Abstract
Acropora is the most diverse genus of reef-building corals in the world today. It occurs in all three major oceans; it is restricted to latitudes 31° N–31° S, where most coral reefs occur, and reaches greatest diversity in the central Indo-Pacific. As an exemplar genus, the long-term history of Acropora has implications for the evolution and origins of present day biodiversity patterns of reef corals and for predicting their response to future climate change. Diversification of Acropora was thought to have occurred in the central Indo-Pacific within the previous two million years. We examined Eocene fossils from southern England and northern France and found evidence that precursors of up to nine of 20 currently recognized Acropora species groups existed 49–34 Myr, at palaeolatitudes far higher than current limits, to 51° N. We propose that pre-existing diversity contributed to later rapid speciation in this important functional group of corals.
Keywords: Eocene, coral reefs, biogeography, climate change, molecular clock
1. Introduction
The purpose of this paper is to draw attention to the currently overlooked deep fossil record of Acropora (Oken 1815), and its implications for the evolution of modern diversity patterns of reef corals. Acropora is the most diverse modern coral genus (more than 120 extant species; Wallace 1999; Veron 2000) and provides a major element of modern reef coral diversity and coral reef frameworks throughout the world, reaching its greatest diversity in the central Indo-Pacific (Veron 1995; Wilson & Rosen 1998; Wallace 1999). Owing to this diversity and abundance, Acropora has been the subject of a number of evolutionary studies (e.g. Fukami et al. 2000; van Oppen et al. 2001; Volmer & Palumbi 2002; Wolstenholme 2003). The genus also has an extensive fossil history, which is becoming more accessible as new studies provide more data and finer resolution (e.g. Carbone et al. 1994; McCall et al. 1994; Wilson & Rosen 1998; Budd 2000; Schuster 2002). It is thus an ideal exemplar for studying the origins of modern diversity and distribution patterns for reef corals.
Although Southeast Asia is the current locality of the greatest diversity of Acropora and other modern Indo-Pacific reef coral genera, this region was lacking in most zooxanthellate coral genera (‘z-corals’) and z-coral-dominated carbonates up until the latest Oligocene to earliest Miocene (around 26 Myr), a phenomenon dubbed the ‘Paleogene gap’ by Wilson & Rosen (1998). While future discoveries could conceivably show that the Paleogene gap is an observational artefact, Wilson & Rosen (1998) pointed out that this pattern is founded on numerous preceding studies over 80 years, and that it also applies to other tropical shallow water marine organisms. Moreover, this biotic and palaeoenvironmental gap corresponds to a real oceanic gap. Wilson & Rosen (see also Wilson 2002) reviewed the carbonate record of the entire region in the context of plate movements and palaeogeographical changes through the Cenozoic. Significantly, a 3000 km wide oceanic void, with few shallow water areas and landmasses, existed in the earlier Paleogene between Australasia and the Southeast Asia mainland. This gap gradually narrowed through the Paleogene, and shallow water areas increasingly emerged as the Australasian craton moved northwards, colliding with the Southeast Asian craton during the Paleogene–Neogene transition time.
The distribution pattern of Acropora in particular, also shows a Paleogene gap, since there are no records of it to date in the Indo-West Pacific or Pacific regions until the Paleogene–Neogene transition. In contrast, in addition to the English and French Eocene material examined in this paper, Acropora species occurred in Eocene shallow water carbonates in Mediterranean France, Spain, Italy and the former Yugoslavia (see Wallace 1999, p. 20). The earliest known record of the genus occurs in the Late Paleocene (65–54 Myr) from Somalia (Carbone et al. 1994). The first record of Acropora dominating a reef structure comes from the Late Oligocene (28–23 Myr) in Greece (Schuster 2002), but by the Mid-Miocene (16–12 Myr) the genus is no longer represented in the Mediterranean region, having disappeared during the progressive Miocene extinction of all reefal biotas throughout this region (Rosen 1999).
