Abstract
Transmission of parasites to new hosts crucially depends on the timing of production of transmission stages and their capacity to start an infection. These parameters may be influenced by genetic factors, but also by the environment. We tested the effects of temperature and host genotype on infection probability and latency in experimental populations of the ciliate Paramecium caudatum, after exposure to infectious forms of its bacterial parasite Holospora undulata. Temperature had a significant effect on the expression of genetic variation for transmission and maintenance of infection. Overall, low temperature (10 °C) increased levels of (multiple) infection, but arrested parasite development; higher temperatures (23 and 30 °C) accelerated the onset of production of infectious forms, but limited transmission success. Viability of infectious forms declined rapidly at 23 and 30 °C, thereby narrowing the time window for transmission. Thus, environmental conditions can generate trade-offs between transmission relevant parameters and alter levels of multiple infection or parasite-mediated selection, which may affect evolutionary trajectories of parasite life history or virulence.
Keywords: experimental epidemiology, host–parasite interaction, multiple infection, parasite growth
1. Introduction
Transmission to new hosts is a crucial step in the life cycle of a parasite and a major determinant of the spread of infection (Anderson & May 1979). In parasites with free transmission propagules, important components of transmission are the timing of the production of these propagules, as well as their quantity and quality. Once released, they encounter and infect new hosts with a certain probability. Most of these components have a genetic basis in hosts and/or parasites (Thompson & Burdon 1992; Kaltz & Shykoff 2002; Little 2002; Bull et al. 2004; Lambrechts et al. 2005), and can thus be shaped by (co)evolution.
However, variation in these traits is also likely to be modulated by the physical environment of the organisms. Resource availability, light, humidity, temperature, etc. influence growth and development of both host and parasite, and this may feedback on the host's defence system or the parasite's virulence and capacity to invest in transmission (Burdon 1987; Koella et al. 1998; Ebert et al. 2000; Kaltz & Koella 2003; Jokela et al. 2005). Environmental conditions can also directly affect the process of transmission, for example, by determining the lifespan of transmission propagules (Burdon 1987; Gannicott & Tinsley 1998b; Mouritsen 2002) or exposure of the host to the parasite (Decaestecker et al. 2002; Fels et al. 2004; Fels 2005).
Finally, host and/or parasite genotypes may respond differently to different environmental conditions (Ferguson & Read 2002; Blanford et al. 2003; Price et al. 2004; Mitchell et al. 2005). The dynamics of transmission in a population will then depend on its precise genetic composition and the profile of environmental variation. Strong genotype×environment interactions may complicate patterns of antagonistic selection, and thus promote the maintenance of genetic variation in both host and parasite.
Variation in temperature represents one of the most ubiquitous sources of environmental heterogeneity. In various systems, temperature affects the parasite's ability to establish or maintain infection, its latency as well as its virulence (Burdon 1987; Thomas & Blanford 2003). For example, the fungal pathogen Microbotryum violaceum requires low temperatures for successful infection of its host plant, Silene latifolia, and, thus, limited transmission during hot periods of the season (Altizer et al. 1998; Kaltz & Shykoff 2001) may restrict its geographical distribution. Furthermore, effects of temperature are often nonlinear, and different traits may respond differently (Thomas & Blanford 2003). In the crustacean Daphnia magna and its bacterial parasite Pasteuria ramosa, infection success is highest at intermediate temperatures, whereas higher temperatures lead to faster parasite development and higher virulence (Mitchell et al. 2005). Moreover, these patterns vary with host genotype, suggesting that transmission and the outcome of parasite-mediated selection are strongly context-dependent (see also Blanford et al. 2003; Stacey et al. 2003; Mitchell et al. 2005).
We investigated the effects of temperature on horizontal transmission of Holospora undulata, a bacterial parasite of the protozoan Paramecium caudatum (Görtz & Brigge 1998; Fokin 2004). We set up experimental populations of different host clones at three temperatures (10, 23 and 30 °C). After introducing the parasite in these populations, we monitored initial transmission success and subsequent patterns of parasite development and prevalence over one month. A separate experiment tested the activity of infectious forms after 1–3 days of incubation at these temperatures.
2. Material and methods
(a) Study system
Paramecium caudatum inhabits Eurasian freshwater ponds and lakes. It feeds on bacteria and detritus filtered from the water. Like all ciliates, P. caudatum has two nuclei: the somatic, polyploid macronucleus and the diploid micronucleus, activated mainly during sexual reproduction.
The Gram-negative H. undulata belongs to the alpha-Proteobacteria, together with other symbionts, such as Rickettsia and Wolbachia (Amann et al. 1991). An overview of its life cycle is given by Fokin (2004). This parasite is micronucleus-specific. Infection occurs when a paramecium ingests infectious forms (15–20 μm) of the parasite during food uptake. The infectious form leaves the digestive vacuole in a transport vesicle and migrates to the micronucleus. Within 20 h, it differentiates into round-shaped (5 μm) reproductive forms, which multiply in the micronucleus. Reproductive forms differentiate into infectious forms, released during cell division or after host death. When the host divides, bacterial cells segregate into the daughter nuclei and are thus vertically transmitted.
