 7.5). The ovaries of control females were fixed for 1 h in 20% paraformaldehyde and then teased apart to aid counting. Germ line-less ovaries were stained with DAPI, which stains all cell nuclei regardless of origin, to aid visualization of the nascent ovarioles. They were then viewed using a confocal miscroscope (Zeiss LSM 510). Ovarioles in the acquired images were counted using Zeiss LSM software. 10 control (two ovaries per fly) and eight gcl germ line-less (one ovary per fly) females were tested (figure 1).
Figure 1.
Survivorship of virgin germ cell-less, tudor and genetically matched control males and females against age in days since the emergence of the adult from the pupae. Survivorship is the proportion of the original cohort surviving to a given day. (a) gcl virgin females and controls (the latter being the offspring of gcl heterozygote (1) or homozygote (2) mothers possessing a germ line). (b) tud virgin females and controls. (c) gcl virgin males and controls. (d) tud virgin males and controls.
(ii) Ovary staining of females lacking a germ line
We examined the structure of mutant ovaries by dissection and analysis of specific reproductive tissues following chemical or antibody staining. We tested for the absence of the germ line, and for any abnormalities in the structure of the somatic gonad arising from the somatic stem cells (the proliferating somatic cells) in tud and gcl females. Ovaries were visualized using DAPI, which stains all cell nuclei, vasa antibody, which stains all germ line derived tissue (Hay et al. 1990) and fasciclinIII (FasIII) antibody, which exclusively stains somatic cells derived from the somatic stem cells within the germarium (region 2b, see figure 3b; Patel et al. 1987). Monoclonal rabbit anti-vasa antibody was donated by Dr C. Extavour (University of Cambridge). The monoclonal mouse 7G10 anti-Fasciclin antibody, developed by C. Goodman, was obtained from the Developmental Studies Hybridoma Bank (University of Iowa—developed under the NICHD). Ovaries were dissected in PBS (10 mM phosphate buffer, 2.7 mM KCl, 137 mM NaCl, pH 7.4) and fixed in 4% paraformaldehyde in PBS for 30 min at room temperature. Dissected ovaries were washed several times in 0.2 M phosphate buffer (NaH2PO4/Na2HPO4) and pre-incubated in TNT (0.1 M Tris–HCl/0.3 M NaCl (pH 7.4), 0.5% Triton X-100) supplemented with 4% normal donkey serum (Jackson Immunoresearch Laboratories). Immunohistological staining was performed with the following antisera: monoclonal mouse 7G10 anti-fasciclin III at a 1 : 20 dilution; monoclonal rabbit anti-vasa antibody at a 1 : 1000 dilution. Texas Red labelled mouse and rabbit secondary antibodies (Jackson Immunoresearch Laboratories) were used at a 1 : 200 dilution from a 1 mg ml−1 stock. DAPI (Sigma) was used as a counter-stain at a final concentration of 0.5 mg ml−1 immediately after incubation with the secondary antibody. Following staining, all samples were mounted in 2% n-propyl gallate in 80% glycerol/20% 0.2 M phosphate buffer for microscopy. Samples were viewed with a Zeiss Axioskop 2 plus compound microscope and a Zeiss LSM 510 confocal microscope, and images stored and viewed using Zeiss LSM software.
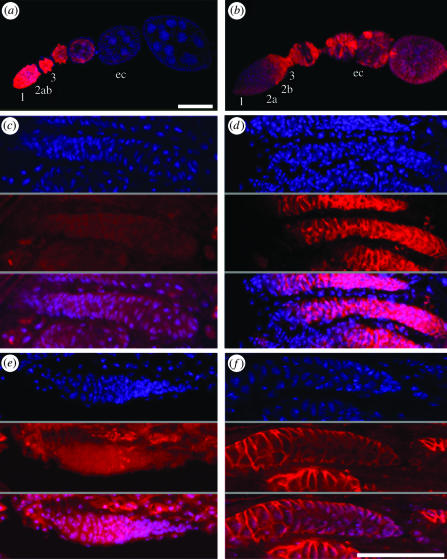
Figure 3.