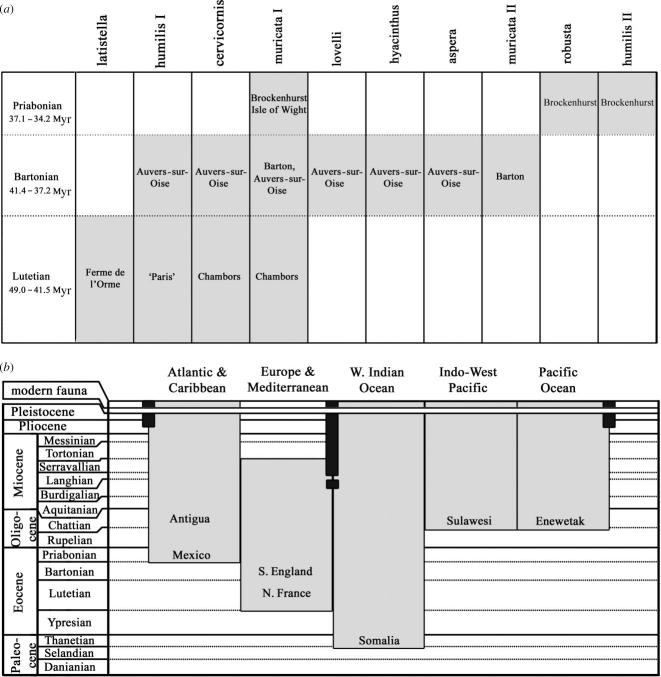
It is commonly assumed that the species composition of extant reef corals, including Acropora, has been most strongly influenced by Neogene evolutionary events, particularly the Panama closure and Plio-Pleistocene sea level fluctuations of the last two million years (Stehli & Wells 1971; Potts 1985; Veron 1995; Fukami et al. 2000). An Eocene appearance date (54 Myr) for Acropora has been used as a molecular clock calibration (Fukami et al. 2000), but potentially the fossil record could provide much further information about the relationship between past and present taxonomic composition. For an indication of Eocene coral diversity, we examined Eocene Acropora fossils from England and France in the collections of the Natural History Museum, London (NHM). These fossils occurred in assemblages 49–34.2 Myr old, during the Lutetian, Bartonian and Priabonian (figure 1, Berggren et al. 1995), i.e. after Early Eocene extreme global warmth but before dramatic cooling in the Oligocene (Huber & Caballero 2003).
Figure 1.
Reconstructed Lutetian–Bartonian world landforms (solid) after Golonka (2000) with permission, indicating locality of Eocene fossil localities (arrow) and locality of earliest recorded Acropora fossil (star). Inset: area covering fossil localities from this study. Deposit age indicated as L (Lutetian), B (Bartonian) and P (Priabonian).
The current distribution of Acropora is almost exclusively between latitudes 31° N and S of the Equator (figure 2), within the temperature and calcium carbonate saturation limits suitable for modern reef development (Opdyke & Wilkinson 1993; Kleypas et al. 2001); however, the Eocene fossils of Acropora studied here, as well as other z-corals and tropical organisms, occurred at palaeolatitudes 49–51° N. Although such records have existed for some time (Duncan 1866), their implications for modern reefs and z-corals in the context of global warming and diversity patterns have not been previously realized, and much relevant material in museum collections has not been studied or identified. The high-latitude fossils have unusually good preservation, because they are preserved in muds or silty and marly sands (table 1). They, therefore, give an insight into Eocene coral diversity, which is not readily available from indurated carbonates typical of many other coral-bearing deposits.
Figure 2.
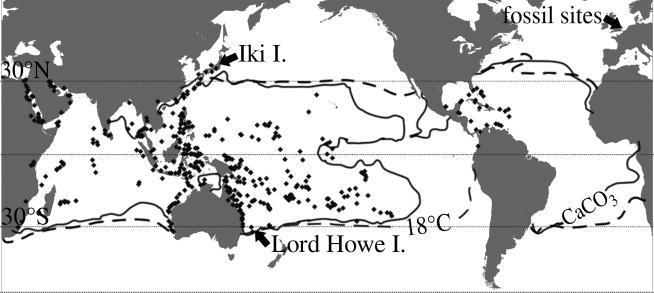
Present distribution of Acropora, from Worldwide Acropora Collection database. Lines indicate critical boundaries of SST (18 °C) and aragonite saturation for coral reef accretion (CaCO3) as given by Kleypas et al. (1999). Highest north and south latitude reefs (Iki I., Lord Howe I.) and Eocene fossil localities in this paper are indicated.