We define latency as the period during which only reproductive forms are produced. The onset of differentiation of reproductive into infectious forms marks the end of the latent period. The production of infectious forms can lead to a heavily swollen micronucleus, carrying hundreds of infectious forms. At this stage, the parasite reduces host division rates and survival (Fokin 2004). Differentiation into infectious forms appears to occur when bacterial density in the micronucleus reaches a threshold, in cultures at or near carrying capacity after 7–10 days at 23 °C. In contrast, rapid host division can reduce bacterial densities, and thus latency is influenced indirectly by host growth conditions (Kaltz & Koella 2003).
(b) Origin of material
Paramecium mass cultures were grown in a sterile medium made of Protozoan Pellets (Carolina Biological Supply Co., Burlington, NC, USA; 0.7 g l−1 in Volvic mineral water), supplied with the bacterium Serratia marcenscens (Institut Pasteur, Paris, France) as food resource. Our standard culture temperature is 23 °C, which is near the optimum reported for paramecia (Wichtermann 1986). Four host clones were used. Two of them (K8 and K9) originate from single exconjugant cells following conjugation between two Japanese strains, provided by T. Watanabe, Tohoku University, Japan. The clones O3 and OB-1 originate from single individuals from two German cultures, provided by H.-D. Görtz (University of Stuttgart, Germany). Inocula were prepared from an infected K8 mass culture. The original parasite isolate was collected in Germany and provided by H.-D. Görtz.
(c) Experimental design
Experiment 1 tested the viability of infectious forms over a range of temperatures (10, 23 and 30 °C) likely to occur under natural conditions (Lampert & Sommer 1999). Experiment 2 investigated the epidemiology at these temperatures.
(i) Experiment 1
Infected individuals were ground with a tissue homogenizer, infectious forms concentrated by density gradient centrifugation (25 min at 2500 g, with 90% Percoll, Fisher Labosi, as the dense phase) and washed twice with sterile Volvic. We then prepared 27 identical tubes, each containing 5×105 infectious forms in a volume of 500 μl, and distributed nine tubes at random to three incubators at 10, 23 and 30 °C. Three tubes were removed after either 24, 48 and 72 h and used for inoculation at 23 °C. Inocula were acclimatized to 23 °C for 1 h, then 15 μl were added to 100 uninfected paramecia of clone K8 (in 1 ml of Volvic). These individuals were taken from a culture at carrying capacity so as to minimize cell division (and thus vertical transmission) during exposure to the parasite. After 24 h, paramecia were fixed with lacto–aceto orcein (Görtz & Dieckmann 1980) and infection status was determined at 1000× magnification.
(ii) Experiment 2
Mass cultures of the four clones were grown up in 50 ml Falcon tubes, with medium added in 2-day intervals until carrying capacity was reached (i.e. 1–2 days after the volume reached 50 ml). Then three uninfected replicate tubes of each clone were acclimatized for 3 days at 10, 23 and 30 °C, respectively. Replicates consisted of 500 paramecia in a volume of 5 ml, except for clone K9, which was started with only 250 individuals because of low densities in the base culture. The 36 replicate tubes were inoculated with freshly prepared infectious forms, as described above (dose: 130 infectious forms per paramecium), and put back to the incubators. Once a week, tubes were supplied with 500 μl of growth medium. Samples of 200 μl were taken and fixations made on day 1, 2, 3, 7, 14 and 28 post-inoculation to determine host densities, prevalence (proportion of infected individuals) and parasite development (initial number of infectious forms invading the micronucleus; presence or absence of reproductive forms and of newly produced infectious form in established infections).
(d) Data analysis
For experiment 1, we used a factorial analysis of deviance (ANODEV, based on a logistic regression) to test for effects of temperature and time of incubation on infection success (absence or presence of infection). Temperature was considered categorial in all analyses, and results did not change when treating it as continuous (not shown). Mean deviances (Pearson chi-square values divided by degrees of freedom) were used for quasi-F-statistics. In experiment 2, variation in (changes in) host density was analysed by means of factorial analysis of variance (ANOVA), with host clone as random and temperature as fixed factor. Factorial ANODEV's analysed variation in infection success, parasite development (proportion of infected individuals with multiplying reproductive forms) and latency of infection (proportion of infected individuals producing new infectious forms at day 7). Variation in initial parasite load (square-root-transformed number of infectious forms present in the micronucleus 24 h post-inoculation) was analysed in a factorial ANOVA. We used the SAS statistical package (SAS 1996).
