Abstract
Paedocypris is a new genus of paedomorphic cyprinid fish from highly acidic blackwater peat swamps in Southeast Asia. It includes two new species, one of which (Paedocypris progenetica) appears to be the smallest fish and vertebrate known, with the smallest mature female measuring a mere 7.9 mm. Paedocypris has many ’larval’ features typically associated with paedomorphic fish (e.g. narrow frontals that leave the brain unprotected dorsally by bone and a precaudal larval-fin-fold), but, uniquely among fishes, males also possess highly modified pelvic fins with hypertrophied muscles and a keratinized pad in front of the pelvic girdle, which, we hypothesize, function together as a clasping or holding device, thereby suggesting an unusual reproductive mode. Unfortunately, habitat destruction jeopardizes the survival of these fishes and thus opportunities for further research.
Keywords: Paedocypris, Cyprinidae, peat swamps, smallest vertebrate, sexual dimorphism
1. Introduction
Miniaturization is an evolutionary phenomenon observed in all vertebrate lineages, but it is more frequently encountered in fishes. Fish species maturing at sizes under 20 mm have been termed ‘miniature fishes’ by Weitzman & Vari (1988), who observed that miniaturization is commonly accompanied by a reduction of the laterosensory canal system of the head and body and reductions in the number of fin rays and body scales. Often, it is also combined with a poorly ossified, largely cartilaginous skeleton or a complete loss of bones (Johnson & Brothers 1993; Britz & Kottelat 2003).
We here describe two new species representing a new genus of miniature fishes (Paedocypris) from Southeast Asian peat swamp forests. In contrast to the general trend to simplification observed in miniature fishes, Paedocypris has evolved complex structural novelties in the pelvic girdle. Further, one of the new species, Paedocypris progenetica (figure 1), is the smallest recorded vertebrate, with a fully mature female measuring just 7.9 mm.
Figure 1.
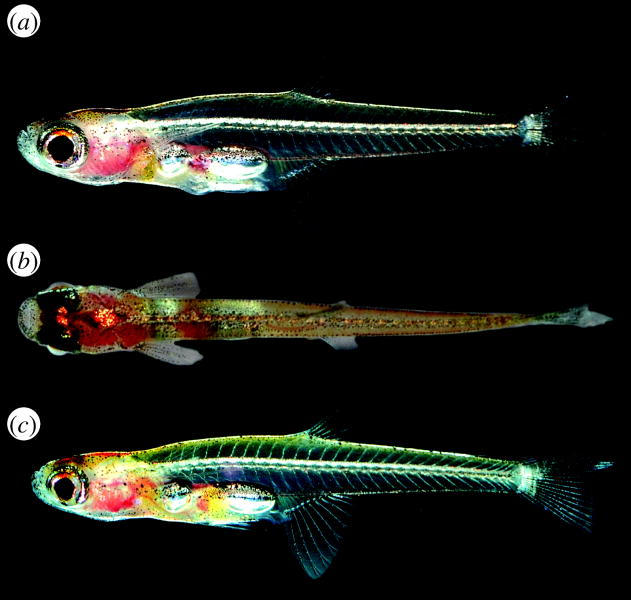
Living Paedocypris progenetica, CMK 18496, (a, b) male, ca 9 mm; (c) female, ca 8.8 mm.
2. Material and methods
All sizes in millimetres are standard length (from tip of lower jaw to end of hypural complex). Measurements were made at 10× magnification using a stereomicroscope equipped with an ocular micrometre calibrated with a microscope slide graduated to 0.01 mm. Sexual maturity was determined by dissection and/or examination with transmitted light. Selected specimens were cleared and double stained according to the protocol of Taylor & van Dyke (1985) (abbreviations as in table 1).
Table 1.
Abbreviations.
| BMNH | Natural History Museum, London |
| CMK | collection of the first author |
| MZB | Research and Development Centre for Biology, Indonesian Institute of Science, Cibinong |
| SM | Sarawak Museum, Kuching |
| ZRC | Raffles Museum of Biodiversity Research, National University of Singapore |
In accordance with Article 8.6 of the International Code of Zoological Nomenclature, copies of the PDF file of this work have been deposited in the following five publicly accessible libraries: 1. Raffles Museum of Biodiversity Research, Singapore; 2. Natural History Museum, London; 3. Muséum d'Histoire Naturelle, Genéve; 4. California Academy of Sciences, San Francisco; 5. National Museum of Natural History, Smithsonian Institution, Washington D.C.