Table 1.
Characteristics of localities from which the Eocene Acropora fossils in this study had been collected. (Quotation marks indicate poor precision of locality or age. NP refers to nannoplankton zone and P to planktonic foraminiferal zone after Berggren et al. (1995). Palaeolatitudes, ages and facies have been inferred from locality information in column 1 using sources including: 1, Davis (1952); 2, Insole & Daley (1985); 3, Melville & Freshney (1982); 4, Gale et al. (1999); 5, Cavelier et al. (1980); 6, Mégnien (1980); 7, Harris & Burrows (1891); 8, Pomerol (1982); 9, Debrand-Passard (1980); 10, Mégnien & Mégnien (1980).)
| locality | palaeolatitude | estimated age (Myr) | relative (biostratigraphical) age | facies | sources |
|---|---|---|---|---|---|
| Brockenhurst, Hampshire, England | 51° N | 36.0–34.2 | Late Eocene (Late Priabonian, NP19–20) | mainly composed of muds, sandy muds and muddy sands | 1, 2, 3 |
| Whitecliff Bay, Isle of Wight, England | 51° N | 36.0–34.2 | Late Eocene (Late Priabonian, NP19–20) | mainly composed of muds, sandy muds and muddy sands | 2, 5 |
| Auvers-sur-Oise, France | 49° N | 41.4–37.0 | Late Mid-Eocene (Bartonian, NP 16–18, P12–15) | sands of variable character, horizontal or false-bedded, buff, grey or ferruginous | 5, 6, 7, 8 |
| Barton, England | 51° N | 41.4–37.2 | Late Mid-Eocene (Bartonian, NP 16–18, P12-15) | fine sands and muds | 2, 3, 4 |
| Chambors, France | 49° N | 47.3–45.5 | Early Mid-Eocene (Lutetian, NP15) | fine sands and muds | 7, 8, 9, 10 |
| La Ferme de l'Orme, France | 49° N | 49.0–41.3 | Lutetian | fine sands and muds | 6, 7 |
| ‘Paris Basin’, France | ‘45° N’ | 49.0–37.0 | Mid-Eocene | not available | |
| Paris, France | ‘45° N’ | 55.0–33.7 | ‘Eocene’ | not available |
Modern coral species are defined primarily by morphological discontinuities in skeletal structures; although molecular techniques are increasingly used to explore species boundaries (Wallace & Willis 1994; Volmer & Palumbi 2002; Wolstenholme et al. 2003), clearly these cannot be applied to fossils. On the other hand, fossil material is variously subject to taphonomic and diagenetic processes, and this often obscures some of the taxonomic characters that are available to those studying modern counterparts of fossil taxa. This can also lead to different methodologies (e.g. studies of skeletal sections are more widely used for fossil than modern corals). While successful integrated studies of corals at species level are possible (e.g. Budd 1991), these can be labour-intensive. Forey et al. (2004) recommend that, in general, for studies based on compilations of fossil taxa, generic level is a more pragmatic approach. However, to identify the present Eocene Acropora fossils, we adopted a level of classification not previously used for fossil corals, but in common use for living Acropora (Wallace 1999). The genus has two subgenera, only one of which is represented in the Eocene: the larger subgenus Acropora (Acropora) (hereafter shortened to Acropora). This has been subdivided into 20 ‘species groups’, based on shared skeletal character states (Veron & Wallace 1984) and on clades in a morphological phylogeny (Wallace 1999). We employed this species group level to make direct comparisons between modern and Eocene faunas.