3. Results
(a) Experiment 1
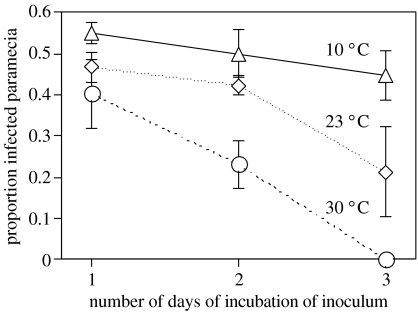
Infectivity of inocula declined during the 3 days of incubation at all three temperatures (effect time: F1,21=29.35, p<0.0001; figure 1). However, this effect depended strongly on incubation temperature (effect time×temperature: F2,21=8.58, p=0.0019). Infectious forms from 10 °C always showed the highest infection rates, and their infectivity declined by less than 20% between the first and third day of incubation. In contrast, at 23 °C, infectivity declined by more than 50%, and no infections were observed after the third day at 30 °C.
Figure 1.
Infection success after 1–3 days of incubation of infectious forms of Holospora undulata at three temperatures. Inoculation tests were carried out at 23 °C. Each point represents the mean (±s.e.) of three replicates.
(b) Experiment 2
(i) Host densities
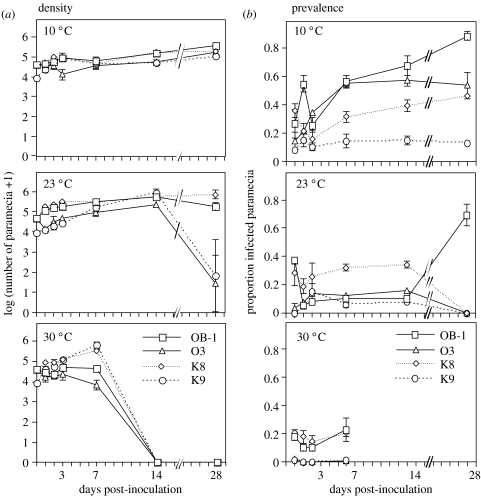
Unexpectedly, nearly all experimental populations gradually increased in density during the first half of the experiment, and those at 10 °C continued to do so over the whole experiment (figure 2a). We suppose that the fresh medium added to adjust starting densities allowed the paramecia to go through one or two rounds of division. To analyse variation in growth rate during the first 3 days, we regressed log-transformed densities on observation date. Analysis of the slopes of these regressions revealed a significant clone×temperature interaction (F6,24=3.62, p=0.0106), but no consistent overall effect of temperature (F2,6=0.32, n.s.). Similarly, a highly significant clone×temperature interaction (F6,24=3.62, p=0.0106), but no temperature main effect (F2,6=0.76, n.s.), was found when analysing the difference in log-transformed density between day 7 and day 3. By day 14, densities at 23 °C were significantly higher than those at 10 °C (F1,3=15.26, p=0.0298). At 30 °C, populations crashed after day 7 and all populations had gone extinct by day 14. At 23 °C, four populations (two K9 and O3, respectively) went extinct after day 14.
Figure 2.
(a) Host density and (b) prevalence for four host clones and three temperatures over the course of the experiment. Most points represent the mean (±s.e.) of three replicates. Populations were inoculated at day 0.
(ii) Infection success
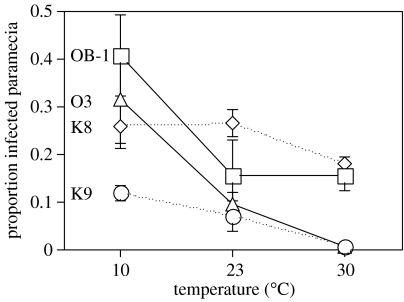
For five of the six assay dates, we obtained significant temperature×host clone interactions for infection success (day 1: F6,23=2.35, p=0.0642; day 2: F6,23=8.31, p<0.0001; day 3: F6,24=5.34, p=0.0013; day 7: F6,24=9.87, p<0.0001; day 14 (10 and 23 °C): F3,16=21.96, p<0.0001; day 28 (10 and 23 °C): F3,12=10.79, p<0.0001). Thus, different clones were more or less susceptible to infection, depending on temperature (figure 2b). Among-clone differences within each temperature regime were relatively constant over the first four assays (except for two exceptionally high values of the OB-1 clone at 10 °C (day 2) and at 23 °C (day 1)). Combining prevalence over this period showed that the ranking of strains was very similar at 23 and 30 °C (figure 3), and that the temperature×clone interaction (F6,24=11.16, p<0.0001) was mainly caused by the high susceptibility of the German clones (OB-1 and O3) at 10 °C. Although temperature effects were highly clone-dependent, there was a clear general trend of increased infection success at 10 °C (significant effect of temperature: F2,6=6.37, p=0.0328; figures 3 and 4).
Figure 3.
Mean prevalence for the four host clones and three temperatures. For each replicate, prevalences were combined over the first week post-inoculation, then means (±s.e.) taken for each clone and temperature.
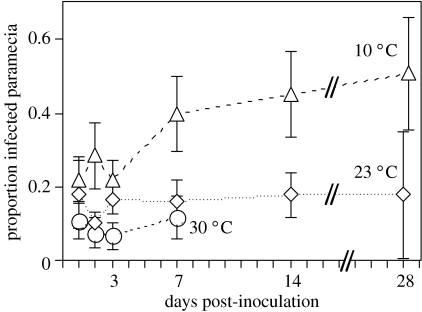
Figure 4.