3. Results
(a) Paedocypris gen. nov.
(i) Type species
Paedocypris progenetica sp. nov.
(ii) Diagnosis
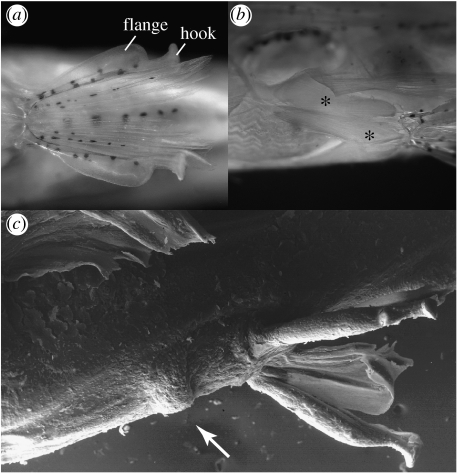
The following characters of Paedocypris are unique among fishes: outermost pelvic-fin ray of male highly modified with ventral hemitrich greatly expanded, flattened, supporting keratinized skin-pad and tip of dorsal hemitrich supporting small hook-like projection of keratinized skin directed outwards (figure 3a); abductor and ventral arrector muscles of pelvic girdle of male hypertrophied (figure 3b), the former attached to ventral extremity of os suspensorium (versus not hypertrophied and abductor muscle restricted to basipterygia); pad of keratinized skin in front of pelvic fin in male (figures 2a,c and 3a,c). The following characters are unique within Cypriniformes: presence in adults (versus only in larvae) of a long post-anal larval-fin-fold along ventral edge of caudal peduncle, from posterior extremity of anal-fin base to caudal-fin base (figure 2); basipterygium of male hypertrophied (versus not hypertrophied) (figure 4); abdominal vertebrae 7–13 with short haemal spines (versus haemal spines only on caudal vertebrae); genital papilla of male hypertrophied, in form of small bag surrounding and including anterior 2 or 3 anal-fin rays, posteriorly confluent with fin membrane (figure 2c); pharyngeal teeth tricuspid.
Figure 3.
(a) Paedocypris micromegethes, paratype male, ZRC 49869, 10.4 mm; pelvic fins, anteroventral view, showing hook and flange on anterior ray. (b) Paedocypris micromegethes, paratype, male, BMNH 2004.11.16.1-40, 10.9 mm, ventrolateral view on hypertrophied pelvic arrector and abductor muscles marked by asterisk symbols. (c) Paedocypris progenetica, paratype male, ZRC 43199, 8.5 mm, scanning electronic micrograph of pelvic region in ventrolateral view, arrow points to keratinized prepelvic knob.
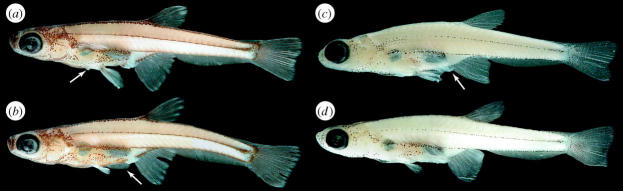
Figure 2.
(a) Paedocypris progenetica holotype male, MZB 5998, 8.6 mm; arrow points to keratinized abdominal knob; (b) Paedocypris progenetica paratype female, ZRC 43199, 8.0 mm; arrow points to preanal larval-fin-fold; (c) Paedocypris micromegethes holotype male, ZRC 49869, 10.4 mm; arrow points to genital papilla; (d) paratype female, BMNH 2004.11.16.1-40, 10.0 mm.
Figure 4.