Germ line-less and WT female ovarioles. Ovarioles are from (a, b) WT, (c, d) gcl germ line-less and (e, f) tud germ line-less females. Each ovariole is shown singly stained with DAPI (top, blue), either (a, c, e) vasa or (b, d, f) FasIII (middle, red) and with the two stains combined (bottom, red and blue). Normal ovarioles develop from the anterior end, which is to the left in all images. Germ stem cells proliferate in region 1, and somatic stem cells are on the border between regions 2a and 2b. In region 3 the somatic cells surround the germ cells to form a nascent egg chamber (EC). (a) Vasa staining (red) indicates the presence of germ cells throughout the early WT ovariole. Vasa staining is completely absent in (b) gcl and (c) tud germ line-less flies. (b) FasIII staining (red) indicates somatically derived cells proliferating posterior of the 2a/2b border. They are confined to the poles of later egg chambers. In (e) gcl and (f) tud germ line-less flies, FasIII staining reaches the anterior end of the germarium, indicating that the whole of the ovariole has become an area of somatic stem cell proliferation (effectively equivalent to the WT region 2b). The white scale bars represent (a–d) 50 μm or (e, f) 25 μm.
The volume of FasIII expressing cells in control and gcl germ line-less ovary germaria was assessed by staining ovaries with FasIII, and viewing them as three-dimensional ‘z stack’ images by confocal microscopy. Ovaries were removed from virgin female gcl germ line-less and control flies that were less than 4 days old by dissection in PBS under light microscopy. Flies had previously been kept at low density on 1.0 SY food. Three germaria per ovary were selected for measurement. Objective, image size and laser power were constant throughout the measurements. The stacked images were then imported into Imaris software (Bitplane AG, Zurich), and a threshold was set to eliminate background fluorescence. Any fluorescence above the threshold on the confocal image was incorporated into the measurements of volume, any below was excluded. The fluorescence threshold was set by reading the background fluorescence (i.e. outside of the germaria) and the peak fluorescence (i.e. inside the germaria), and setting a threshold at 30% of the difference between the two. The 30% threshold for eliminating background fluorescence was the value, which gave consistency between the three-dimensional images of germaria from confocal microscopy and the three-dimensional shapes from which FasIII volume was measured. The same 30% threshold value was applied across all samples. Once the three-dimensional images had been constructed from the stack images, germaria volumes were measured using Imaris software. FasIII expressing tissue in the germarium was measured in nine gcl germ line-less and seven control flies, and three germaria per fly were assessed (apart from a single control fly, in which only two germaria could be analysed).
(d) Statistics
Survival analysis was performed using log-rank tests (Peto & Peto 1972) and structural analyses using analyses of variance/t-tests. Nested analyses of variance were used where multiple structures had been measured within a single individual. The relationship between abdominal segment deletions and longevity in tud flies was assessed using a Wilcoxon rank sum test. All analyses were performed in JMP 5.0 statistical software (SAS Institute Inc.).
3. Results
(a) Longevity of gcl and tud virgin males and females
gcl germ line-less virgin females were significantly shorter lived than control females derived from heterozygote parents (n=197, , p<0.0001) but not significantly different in longevity from control females derived from homozygous mothers (n=75, χ2=2.30, p=0.12). The two control groups were not significantly different in longevity from one another (median longevities=45 and 42 days, respectively; log rank test: , p=0.069) and we therefore combined them to increase the sample size. Analysis using these data showed that the gcl germ line-less virgin females were significantly shorter lived than the combined controls (combined data: germ line-less median=38 days, control median=44 days; log rank test: , p<0.0001; figure 1a).
tud germ line-less virgin females were significantly shorter lived than virgin controls (germ line-less median=57 days, control median=71 days; log rank test: , p<0.0001; figure 1b).