Mean prevalence at three temperatures over the course of the experiment. Each point represents the mean (±s.e.) of four host clones. Populations died out after day 7 at 30 °C.
Unlike at 10 °C, marked changes in prevalence at 23 °C occurred between day 14 and day 30. In populations of the OB-1 clone, prevalence increased from around 10% to more than 60%, whereas infection disappeared in the other clones (figure 2b).
Clone K9 was least infected at all three temperatures (F3,24=45.61, p<0.0001; figure 3). This clone was initiated with a lower density, and because inocula were prepared on a per-paramecium rather than a per-volume basis, the lower overall infection success on this clone may have resulted from reduced encounter rates with the parasite. Therefore, statistical effects of ‘clone’ are potentially confounded with starting density. However, repeating the above analyses without K9 showed that the main results were not bound to this clone (not shown).
Changes in host density potentially affect prevalence. For example, if infected individuals divide less rapidly than uninfected ones, prevalence will decrease and we would thus underestimate horizontal transmission. Such a bias would become more accentuated with increasing division rates. However, changes in population density during the first 3 days (=the slopes from the above analysis of host density) did not significantly affect prevalence at day 3 (ANCOVA of arcsine-transformed prevalence with slope as covariate and clone and temperature as additional factors: no significant effect of slope (F1,12=2.11, n.s.), no significant interactions with slope (all F<1)). Further, changes in densities between days 3 and 7, or between days 7 and 14, were not significantly related to the respective changes in prevalence (ANCOVAs, as above: no significant main effect of density change, nor interactions (all F<1, n.s.)). Thus, within combinations of host clone and temperature, changes in host density did not explain (changes in) prevalence. Similar analyses carried out on the means per combination of clone and temperature also failed to detect significant effects of changes of density (not shown). This suggests that differences in prevalence among temperatures or clones were caused by differential infection rates rather than differential growth rates post-infection. These patterns indicate that the parasite has little effect on host division at this stage of infection (O. Restif & O. Kaltz, in press; Le Cocq 2004)
(iii) Parasite loads, development and latency
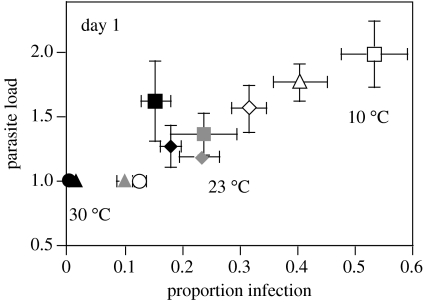
Twenty-four hours post-inoculation, we found significant effects of temperature (F2,19=12.60, p=0.0003) and host clone (F3,19=7.15, p=0.0021) on the number of infectious forms entering the micronucleus. Highest loads occurred at 10 °C, the lowest at 30 °C. There was a significant positive correlation between infection success (arcsine-transformed proportion of individuals infected) and parasite loads (r=0.66, n=11, p=0.0268, based on host clone means in each temperature; figure 5). Thus, at 10 °C we observed both increased transmission and multiplicity of infection.
Figure 5.
Relationship between prevalence and multiplicity of infection (mean number of infectious forms in the micronucleus) on day 1 post-infection. Means (±s.e.) were taken over the three replicates per host clone and temperature. Different symbols correspond to the four host clones (see figure 2).
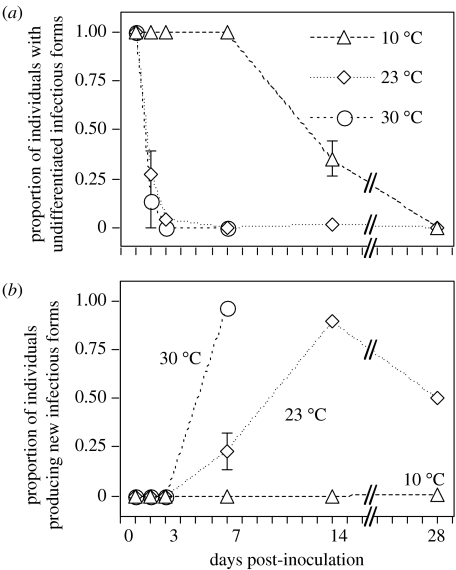
Subsequent development of infection was temperature-dependent. At 23 and 30 °C, differentiation of infectious forms began within 24–48 h post-inoculation, and by day 3, the majority (75%) of infected micronuclei carried multiplying reproductive stages (figure 6a). After one week, all infections were at the reproductive or infectious stage. The absence of new infections at this point indicates that most, if not all, infections at 23 and 30 °C occurred during the first few days post-inoculation. In contrast, at 10 °C, parasite development was slowed down and multiplication of reproductive stages only began during the second week (figure 6a). The concomitant increase in prevalence over time (figures 2 and 4) indicates that new infections occurred beyond the first week post-inoculation. The timing of these events was not significantly affected by host clone, nor its interaction with temperature (all analyses: F<1, n.s.).
Figure 6.