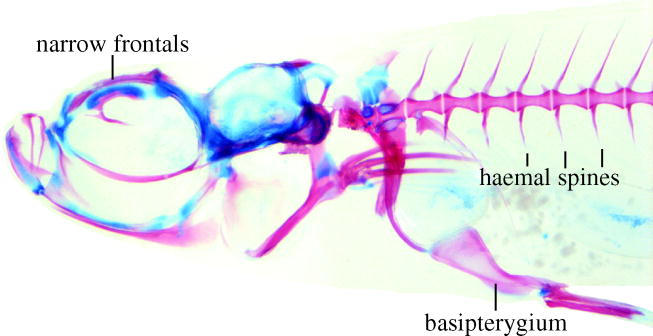
Paedocypris micromegethes, cleared and double stained paratype, male, BMNH 2004.11.16.41-60, 10.7 mm, cartilage in blue, bone in red, note largely cartilaginous roofless skull, haemal spines on abdominal vertebrae and hypertrophied basipterygium of pelvic girdle.
The following characters are also diagnostic, although not unique to the genus: miniature adult size (maximum 10.5 mm); males with large conical tubercles along dentary, preopercle/interopercle, subopercle/opercle, frontal and lachrymal; caudal peduncle very slender; dorsal-fin rays ii,3,ii or ii,4,i; anal-fin rays iii,5,ii, iii,6,i, iii,5,iii, iii,6,ii or iii,7,i; caudal-fin slightly emarginate, with 14 branched rays; 5 pelvic-fin rays; 8 pectoral-fin rays; 12−13 abdominal+21−23 caudal=33−35 vertebrae; absence of the following bones and cartilages in the neurocranium (parietals, nasals, vomer, preethmoid, circumorbitals (except lachrymal)), in the lower jaw (angular), in the upper jaw (bony kinethmoid), in the hyopalatine arch (ectopterygoid), in the shoulder girdle (post-temporal, post-cleithrum, cartilaginous distal pectoral radials) and in the axial skeleton (supraneural 2, epural); frontals narrow leaving the brain exposed; intercalarium reduced to a splint; basibranchials, ceratobranchials, epibranchials and pharyngobranchials not ossified, except ceratobranchials 4 and 5; proximal–middle radials of dorsal and anal-fins remain mainly cartilaginous; scales absent. Coloration in life: translucent orange; males with orange iridescent spot between eyes and one on nape; preserved, yellowish grey with black pigments organized as in figures 1 and 2.
(iii) Etymology
Paideios (Greek), children; Cypris (Greek), Venus, common suffix for cyprinid genera; gender feminine.
(b) Paedocypris progenetica sp. nov.
(i) Material
Holotype: see figures 1 and 2a,b. MZB 5998, male, 8.6 mm; Sumatra: Jambi Province: peat swamp, 15 km from Muara Sabak on road to Jambi; 1°14′17.8″ S, 103°35′56.8″ E; coll. H. H. Tan et al., 22 November 1996. Paratypes: MZB 5999, 12 specimens; ZRC 43199, 54 specimens; 10 males, 8.0–9.6 mm, 56 females, 5.8–8.3 mm; same data as holotype. ZRC 43130, 2 males, 8.8–9.1 mm, 4 females, 7.9–9.4 mm; Sumatra: Jambi Province: Bayou Rantau Panjang; 1°23′03.1″ S, 103°55′10.7″ E; coll. H. H. Tan, 28 November 1996. CMK 11260, 11 males, 8.1–10.1 mm, 17 females, 7.8–10.3 mm; Sumatra: Jambi Province: swamp near Pematang Lumut, 40 km before Kuala Tungkal on road to Jambi (95 km) and Simpangtuan (36 km); coll. M. Kottelat, 7 June 1994.