We tested for an association between longevity and abdominal segment number in the tud flies, to assess any potential confounding effect of the abdominal segment pleiotropy. There was no significant relationship between the number of abdominal deletions and the longevity of tud flies of either sex (Wilcoxon rank sum test: males: , p=0.08; females: , p=0.57; table 1).
Table 1.
Relationship between the number of abdominal segments present in germ cell-less tud individuals, and longevity.
| sex | segments present | n | median lifespan (days) | 25% interval | 75% interval | χ2 | p value |
|---|---|---|---|---|---|---|---|
| male | 6 | 4 | 60.5 | 57.25 | 67.5 | 5.09 | 0.08 |
| 7 | 16 | 56.5 | 50 | 69 | |||
| 8 | 39 | 65 | 60 | 69 | |||
| female | 5 | 1 | 44 | 44 | 44 | 2.08 | 0.56 |
| 6 | 5 | 54 | 39.5 | 62.5 | |||
| 7 | 22 | 56 | 50.5 | 63.25 | |||
| 8 | 38 | 57 | 53 | 60 |
The longevity of gcl germ line-less virgin males was not significantly different compared to that of gcl control males (germ line-less median=40 days, control median=40 days; log rank test: , p=0.10; figure 1c). However, tud germ line-less male virgins showed a significant (8.6%) extension in longevity compared to controls (germ line-less median=58 days, control median=63 days; log rank test: , p=0.002; figure 1d).
(b) Analysis of the reproductive structures of female germ line-less flies
(i) Ovariole number in gcl females
Ovariole number did not differ significantly between gcl germ line-less and control females (nested ANOVA: d.f.=1, F=2.6909, p=0.1158; table 2). Hence, removal of the germ line in females had no detectable effect on the number of ovarioles that were formed by the somatic gonad.
Table 2.
Analysis of gonad structure in gcl germ line-less and wild-type flies. (Figures shown are means±s.e.)
| gonad type | ovariole number per ovary | germarium region 2b volume (μm3) |
|---|---|---|
| gcl germ line present | 16.1±0.49 | 4623±1698 |
| gcl germ line less | 17.8±0.70 | 16 230±1677 |
| test statistic | F=2.69 | F=20.13 |
| p value | p=0.116 | p=0.0005 |
(ii) Ovary staining of females lacking a germ line
Nascent ovarioles were clearly visible at the anterior pole of germ line-less ovaries with DAPI staining (figure 2d–f). Vasa staining was clearly visible in the germaria and early egg chambers of WT ovaries. As expected, no vasa staining above background levels was detectable in gcl or tud germ line-less ovaries, confirming the absence of the germ line in these flies (figure 3a,c,e). However, the area of proliferating somatic cells within germarium region 2b, as indicated by FasIII expressing cells in the germarium, showed very different patterning between WT and germ line-less ovarioles (figure 3b,d,f). The total volume of the proliferative region was significantly larger in gcl germ line-less females (mean=16 230 μm3) than in WT controls (mean=4665 μm3; nested ANOVA: d.f.=1, F=20.1317, p=0.0005; table 2).
Figure 2.

Germ line-less and WT adult gonads. (a–c): male reproductive organs from (a) WT, (b) gcl germ line-less and (c) tud germ line-less males. tst, testes; vd, vas deferens; ag, accessory gland; ed, ejaculatory duct. The testes are greatly reduced in size in germ line-less flies, but accessory gland size is unaffected (see text). (d–f): paired ovaries from (d) WT, (e) gcl germ line-less and (f) tud germ line-less females. (d) is viewed directly under light microscopy (e) and (f) have been DAPI stained. a, anterior; p, posterior. Ovariole structure is clearly visible at the anterior end of all ovaries, but the mature eggs, which make up the bulk of the WT ovary, are lacking in the germ line-less females.
We did not perform similar quantification on tudor ovaries because of difficulties with the stock. However, we have visualized tudor ovaries with DAPI, vasa and FasIII staining, and their structure is very similar to gcl ovaries, including an apparent over-proliferation of FasIII expressing cells in the germaria (see figures 2f and 3f).