Parasite development at three temperatures over the course of the experiment. (a) Proportion of host individuals, where infectious forms of the parasite have not yet differentiated into reproductive forms; (b) proportion of individuals, where the parasite has begun to differentiate from reproductive into infectious forms. Each point represents the mean (±s.e.) of four host clones.
The onset of the production of infectious forms (=end of latency period) began after day 3, and it was highly temperature-dependent (significant effect of temperature on the proportion of established infections with infectious forms: F2,20=384.4, p<0.0001; figure 6b). On day 7, nearly all infected individuals at 30 °C produced infectious forms (by day 14, all populations had gone extinct). At 23 °C, only about 25% of the individuals produced infectious forms on day 7, then values peaked at day 14. At 10 °C, differentiation into infectious forms had still not occurred at the end of the experiment (figure 6b). At day 7, latency was not significantly affected by host clone (F3,20<1, n.s.), nor its interaction with temperature (F6,20<1, n.s.). Similarly, at day 14 and 23 °C, clones did not differ in the proportion of individuals producing new infectious forms (F3,8=1.06, n.s.).
4. Discussion
(a) Transmission
Prevalence varied among the four P. caudatum clones, corroborating earlier reports of variation in resistance against this and other Holospora species (Fokin 2004). However, the expression of genetic variation was temperature-dependent. The two German clones (OB-1 and O3) were particularly susceptible to infection at 10 °C, resulting in crossing reaction norms (figure 3). Such genotype×temperature interactions have also been reported for other host–parasite systems (Burdon 1987; Blanford et al. 2003; Stacey et al. 2003; Mitchell et al. 2005). Clearly, if host genetic variation is expressed in some environments, but not in others, or if genotypes switch ranks between environments, the outcome of parasite-mediated selection will differ among environments. Hence, genotype×environment interactions can be an important mechanism responsible for the maintenance of genetic polymorphism.
Resistance of paramecia may have a physiological and/or behavioural component, and both may be temperature-sensitive. For example, the two German clones may be generally less adapted to low temperature and, therefore, have less energy available to invest into physiological resistance, such as lysis of the parasite early after infection (Fokin & Skovorodkin 1997). Alternatively, these clones may in fact be better adapted to low temperatures, thus be more active and consequently experience higher contact rates with the parasite. To differentiate between these hypotheses more detailed analyses are required on feeding and uptake rates or bacterial lysis.
For all host clones, parasite infection success was highest at low temperature (10 °C). This result is counterintuitive, because low temperature should downregulate activity and feeding rates, thereby reducing contact rates with the parasite. One explanation may be the temperature-dependent viability of infectious forms. Experiment 1 showed much stronger declines of infectivity of inocula incubated at 23 and 30 °C than did those incubated at 10 °C (figure 1). Thus, higher temperatures limit the time window open for the parasite to acquire new infections, which also explains the rapid decline of newly infected individuals at 23 and 30 °C in experiment 2 (figure 6a).
Indeed, temperature-dependent viability (and infection success) of transmission stages is a common phenomenon, although the shape of this relationship varies among systems (Evans 1985; Burdon 1987; Ebert 1995; Gannicott & Tinsley 1998a; Meissner & Bick 1999; Mouritsen 2002). Here, the correlation is negative and its shape indicates that temperatures optimal for the host impose a constraint on horizontal transmission of this parasite.
(b) Parasite loads and latency
The number of parasite propagules initially entering a host can influence subsequent within-host dynamics and expression of disease symptoms (Poulin 1998; Read & Taylor 2001). Theoretical models consider multiple infection as a key factor of the evolution of parasite life history and virulence (Schjørring & Koella 2002 and references therein). Our experiment illustrates the potential context-dependency of multiple infection, as its degree varied with both host clone identity and temperature.
In the short run, variation in the multiplicity of infection translates into variation in parasite dose, which can affect the timing of disease symptoms (Ebert et al. 2000; Timms et al. 2001). In our system, higher initial parasite dose reduces latency (at 23 °C), presumably because the bacterial threshold density triggering the production of infectious forms is reached earlier (O. Kaltz & O. Sakwinska, unpublished data). Here, low temperature led to a higher degree of (multiple) infection, but subsequent development was extremely slow and newly produced infectious forms still absent after four weeks. In contrast, despite lower initial loads, infectious forms were produced within a week at 30 °C. Thus, the temperature at which the parasite developed was by far the more important determinant of latency than initial parasite load. Of course, latency is also determined by relative growth rates of host and parasite. In favourable conditions, rapid host division dilutes bacterial densities in the micronucleus, thereby postponing the production of infectious forms, at least at 23 °C (Kaltz & Koella 2003). It remains to be tested whether the host has the potential to outgrow the parasite also at 30 °C.