(ii) Diagnosis
Paedocypris progenetica is distinguished from Paedocypris micromegethes by: a smaller maximum size (male 9.8 mm, versus 11.6; female 10.3 mm, versus 11.4), keratinized pad in front of male pelvic fins as a projecting knob (versus flat to slightly swollen), breeding tubercles on the dentary arranged in a straight row, without forming a cluster (versus forming a cluster of up to five closely set individual tubercles, subdistally on the lateral side of the dentary, supported by a conspicuous lateral bony extension), pelvic fins of female vestigial, or often missing (versus smaller than in male but similarly shaped), presence of pre-anal-fin-fold in female (a character unique among teleosts; versus absent in both sexes), dense small chromatophores in deep layer on abdomen appearing as a continuous patch, tiny black vermiculations on belly from throat to anus, including on male's pre-pelvic keratinized knob (versus large superficial, isolated chromatophores on belly from throat to anus, absent on male's keratinized pad), numerous small, contiguous chromatophores on middorsal line, organized in 3–5 rows, forming a broad stripe (versus few large isolated chromatophores, forming 1–3 irregular rows), pelvic-fin rays plain brown (versus with rows of spots).
(iii) Distribution
Paedocypris progenetica is known from Sumatra and Bintan Island (Indonesia).
(iv) Etymology
Progenetica, progenetic; an adjective.
(c) Paedocypris micromegethes sp. nov.
(i) Material
Holotype: see figure 2c,d. ZRC 49869, male, 10.4 mm; Borneo: Sarawak: Sungai Gayao, ca 40 km from Mukah (128 km from Sibu) on road Mukah Sibu; 2°54′29″ N, 112°19′32″ E; coll. M. Kottelat et al., 14 May 1994. Paratypes: ZRC 49870, 166 specimens; SM, 20 specimens; BMNH 2004.11.16.1-40, 40 specimens; CMK 10942, 160 specimens; total 386 specimens, males 9.7–11.6 mm, females 6.5–11.4 mm; same data as holotype. BMNH 2004.11.16.41-60, 20 specimens cleared and double stained, 10 males, 10.0–11.1 mm, 10 females 8.7–11.1 mm; same data as holotype. ZRC 39852, 6 males, 9.3–10.2 mm, 13 females, 7.3–10.4 mm; Borneo: Sarawak: Batu Kawa-Matang, Taman Koperkasa, ca 10 km from Kuching; 1°34′42.0″ N, 110°16′24.7″ E; coll. H. H. Tan et al., 13 January 1996.
(ii) Diagnosis
The characters distinguishing P. micromegethes from P. progenetica are listed in the diagnosis of the latter.
(iii) Distribution
Paedocypris micromegethes is known from Sarawak (Malaysia).
(iv) Etymology
Micromegethes (Greek), small in size; a noun in apposition.
(d) Notes on habitat, food and reproductive biology
The habitat of both Paedocypris species is slow-flowing blackwater streams or pools in peat swamp forests, where they inhabit the deeper, cooler water layers, in the lower half of the water column close to the bottom (but not on the bottom). The two species were observed only in shaded areas, in primary or secondary forest, and were absent from light-exposed open areas.
Gut dissections indicate that Paedocypris feeds mainly on planktonic rotifers and cladocerans (60–500 μm).
The smallest female with ripe eggs measured 7.9 mm in P. progenetica and 8.8 mm in P. micromegethes. In a 9.1 mm female P. progenetica, the ovaries contained four opaque eggs (diameter 0.3 mm) and 53 transparent eggs (diameter less than 0.15 mm). A 10.3 mm female of P. micromegethes had ovaries with 21 opaque eggs (diameter 0.40–0.45 mm (n=5), 0.30–0.40 mm (n=16)) and about 30 transparent eggs (0.10–0.20 mm diameter). There were no clear-cut size classes in eggs larger than 0.30 mm, with all smaller ones of similar sizes. The two ovaries were simultaneously functional. The presence of only few fully developed eggs in the ovaries of both species indicates that eggs are most probably deposited individually.
The smallest male in which modified pelvic fins were unambiguously observed measures 8.2 mm in P. progenetica (largest male 9.6 mm) and 9.1 mm in P. micromegethes (largest male 11.6 mm). In P. progenetica, the prepelvic pad appears as a small knob (ca 0.3 mm diameter), sometimes with a small groove and fold behind; the space between knob and pelvic-fin base is about equal to the egg size. In some specimens of P. micromegethes, the pad is slightly concave and pieces of unidentifiable tissue are attached.