4. Discussion
We tested the effect on adult male and female lifespan of ablating the germ line tissue in D. melanogaster. The most important finding was that there was little or no longevity extension in the absence of the germ line. Female flies lacking a germ line showed reduced longevity relative to controls. Male flies lacking a germ line showed either no difference or a small, but significant, increase in longevity as compared to controls. These effects were manifest in mutants in two different genetic backgrounds. gcl and tud females lacked a germ line and any mature egg chambers as expected, and did not differ significantly from WT females in ovariole number. However, we found that the volume of proliferating (FasIII expressing) somatic cells in the ovary germaria was much greater in germ line-less than in WT ovaries. Our results, both in terms of the lack of longevity extension and the gonad structure contrast to the findings reported for C. elegans (see below).
The finding that the abolition of the germ line and hence removal of egg laying in females did not lead to an extension in longevity also contrasts with the widely held view that the production and laying of eggs is necessarily costly to females. For example, in D. subobscura a grandchildless mutant with a germ line-less phenotype (Spurway 1948) was reported to show extended lifespan relative to virgin controls (Maynard Smith 1958). However, there is some doubt about this interpretation as grandchildless shows incomplete penetrance (much like gcl) and it is not clear what proportion of females had a functioning germ line. There were also no controls for genetic background, sample sizes were very small (less than 30 flies) and no statistical analyses were performed. More recently, Sgró & Partridge (1999) showed that eliminating egg laying in females via X-irradiation or by introduction of the ovoD mutation, had either no effect on, or decreased, mortality. Increasing evidence suggests that interventions that extend longevity may not always have correlated effects on fecundity (e.g. Rogina et al. 2000; Simon et al. 2003; Hwangbo et al. 2004; Wood et al. 2004) and hence can be manifested even in sterile females (Clancy et al. 2001; Mair et al. 2004). These findings lead to the hypothesis that it is not egg production per se that is costly, but the specific signalling processes that contribute to the enabling of reproduction, including egg production. Changes in the magnitude of the cost of reproduction generated by experimental manipulations will, therefore, depend on the way these enabling processes are disrupted, regardless of the ultimate effect on egg laying itself.
Although the lack of an extension in longevity in females with no germ line could be explained by deleterious pleiotropic effects of the tud and gcl mutations, the data do not support this interpretation. Even though germ line-less tud flies exhibited abdominal defects, including deleted abdominal segments, the severity of the effect (scored as the number of abdominal segments missing) did not correlate with lifespan (table 1). This suggests that the pleiotropic effects of tud were not important in determining longevity. gcl has no known phenotypes other than its effect on germ tissue (Robertson et al. 1999).
The longevity results for males contrasted with those for females. Males lacking a germ line showed either no difference in longevity relative to controls (gcl), or slightly, but significantly, increased longevity (tud). It should be noted that the male gcl germ line-less groups included about 25% of flies that possessed a germ line, and this could have minimized small increases in longevity in these males. In addition, any pleiotropic effects of the mutations could be less severe than in females. Ablation of the germ line appeared to lead to a slowing down in mortality late in life and an extension of maximum (but not necessarily median) lifespan. This tendency for a late slowing of late age mortality is indicated in three replicates of lifespan studies for both gcl and tud males (data not shown).
The removal of the germ line in gcl (and probably tudor) females led to an over-proliferation in the FasIII expressing cells in the somatic gonad, although ovariole number itself was unchanged. A similar phenotype has previously been observed in another agametic mutant oskar (Margolis & Spradling 1995). These observations support the idea that, in a normal ovariole, an intimate association exists between proliferating somatic and germ line tissue, including, presumably, molecular signals passed between them. We did not test whether there is a similar over-proliferation of the somatic cyst cells in the testes of germ line lacking males, although the size of the major part of the somatic gonad (i.e. the accessory glands) is unaffected following germ line removal (figure 2a–c). The possibility that differences in proliferation of parts of the somatic gonad could explain sex differences in the response of lifespan to germ line removal remains to be tested.