(c) Epidemiological and evolutionary implications
Higher temperatures generally speed up parasite development and thus reduce latency (Burdon 1987; Gannicott & Tinsley 1998a; Mouritsen 2002; Thomas & Blanford 2003; Mitchell et al. 2005). It has therefore been suggested that climatic changes, such as global warming, facilitate the spread of infectious diseases (Dobson & Carper 1992). However, interactions between temperature and parasite life history may be complex. We found a temperature-dependent, epidemiological trade-off between transmission and latency. Low temperature facilitated transmission, but arrested parasite development, whereas higher temperatures allowed rapid production of new infectious forms, but limited their chance of transmission. Indeed, these kinds of trade-offs may be common, especially for parasites with free transmission stages, which are likely to encounter variable environmental conditions (Evans 1985; Ebert 1995; Mouritsen & Jensen 1997; Gannicott & Tinsley 1998a).
One aspect of the trade-off is its potentially compensatory nature. If at least one parasite fitness component is well functioning in the different temperature regimes, a minimum level of transmission may be assured over a relatively wide range of temperatures. The trade-off further suggests the existence of an optimal temperature that maximizes the spread of infection. Where this balance lies depends not only on the gain through new infections, but also on the loss through natural and parasite-induced mortality (Anderson & May 1979). Our data indicate a positive relationship between temperature and natural host mortality, and this may limit the success of this parasite in populations at high temperature. However, populations at 30 °C died out too rapidly to provide a full picture of the epidemiological dynamics. Given the rather low prevalence, it is unlikely that extinction was caused by the parasite (alone). In fact, even the opposite may be the case. If investment into horizontal transmission is less rewarding and multiple infection less frequent, the evolution of more benevolent parasites may be favoured. For example, at high temperatures (greater than 35 °C), the macronucleus-specific parasite Holospora obtusa induces the production of heat-shock proteins, thereby increasing survival of P. caudatum (Hori & Fujishima 2003).
In contrast, populations of both host and parasite were stably maintained at 10 °C. However, the slow development of infection indicates that the parasite is essentially locked inside the host. This, together with increased multiplicity of infection, may intensify within-host competition and thus facilitate selection for more rapid development, shorter latency and, possibly, higher virulence (e.g. Gandon 1998).
At 23 °C, we obtained a more complete picture of the epidemiological dynamics, and the results highlight the importance of the genetic component in this process. In three of the four clones, infection disappeared during the second half of the experiment, possibly because parasite-induced mortality was not compensated by new infections. In the clone OB-1, however, a threefold increase in prevalence occurred (figure 2b); about 50% of the infections were at the reproductive stage (figure 6b), suggesting that they were recently established. Hence, shorter latency and additional rounds of horizontal transmission resulted in prevalences similar to those at 10 °C. Altogether, the variable outcomes in the different host clones suggest that a single temperature optimum does not exist for this parasite.
(d) Perspectives
We are currently measuring parasite virulence over a range of temperatures. Less restrictive protocols of these experiments (e.g. regular supply of fresh medium) will also allow us to follow long-term infection dynamics. Furthermore, here we simulated the arrival of a single, massive wave of parasites. Perhaps more realistic scenarios may test the effects of variation in initial parasite dose or the frequency of exposure. We also plan to impose temporal fluctuations in temperature, which certainly occur in natural populations. The importance of this type of variation for population dynamics has recently been shown in Paramecium aurelia (Gonzalez & Holt 2002).
(e) Conclusions
We found that temperature affected two key determinants of disease spread, transmission and latency, and these effects varied with host genetic background. These results are in line with recent studies in other host–parasite systems (Blanford et al. 2003; Stacey et al. 2003; Mitchell et al. 2005), thus illustrating the need to consider environmental and genetic variation of epidemiological parameters, which theoretical models often treat as ‘constants’.
Acknowledgments
This project was financed by a research grant ‘ACI Jeunes Chercheurs’ (Ministère de Recherche, France) to O.K. Tom Little and two anonymous reviewers gave helpful comments on earlier versions of this manuscript.