4. Discussion
(a) Miniature fishes
Miniature fishes have been defined by Weitzman & Vari (1988) as those species reaching sexual maturity at a size of under 20 mm or, when maturity data are not available, not exceeding 26 mm in the wild. A number of fish species discovered over the past few years have maximal known sizes ranging from 8.0 to 15.0 mm (Kottelat & Vidthayanon 1993; Britz 2003; Watson & Walker 2004). An Australian marine gobioid fish, Schindleria brevipinguis (family Schindleriidae), was recently presented as ‘almost certainly’ the world's smallest vertebrate maturing ‘by 7 mm’, at ‘7–8 mm’ or ‘6.5–7 mm’ on different pages in the paper (Watson & Walker 2004). Of the six known specimens, however, sexual maturity was objectively established only for the single female measuring 8.4 mm. Maturity of the smaller males seems to have been inferred from the presence of the genital papilla as specimens were not dissected. Without histological examination, sexual maturity can be objectively established only by the presence of ripe eggs in the female's ovaries. That means, however, that Trimmatom nanus, another marine gobioid (Gobiidae), which has ‘fully developed eggs … present from a standard length of 8 mm and greater’ (largest known individual 10.2 mm) (Winterbottom & Emery 1981), is the smallest previously recorded vertebrate.
Fishes are also the smallest known freshwater vertebrates, the current record being held by the Burmese cyprinid Danionella translucida (12.0 mm, size at maturity unknown), followed by the Southeast Asian cobitid Kottelatlimia katik (mature at 13.0 mm) and cyprinid Boraras micros (13.3 mm, size at maturity unknown) (Kottelat & Lim 1992; Kottelat & Vidthayanon 1993) and the South American characid Xenurobrycon polyancistrus (13.1 mm) (Weitzman & Vari 1988).
The discovery of P. progenetica, with a mature female of just 7.9 mm and a maximum size of 10.3 mm, makes it the smallest recorded vertebrate species, slightly smaller than the marine goby T. nanus. Paedocypris micromegethes, the females of which mature at 8.8 mm (maximum 11.6 mm), comes a close second as the smallest freshwater vertebrate.
The 7.9 mm mature female of P. progenetica is not an unusually small individual. The 1 mm mesh size that we use in peat swamps only rarely catches specimens smaller than 7 mm and, therefore, introduces a size bias in our samples. Our largest sample contains 56 females (MZB5998, 5999, ZRC 43199), including the 7.9 mm one and the individuals unambiguously identifiable as females are 5.9–8.3 mm. The largest female is only slightly larger than the smallest mature one.
Typical features of miniature fishes include a tendency to simplify the skeleton and other structures, the production of very few, comparatively large eggs and (in freshwater species) a preference for standing or slow-flowing waters, often in nutrient-poor habitats (Weitzman & Vari 1988). A number of miniature fishes are paedomorphic and show a truncated development, but accelerated maturation leading to dwarfed adults with larval features (progenetic paedomorphosis).
The conspicuous skin fold along the lower edge of the caudal peduncle of Paedocypris is observed in many fish larvae (Moser et al. 1984), but is normally lost early in ontogeny. Paedocypris is the only known cypriniform fish to retain it as an adult. The long caudal peduncle, the translucent body and the brain not protected by frontals are additional progenetic attributes.
Most progenetic fishes tend to lose bones and scales, and to evolve bones that are very thin or perforated and part of the skeleton not ossifying but remaining cartilaginous (Johnson & Brothers 1993; Kottelat & Lim 1994; Britz & Kottelat 2003). Paedocypris lacks scales and numerous bones and cartilages in the neurocranium (parietals, nasals, vomer, preethmoid, most circumorbitals), lower jaw (angular), hyopalatine arch (ectopterygoid), shoulder girdle (post-temporal) and axial skeleton (supraneural 2, epural); its reduced bones include the very narrow frontals. None of the basi-, cerato-, epi- or pharyngobranchials are ossified, except ceratobranchials 4 and 5. The number of branched dorsal rays is reduced to five (versus at least seven in most Cypriniformes), with that of the branched caudal rays to 14 (versus 17 in most Cyprinidae).