Our results suggest that the negative relationship between reproduction and longevity seen in flies (Rose 1984; Partridge et al. 1987; Chapman et al. 1995) is controlled by a different signalling pathway than that seen in the worm (Hsin & Kenyon 1999). A marked difference in the worm and fly reproductive systems concerns the role and activity of somatically derived cells. In the worm, somatic cells proliferate only during development, and once the adult reproductive structures have been formed, they cease mitosis. Somatic (‘distal tip’) cells signal to control germ cell proliferation, but do not themselves actively proliferate (Hirsch et al. 1976; Kimble & White 1981; Hansen et al. 2004). In the fly, sperm and eggs are formed by proliferating germ line tissue, which becomes surrounded by proliferating somatic tissue (cyst cells for sperm, follicle cells for eggs) which is the product of the somatic stem cells (reviewed in Spradling 1993). In flies therefore, the activity of some somatic tissue mirrors that of germ tissue and could also be a potential source of information to the fly about its level of reproductive activity. Somatic gonad tissue is transcriptionally active in females (Roth 2001; Lin 2002), suggesting that it may be a likely candidate for a reproductive signal in the fly. Our observation that somatic tissue over-proliferates in germ line-less females, could support a model in which reproductive status in females is signalled via the proliferating soma, and not the proliferating germ line as in worms and as generally predicted. This could explain the reduction in lifespan in germ line-less female flies. Interestingly, irradiation (which blocks both somatic and germ cell proliferation in the ovary) has little or no affect on patterns of mortality in WT flies (Sgró & Partridge 1999), perhaps suggesting that removal of both types of reproductive tissue eliminates all reproductive signalling and returns the flies to a default rate of ageing. This interpretation concurs with the results of whole gonad ablations in C. elegans (Hsin & Kenyon 1999). The increased mortality observed in irradiated flies selected for decreased reproductive rate (Sgró & Partridge 1999), suggests either that the scheduling of reproduction is important in determining trade-offs with longevity, or that the selected flies showed increase sensitivity to the deleterious effects of irradiation.
There are also sex differences in the response to the removal of the germ line between the worm and fly. Worms are either male or hermaphroditic whereas flies are male or female. The differences between male and female flies found in our study could indicate that signalling pathways differ in response to the production of different gamete types. However, germ line ablation extends lifespan in both male and hermaphroditic worms (Hsin & Kenyon 1999). In addition, mutants in which hermaphrodites produce either only sperm (daz-1) or eggs (fem-3, fog-1, fog-2, fog-3) are reported to have a normal lifespan (Kenyon et al. 1993; Hsin & Kenyon 1999). The sex specific effect we saw in germ line ablated flies is therefore further evidence for a lack of conservation in fly and worm signalling pathways.
Previous work has shown that reproduction is a costly process, because it increases mortality early in life (Sgró & Partridge 1999). Our results suggest that the abolition of potentially costly reproductive processes such as egg laying may not inevitably eliminate reproductive costs if upstream signalling processes are left intact (e.g. somatic gonad signalling). The comparison of our results with those of the worm also suggest that variation in life history could partly be due to variation in the underlying proximate mechanisms involved. More work is clearly needed on identifying the proximate signalling mechanisms at work in determining the trade-offs between life history traits.
Acknowledgments
This work was supported NERC (research grant to T.C. and L.P.), the Wellcome Trust (programme grant to L.P.), BBSRC (Professorial Fellowship to L.P.) and the Royal Society (University Research Fellowship to T.C.). We thank Mariana Wolfner and Tom Jongens for providing flies, Cassandra Extavour for providing antibody, Claire Cronmiller, Su Broughton and Martin Sikora for advice about antibody staining and microscopy, Prof. Michael Duchen (UCL—Department of Physiology) for the use of the confocal microscope, and Daniel Cianter (UCL—Department of Anatomy) for assistance with three-dimensional imaging.