References
- Altizer S.M, Thrall P.H, Antonovics J. Vector behavior and transmission of anther-smut infection in Silene alba. Am. Midl. Nat. 1998;139:147–163. [Google Scholar]
- Amann R, Springer N, Ludwig W, Görtz H.-D, Schlaifer K.-H. Identification in situ and phylogeny of uncultured bacterial endosymbionts. Nature. 1991;351:161–164. doi: 10.1038/351161a0. 10.1038/351161a0 [DOI] [PubMed] [Google Scholar]
- Anderson R.M, May R.M. Population biology of infectious diseases: part 1. Nature. 1979;280:361–367. doi: 10.1038/280361a0. 10.1038/280361a0 [DOI] [PubMed] [Google Scholar]
- Blanford S, Thomas M.B, Pugh C, Pell J.K. Temperature checks the red Queen? Resistance and virulence in a fluctuating environment. Ecol. Lett. 2003;6:2–5. 10.1046/j.1461-0248.2003.00387.x [Google Scholar]
- Bull J.J, Pfennig D.W, Wang I.-N. Genetic details, optimization and phage life histories. Trends Ecol. Evol. 2004;19:76–82. doi: 10.1016/j.tree.2003.10.008. 10.1016/j.tree.2003.10.008 [DOI] [PubMed] [Google Scholar]
- Burdon J.J. Cambridge University Press; Cambridge, UK: 1987. Diseases and plant population biology. Cambridge studies in ecology. [Google Scholar]
- Decaestecker E, De Meester L, Ebert D. In deep trouble: habitat selection constrained by multiple enemies in zooplankton. Proc. Natl Acad. Sci. USA. 2002;99:5481–5485. doi: 10.1073/pnas.082543099. 10.1073/pnas.082543099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A, Carper R. Global warming and potential changes in host–parasite and disease–vector relationships. In: Peters R.L, Lovejoy T.E, editors. Global warming and biodiversity. Yale University Press; New Haven, CT: 1992. pp. 201–217. [Google Scholar]
- Ebert D. The ecological interactions between a microsporidian parasite and its host Daphnia magna. J. Anim. Ecol. 1995;64:361–369. [Google Scholar]
- Ebert D, Zschokke-Rohringer C.D, Carius H.J. Dose effects and density dependent regulation of two microparasites of Daphnia magna. Oecologia. 2000;122:200–209. doi: 10.1007/PL00008847. [DOI] [PubMed] [Google Scholar]
- Evans N.A. The influence of environmental temperature upon transmission of the cercariae of Echinostoma liei (Digenea: Echinostimatidae) Parasitology. 1985;90:269. [Google Scholar]
- Fels D. The effect of food on microparasite transmission in the waterflea Daphnia magna. Oikos. 2005;109:360–366. 10.1111/j.0030-1299.2005.13812.x [Google Scholar]
- Fels D, Lee V.A, Ebert D. The impact of microparasites on the vertical distribution of Daphnia magna. Arch. Hydrobiol. 2004;161:65–80. 10.1127/0003-9136/2004/0161-0065 [Google Scholar]
- Ferguson H.M, Read A.F. Genetic and environmental determinants of malaria parasite virulence in mosquitos. Proc. R. Soc. B. 2002;269:1217–1224. doi: 10.1098/rspb.2002.2023. 10.1098/rspb.2002.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokin S.I. Bacterial endocytobionts of ciliophora and their interactions with the host cell. Int. Rev. Cytol. 2004;236:181–249. doi: 10.1016/S0074-7696(04)36005-5. [DOI] [PubMed] [Google Scholar]
- Fokin S.I, Skovorodkin I.N. Experimantal analysis of the resistance of Paramecium caudatum (Ciliophora) against infection by bacterium Holospora undulata. Eur. J. Protistol. 1997;33:214–218. [Google Scholar]
- Gandon S. The curse of the pharaoh hypothesis. Proc. R. Soc. B. 1998;265:1545–1552. doi: 10.1098/rspb.1998.0470. 10.1098/rspb.1998.0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannicott A.M, Tinsley R.C. Environmental effects on transmission of Discocotyle sagittata (Monogenea): egg production and development. Parasitology. 1998a;117:499–504. doi: 10.1017/s0031182098003205. 10.1017/S0031182098003205 [DOI] [PubMed] [Google Scholar]
- Gannicott A.M, Tinsley R.C. Larval survival characteristics and behaviour of the gill monogenean Discocotyle sagittata. Parasitology. 1998;117:491–498. doi: 10.1017/s0031182098003217. 10.1017/S0031182098003217 [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Holt R.D. The inflationary effects of environmental fluctuations in source–sink systems. Proc. Natl Acad. Sci. USA. 2002;99:14 872–14 877. doi: 10.1073/pnas.232589299. 10.1073/pnas.232589299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görtz H.-D, Brigge T. Intracellular bacteria in protozoa. Naturwissenschaften. 1998;85:359–368. doi: 10.1007/s001140050517. 10.1007/s001140050517 [DOI] [PubMed] [Google Scholar]
- Görtz H.-D, Dieckmann J. Life cycle and infectivity of Holospora elegans Haffkine, a micronucleus-specific symbiont of Paramecium caudatum (Ehrenberg) Protistologia. 1980;16:591–603. [Google Scholar]
- Hori M, Fujishima M. The endosymbiotic bacterium Holospora obtusa enhances heat-shock gene expression of the host Paramecium caudatum. J. Eukaryot. Microbiol. 2003;50:293–298. doi: 10.1111/j.1550-7408.2003.tb00137.x. 10.1111/j.1550-7408.2003.tb00137.x [DOI] [PubMed] [Google Scholar]
- Jokela J, Taskinen J, Mutikainen P, Kopp K. Virulence of parasites in hosts under environmental stress: experiments with anoxia and starvation. Oikos. 2005;108:156. 10.1111/j.0030-1299.2005.13185.x [Google Scholar]
- Kaltz O, Koella J.C. Host growth conditions regulate the plasticity of horizontal and vertical transmission in Holospora undulata, a bacterial parasite of the protozoan Paramecium caudatum. Evolution. 2003;57:1489–1497. doi: 10.1111/j.0014-3820.2003.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Kaltz O, Shykoff J.A. Male and female Silene latifolia plants differ in per-contact risk of infection by a sexually transmitted disease. J. Ecol. 2001;89:99–109. 10.1046/j.1365-2745.2001.00527.x [Google Scholar]
- Kaltz O, Shykoff J.A. Within- and among-population variation in infectivity, latency and spore production in a host–pathogen system. J. Evol. Biol. 2002;15:850–860. 10.1046/j.1420-9101.2002.00433.x [Google Scholar]
- Koella J.C, Agnew P, Michalakis Y. Coevolutionary interactions between host life histories and parasite life cycles. Parasitology. 1998;116:S47–S55. doi: 10.1017/s0031182000084936. [DOI] [PubMed] [Google Scholar]
- Lambrechts L, Halbert J, Durand P, Gouagna L.C, Koella J.C. Host genotype by parasite genotype interactions underlying the resistance of anopheline mosquitoes to Plasmodium falciparum. Malar. J. 2005;4:3–11. doi: 10.1186/1475-2875-4-3. 10.1186/1475-2875-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert W, Sommer U. Thieme; Stuttgart: 1999. Limnoökologie. [Google Scholar]
- Le Cocq, D. 2004 Etude de l'impact du parasitisme de Holospora undulata sur les traits d'histoire de vie de Paramecium caudatum Maîtrise thesis, Université Pierre & Marie Curie, Paris, France.