Sharply contradicting this general tendency of miniature fishes to simplification, Paedocypris possesses one feature that is unique among fishes—a complex pelvic girdle, in which the first pelvic-fin ray and its abductor muscles are hypertrophied and highly modified (figures 2–4). These modifications are present only in males and most probably play a role in reproduction.
The structure of the pelvic fin suggests that it forms a clasping device in conjunction with the prepelvic keratinized pad. It might be used to grab the female during mating, analogous to what is observed in the unrelated priapus fishes (family Phallostethidae) that use a modified pelvic girdle to hold the female in position during copulation (Villadolid & Manacop 1934) and transfer sperm to fertilize the eggs internally (Grier & Parenti 1994). Alternatively, the male Paedocypris might use its modified pelvic girdle and fin to keep position at a spawning site or to manipulate spawned eggs, which are almost certainly laid individually. The hypertrophied male genital papilla is most likely part of this functional complex. All these modifications, taken together, point to an unusual reproductive mode. The iridescent spot on top of the head of the male probably plays a role in female/male recognition or male display.
This unusual suite of characters of Paedocypris poses numerous questions, many of which can only be answered in broader studies. Its systematic position within the Cypriniformes is uncertain; the numerous paedomorphic features make its precise affinities unclear and will require further comparative morphological and molecular investigation. Unfortunately, their habitat's current state of destruction makes it difficult to access new and live material for further study.
(b) Peat swamps, miniature fishes and their future
Peat swamp forests have long been regarded as a species-poor ecosystem with low productivity, low faunal diversity and few endemics (Johnson 1967), an assumption contradicted by the many endemic and highly stenotopic species discovered in recent years (e.g. Kottelat & Lim 1994; Kottelat & Ng 1994). Up to 15% of the known freshwater fish species in Malaysia are associated with peat swamps, with more than 80 stenotopic blackwater fish species, representing more than 20% of this specialized fauna, discovered only in the last 20 years (Ng et al. 1994; M. Kottelat and T. H. Hui 1986–2005, personal observation). Peat swamps also harbour a significant number of miniature fish species.
Of the 47 miniature fishes in Asian freshwaters listed by Kottelat & Vidthayanon (1993), 27 inhabit swamps, of which 11 live in peat swamps. Since then, new discoveries have brought the total up to 20 named miniature peat swamp species and more are not yet formally described (M. Kottelat, R. Britz and T. H. Hui 2005, personal observation). In peat swamps, miniature fishes survive droughts in shallow pools, burrows of other animals, or in the soil, and small size is a considerable advantage when the water level falls. Even in very dry periods, the peat acts as a buffer and retains isolated pools of clean and cold water. In high domes, the waterlogged peat often releases permanent creeks. The permanent presence of water in this loose soil ensures stability of the peat swamp habitat. This stability must have allowed the survival and favoured the evolution of strictly stenotopic species, among them many miniatures.
The patchy distribution and fragmentation of the peat swamp forests contributed to their high taxonomic diversity, with each patch having its own suite of endemic species. Empirical observations are that small and miniature species are more stenotopic and have much smaller ranges than larger ones, and that lineages comprising mainly or exclusively miniature species (Boraras, Sundadanio, Paedocypris, Kottelatlimia katik group, Parosphromenus, Betta coccina group—including a number of still undescribed species), each have allopatric species in several or all of the peat swamp forest patches.
The structurally complex peat swamp forests are disappearing quickly in Southeast Asia, due to logging, urbanization and conversion for agricultural use, especially oil palm plantations and shrimp farms. Peat swamp forests paid a high toll to the forest fires of Sumatra and Borneo in 1997, which lasted for several months. Many of the peat swamps we surveyed throughout Southeast Asia no longer exist and their fauna is eradicated. Populations of all the highly endemic and stenotopic miniature fishes of peat swamps have decreased or collapsed or are extirpated. Besides those listed as type material, we have collected or observed Paedocypris at numerous localities; many populations have disappeared, potentially dooming any efforts to elucidate the enigmatic reproductive biology of these species.