References
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line cells in Caenorhabditis elegans. Science. 2002;295:502–505. doi: 10.1126/science.1065768. 10.1126/science.1065768 [DOI] [PubMed] [Google Scholar]
- Barnes A.I, Partridge L. Costing reproduction. Anim. Behav. 2003;66:199–204. 10.1006/anbe.2003.2122 [Google Scholar]
- Bell P.D, Koufopanou V. The cost of reproduction. In: Dawkins R, editor. Oxford surveys of evolutionary biology. Oxford University Press; Oxford, UK: 1985. pp. 83–131. [Google Scholar]
- Blüher M, Kahn B.B, Kahn C.R. Extended longevity in mice lacking he insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. 10.1126/science.1078223 [DOI] [PubMed] [Google Scholar]
- Boswell R.E, Mahowald A.P. tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster. Cell. 1985;43:97–104. doi: 10.1016/0092-8674(85)90015-7. 10.1016/0092-8674(85)90015-7 [DOI] [PubMed] [Google Scholar]
- Brown-Borg H.M, Borg K.E, Meliska C.J, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. 10.1038/384033a0 [DOI] [PubMed] [Google Scholar]
- Calow P. The cost of reproduction—a physiological approach. Biol. Rev. 1979;54:23–40. doi: 10.1111/j.1469-185x.1979.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Chapman T, Liddle L.F, Kalb J.M, Wolfner M.F, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. 10.1038/373241a0 [DOI] [PubMed] [Google Scholar]
- Charlesworth B. Selection in populations with overlapping generations V. Natural selection and life histories. Am. Nat. 1973;107:303–311. 10.1086/282832 [Google Scholar]
- Charlesworth B. Cambridge University Press; Cambridge, UK: 1980. Evolution in age-structured populations. [Google Scholar]
- Clancy D.J, Gems D, Harshman L.G, Oldham S, Stocker H, Hafen E, Leevers S.J, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. 10.1126/science.1057991 [DOI] [PubMed] [Google Scholar]
- Dillin A, Crawford D.K, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240. 10.1126/science.1074240 [DOI] [PubMed] [Google Scholar]
- Fisher R.A. 1st ed. Clarendon Press; Oxford: 1930. The genetical theory of natural selection. [Google Scholar]
- Flurkey K, Papaconstantinou J, Harrison D.E. The Snell dwarf mutation Pit1(dw) can increase life span in mice. Mech. Ageing Dev. 2002;123:121–130. doi: 10.1016/s0047-6374(01)00339-6. 10.1016/S0047-6374(01)00339-6 [DOI] [PubMed] [Google Scholar]
- Fowler K, Partridge L. A cost of mating in female fruit flies. Nature. 1989;338:760. 10.1038/338760a0 [Google Scholar]
- Gems D, Partridge L. Insulin/IGF signalling and ageing: seeing the bigger picture. Curr. Opin. Genet. Dev. 2001;11:287–292. doi: 10.1016/s0959-437x(00)00192-1. 10.1016/S0959-437X(00)00192-1 [DOI] [PubMed] [Google Scholar]
- Gems D, Sutton A.J, Sundermeyer M, Albert P.S, King K.V, Edgley M, Larsen P.L, Riddle D.L.L. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W.D. The moulding of senescence by natural selection. J. Theor. Biol. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. 10.1016/0022-5193(66)90184-6 [DOI] [PubMed] [Google Scholar]
- Hansen D, Hubbard E.J.A, Schedl T. Multi-pathway control of the proliferation versus meiotic development decision in the Caenorhabditis elegans germline. Dev. Biol. 2004;268:342–357. doi: 10.1016/j.ydbio.2003.12.023. 10.1016/j.ydbio.2003.12.023 [DOI] [PubMed] [Google Scholar]
- Hay B, Jan L.Y, Jan Y.N. Localization of vasa, a component of Drosophila polar granules, in maternal-effect mutants that alter embryonic anteroposterior polarity. Development. 1990;109:425–433. doi: 10.1242/dev.109.2.425. [DOI] [PubMed] [Google Scholar]