- Little T.J. The evolutionary significance of parasitism: do parasite-driven genetic dynamics occur ex silico? J. Evol. Biol. 2002;15:1–9. 10.1046/j.1420-9101.2002.00366.x [Google Scholar]
- Meissner K, Bick A. Laboratory studies of parasite transmission aspects between Hydrobia spp (Gastropoda) and Corophium volutator (Amphipoda) Int. Rev. Hydrobiol. 1999;84:61–72. [Google Scholar]
- Mitchell S.E, Rogers E.S, Little T.J, Read A.F. Host–parasite and genotype-by-environment interactions: temperature modifies potential for selection by a sterilizing pathogen. Evolution. 2005;59:70–80. [PubMed] [Google Scholar]
- Mouritsen K.N. The Hydrobia ulvae–Maritrema subdolum association: influence of temperature, salinity, light, water-pressure and secondary host exudates on cercarial emergence and longevity. J. Helminthol. 2002;76:341–347. doi: 10.1079/JOH2002136. 10.1079/JOH2002136 [DOI] [PubMed] [Google Scholar]
- Mouritsen K.N, Jensen K.T. Parasite transmission between soft-bottom invertebrates: temperature mediated infection rates and mortality in Corophium volutator. Mar. Ecol. Prog. Ser. 1997;151:123–134. [Google Scholar]
- Poulin R. Chapman & Hall; London: 1998. Evolutionary ecology of parasites: from individuals to communities. [Google Scholar]
- Price J.S, Bever J.D, Clay K. Genotype, environment and genotype by environment interactions determine quantitative resistance to leaf rust (Coleosporium asterum) in Euthamia graminifolia (Asteraceae) New Phytol. 2004;162:729–743. doi: 10.1111/j.1469-8137.2004.01082.x. 10.1111/j.1469-8137.2004.01082.x [DOI] [PubMed] [Google Scholar]
- Read A.F, Taylor L.H. The ecology of genetically diverse infections. Science. 2001;292:1099–1102. doi: 10.1126/science.1059410. 10.1126/science.1059410 [DOI] [PubMed] [Google Scholar]
- Restif, O. & Kaltz, O. In press. Condition-dependent virulence in a horizontally and vertically transmitted bacterial parasite. Oikos
- SAS. SAS Institute; Cary, NC: 1996. SAS/STAT User's guide, Release 6.11. [Google Scholar]
- Schjørring S, Koella J.C. Sub-lethal effects of pathogens can lead to the evolution of lower virulence in multiple infections. Proc. R. Soc. B. 2002;270:189–193. doi: 10.1098/rspb.2002.2233. 10.1098/rspb.2002.2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D.A, Thomas M.B, Blanford S, Pell J.K, Pugh C, Fellowes M.D.E. Genotype and temperature influence pea aphid resistance to a fungal entomopathogen. Physiol. Entomol. 2003;28:75–81. 10.1046/j.1365-3032.2003.00309.x [Google Scholar]
- Thomas M.B, Blanford S. Thermal biology in insect–parasite interactions. Trends Ecol. Evol. 2003;18:344–350. 10.1016/S0169-5347(03)00069-7 [Google Scholar]
- Thompson J.N, Burdon J.J. Gene-for-gene coevolution between plants and parasites. Nature. 1992;360:121–125. 10.1038/360121a0 [Google Scholar]
- Timms R, Colegrave N, Chan B.H, Read A.F. The effect of parasite dose on disease severity in the rodent malaria Plasmodium chabaudi. Parasitology. 2001;123:1–11. doi: 10.1017/s0031182001008083. 10.1017/S0031182001008083 [DOI] [PubMed] [Google Scholar]
- Wichtermann R.T. Plenum Press; New York: 1986. The biology of Paramecium. [Google Scholar]