Acknowledgments
Support for fieldwork came from a grant to Peter K. L. Ng and M.K. from the Fauna and Flora Preservation Society (London); T.H.H. was supported by various grants from NUS to Peter K. L. Ng; M.K. was supported partially by a research fellowship from the Raffles Museum of Biodiversity Research, Singapore; K.E.W. was supported partially by a stipend from the Max Planck Society. Thomas Sim provided key assistance in the field, Peter K. L. Ng organized funding and facilities, H. K. Loy helped with SEM work, Olivier Perrin shared his observation on aquarium-kept individuals.
References
- Britz R. Danionella mirifica, a new species of miniature fish from Upper Myanmar (Ostariophysi: Cyprinidae) Ichthyol. Explor. Freshwaters. 2003;14:217–222. [Google Scholar]
- Britz R, Kottelat M. Descriptive osteology of the family Chaudhuriidae (Teleostei, Synbranchiformes, Mastacembeloidei), with a discussion of its relationships. Am. Mus. Novit. 2003;3418:1–62. 10.1206/0003-0082(2003)418%3C0001:DOOTFC%3E2.0.CO;2 [Google Scholar]
- Grier H.J, Parenti L.R. Reproductive biology and systematics of phallostethid fishes as revealed by gonad structure. Environ. Biol. Fish. 1994;41:287–299. [Google Scholar]
- Johnson D.S. Distributional patterns in Malayan freshwater fish. Ecology. 1967;48:722–730. doi: 10.2307/1933729. [DOI] [PubMed] [Google Scholar]
- Johnson G.D, Brothers E.B. Schindleria: a paedomorphic goby (Teleostei: Gobioidei) Bull. Mar. Sci. 1993;52:441–471. [Google Scholar]
- Kottelat M, Lim K.K.P. A synopsis of the Malayan species of Lepidocephalichthys, with descriptions of two new species (Teleostei: Cobitidae) Raffles Bull. Zool. 1992;40:201–220. [Google Scholar]
- Kottelat M, Lim K.K.P. Diagnoses of two new genera and three new species of earthworm eels from the Malay Peninsula and Borneo (Teleostei: Chaudhuriidae) Ichthyol. Explor. Freshwaters. 1994;5:181–190. [Google Scholar]
- Kottelat M, Ng P.K.L. Diagnoses of five new species of fighting fishes from Banka and Borneo (Teleostei: Belontiidae) Ichthyol. Explor. Freshwat. 1994;5:65–78. [Google Scholar]
- Kottelat M, Vidthayanon C. Boraras micros, a new genus and species of minute freshwater fish from Thailand (Teleostei: Cyprinidae) Ichthyol. Explor. Freshwaters. 1993;4:161–176. [Google Scholar]
- Moser H.G, Richards W.J, Cohen D.M, Fahay M.P, Kendall A.W Jr, Richardson S.L, editors. Ontogeny and systematics of fishes. American Society of Ichthyologists and Herpetologists; Lawrence: 1984. [Google Scholar]
- Ng P.K.L, Tay J.B, Lim K.K.P. Diversity and conservation of blackwater fishes in Peninsular Malaysia, particularly in the North Selangor peat swamp forest. Hydrobiologia. 1994;285:203–218. 10.1007/BF00005667 [Google Scholar]
- Taylor W.R, van Dyke G.C. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985;9:107–119. [Google Scholar]
- Villadolid D.V, Manacop P.R. The Philippine Phallostethidae, a description of a new species and a report on the biology of Gulaphallus mirabilis Herre. Philipp. J. Sci. 1934;55:193–217. [Google Scholar]
- Watson W, Walker H.J. The world's smallest vertebrate, Schindleria brevipinguis, a new paedomorphic species in the family Schindleriidae (Perciformes: Gobioidei) Rec. Aust. Mus. 2004;56:139–142. [Google Scholar]
- Weitzman S.H, Vari R.P. Miniaturization in South American freshwater fishes; an overview and discussion. Proc. Biol. Soc. Wash. 1988;101:444–465. [Google Scholar]
- Winterbottom R, Emery A.R. A new genus and two new species of gobiid fishes (Perciformes) from the Chagos Archipelago, Central Indian Ocean. Environ. Biol. Fish. 1981;6:139–149. 10.1007/BF00002777 [Google Scholar]