- Hirsch D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev. Biol. 1976;49:200–219. doi: 10.1016/0012-1606(76)90267-0. 10.1016/0012-1606(76)90267-0 [DOI] [PubMed] [Google Scholar]
- Holmes D.J, Fluckiger R, Austad S.N. Comparitive biology of aging in birds: an update. Exp. Gerontol. 2001;36:869–883. doi: 10.1016/s0531-5565(00)00247-3. 10.1016/S0531-5565(00)00247-3 [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even P.C, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. 10.1038/nature01298 [DOI] [PubMed] [Google Scholar]
- Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. 10.1038/20694 [DOI] [PubMed] [Google Scholar]
- Hwangbo D.S, Gersham B, Tu M.P, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. 10.1038/nature02549 [DOI] [PubMed] [Google Scholar]
- Johnson T.E, Tedesco P.M, Lithgow G.J. Comparing mutants, selective breeding, and transgenics in the dissection of aging processes of Caenorhabditis elegans. Genetica. 2003;91:65–77. doi: 10.1007/BF01435988. 10.1007/BF01435988 [DOI] [PubMed] [Google Scholar]
- Jongens T.A, Hay B, Jan L.Y, Jan Y.N. The germ cell-less gene product: a posteriorly localized component necessary for germ cell development in Drosophila. Cell. 1992;70:569–584. doi: 10.1016/0092-8674(92)90427-e. 10.1016/0092-8674(92)90427-E [DOI] [PubMed] [Google Scholar]
- Jongens T.A, Ackerman L.D, Swedlow J.R, Jan L.Y, Jan Y.N. Germ cell-less encodes a cell type-specific nuclear pore-associated protein and functions early in the germ-cell specification pathway. Genes Dev. 1994;4:905–921. doi: 10.1101/gad.8.18.2123. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tablang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. 10.1038/366461a0 [DOI] [PubMed] [Google Scholar]
- Kimble J.E, White J.G. On the control of germ cell development in Caenorhabditis elegans. Dev. Biol. 1981;81:208–219. doi: 10.1016/0012-1606(81)90284-0. 10.1016/0012-1606(81)90284-0 [DOI] [PubMed] [Google Scholar]
- Kirkwood T.B.L. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. 10.1038/270301a0 [DOI] [PubMed] [Google Scholar]
- Law R. Optimal life histories under age-specific predation. Am. Nat. 1979;113:3–16. 10.1086/283361 [Google Scholar]
- Leroi A.M. Molecular signals versus the Loi de Balancement. Trends Ecol. Evol. 2001;16:24–29. doi: 10.1016/s0169-5347(00)02032-2. 10.1016/S0169-5347(00)02032-2 [DOI] [PubMed] [Google Scholar]
- Levins R. Princeton University Press; Princeton: 1968. Evolution in changing environments. [Google Scholar]
- Lin H. The stem-cell niche theory: lessons from flies. Nat. Rev. 2002;3:931–940. doi: 10.1038/nrg952. 10.1038/nrg952 [DOI] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signalling. Nat. Genet. 2001;28:139–145. doi: 10.1038/88850. 10.1038/88850 [DOI] [PubMed] [Google Scholar]
- Mair W, Sgró C.M, Johnson A.P, Chapman T, Partridge L. Lifespan extension by dietary restriction in female Drosophila melanogaster is not caused by a reduction in vitellogenesis or ovarian activity. Exp. Gerontol. 2004;39:1011–1019. doi: 10.1016/j.exger.2004.03.018. 10.1016/j.exger.2004.03.018 [DOI] [PubMed] [Google Scholar]
- Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. The effects of temperature and of egg-laying on the longevity of Drosophila subobscura. J. Exp. Biol. 1958;35:832–842. [Google Scholar]
- Partridge L, Harvey P.H. Evolutionary biology—costs of reproduction. Nature. 1985;316:20. [Google Scholar]
- Partridge L, Green A, Fowler K. Effects of egg-production and of exposure to males on female survival in Drosophila melanogaster. J. Insect Physiol. 1987;33:745–749. 10.1016/0022-1910(87)90060-6 [Google Scholar]
- Partridge L, Gems D, Withers D.J. Sex and death: what is the connection. Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. 10.1016/j.cell.2005.01.026 [DOI] [PubMed] [Google Scholar]
- Patel N.H, Snow P.M, Goodman C.S. Characterization and cloning of fasciclin III: a glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell. 1987;48:975–988. doi: 10.1016/0092-8674(87)90706-9. 10.1016/0092-8674(87)90706-9 [DOI] [PubMed] [Google Scholar]
- Peto R, Peto J. Asymptotically efficient rank invariant procedures. J. R. Stat. Soc. A. 1972;135:185–207. [Google Scholar]
- Read A.R, Harvey P.H. Life history differences among the eutherian radiations. J. Zool. 1989;219:329–353. [Google Scholar]
- Reznick D.N. Costs of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. [Google Scholar]
- Riddle D.L. Ageing—a message from the gonads. Nature. 1999;399:308–309. doi: 10.1038/20557. 10.1038/20557 [DOI] [PubMed] [Google Scholar]
- Robertson S.E, Dockendorff T.C, Leatherman J.L, Faulkner D.L, Jongens T.A. germ cell-less is required only during the establishment of the germ cell lineage of Drosophila and has activities which are dependent and independent of its localization to the nuclear envelope. Dev. Biol. 1999;215:288–297. doi: 10.1006/dbio.1999.9453. 10.1006/dbio.1999.9453 [DOI] [PubMed] [Google Scholar]
- Rogina B, Reenan R.A, Nilsen S.P, Helfand S.L. Extended life-span conferred by cotransporter gene mutations in Drosophila. Science. 2000;290:2137–2140. doi: 10.1126/science.290.5499.2137. 10.1126/science.290.5499.2137 [DOI] [PubMed] [Google Scholar]
- Rose M.R. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution. 1984;38:1004–1010. doi: 10.1111/j.1558-5646.1984.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Roth S. Drosophila oogenesis: coordinating the germ line and soma. Curr. Biol. 2001;11:R779–R781. doi: 10.1016/s0960-9822(01)00469-9. 10.1016/S0960-9822(01)00469-9 [DOI] [PubMed] [Google Scholar]
- Sgró C.M, Partridge L. A delayed wave of death from reproduction in Drosophila. Science. 1999;286:2521–2524. doi: 10.1126/science.286.5449.2521. 10.1126/science.286.5449.2521 [DOI] [PubMed] [Google Scholar]
- Sibly R.W, Calow P. Blackwell Scientific; Oxford: 1986. Physiological ecology of animals. [Google Scholar]
- Simon A.F, Shih C, Mack A, Benzer S. Steroid control of longevity in Drosophila melanogaster. Science. 2003;299:1407–1410. doi: 10.1126/science.1080539. 10.1126/science.1080539 [DOI] [PubMed] [Google Scholar]
- Spradling A.C. Developmental genetics of oogenesis. In: Bate M, Martinez-Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbour Laboratory Press; New York: 1993. [Google Scholar]
- Spurway H. Genetics and cytology of Drosophila subobscura IV. An extreme example of delay in gene action causing sterility. J. Genet. 1948;49:126–140. doi: 10.1007/BF02986830. [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Tatar M, Carey J.R. Nutrition mediates reproductive trade-offs with age-specific mortality in the beetle Callosobruchus maculatus. Ecology. 1995;76:2066–2073. [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu M.-P, Yin C.-M, Garofalo R.S. A mutant Drosophila insulin receptor homolog that exrtends life-span and impairs nueroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. 10.1126/science.1057987 [DOI] [PubMed] [Google Scholar]
- van Noordwijk A.J, de Jong G. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 1986;128:137–142. [Google Scholar]
- Williams G.C. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 1966;100:687–690. 10.1086/282461 [Google Scholar]
- Wood J.G, Rogina B, Lavu S, Howitz K, Helfand S.L, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. 10.1038/nature02789 [DOI] [PubMed] [Google Scholar]