2. Material and methods
(a) Localities
Material examined came from six main localities (table 1 and figure 1 inset), two Late Priabonian localities: Whitecliff Bay (Isle of Wight) and Brockenhurst in England, two Bartonian: Auvers-sur-Oise in France and Barton in England and two Lutetian: Chambors and La Ferme de l'Orme in France. Data on the most recent interpretations of stratigraphy and facies of these localities were reviewed and compiled from sources given in table 1.
(b) Material examined
We examined 56 fossil fragments of Acropora from the NHM, collected at the localities from mid-nineteenth to late-twentieth centuries. Specimen details are given in the electronic supplementary material, table 1. Most of these were identified on the museum labels as the fossil species Acropora solanderi (Defrance) and the remainder as two other fossil species, non-specific Acropora or another genus (electronic supplementary material, table 1).
(c) Methods
Each fossil was examined in detail, using a Wild binocular microscope with eyepiece graticule, for the presence of features used for recognition of branching type, radial corallite structure and coenosteal structure (see Wallace 1999; Wolstenholme et al. 2003). Examples of morphological characters were examined and photographed using the JEOL JSM-5410LV Scanning Electron Microscope at James Cook University, Townsville. To establish the species group of each fossil, we compared the characters visible on the fossil against those on modern specimens in the Worldwide Acropora Collection (with over 20 000 specimens, located in the Museum of Tropical Queensland (MTQ), Australia: this includes types and mentioned specimens of Veron & Wallace (1984), Wallace (1999), Wolstenholme et al. (2003), Veron (2000), (2003) and others). We assigned the fossil to the species group meeting the diagnostic character combinations (Wallace 1999). We also nominated a ‘most similar extant species’ for each fossil, based on comparisons with the collections.
3. Results
Of 22 skeletal characters already established for interpretation and analysis of extant Acropora, we found that 12 could be determined in the fossils (table 2). Most individual fossils had at least five of the 12 characters visible (see figure 3 for examples). No character states additional to those of present day Acropora were seen, although several modern states were missing. Seventeen specimens were either too small or too degraded to provide sufficient characters. We were able to assign the remaining 39 of the 56 available Acropora fossils to nine currently existing species groups, as well as to nominate a most similar extant species for each of these 39 fossils (electronic supplementary material, table 2). Two species groups, humilis and muricata, were represented by two morphologies presumed to be separate species (figure 4).
Table 2.
Skeletal characters for Acropora which were discernible in fossils, with their character states. (Scheme 1, character numbering used by Wallace (1999), scheme 2 that used by Wolstenholme et al. (2003). States include all known to be possible for extant material; no new states seen in the fossil specimens examined.)
| character | states | scheme | |
|---|---|---|---|
| 1 | 2 | ||
| branch formation | around a single axial corallite/more than one axial corallite | 1 | — |
| branching orders | tertiary or later orders: present/not present | 2 | 1 |
| predominant outline | cuneiform/arborescent/hispidose/encrusting/elkhorn/corymbose/tabulara | 3 | 2 |
| coenosteum | same on and between radial corallites/different on and between radial corallites | 6 | 4 |
| coenosteum on radial corallites | costate or reticulo-costatea/open spinules | 7 | 5 |
| coenosteum between radial corallites | reticulo-costate/reticulatea/open spinules | 8 | 6 |
| radial corallite sizes | one size or graded/two distinct sizesa | 10 | 8 |
| radial corallite inner wall | developed/not developed/neither inner or outer wall developed | 11 | 9 |
| radial corallite shape | nariform/dimidiate/lipped/tubular/appressed tubulara/conical/rounded appressed/round tubular/immersed | 12 | 10 |
| radial corallites relative size | very large/large/medium/small | 16 | 13 |
| maximum branch thickness | >20 mm /20–25 mm/10–19.9 mm/<5–9.9 mm /2.5–4.9 mm/<2.5 mm | 17 | 14 |
| radial crowding | radials do not touch/some radials touch/radials crowded, touching | 20 | 17 |
Character states illustrated in figure 3.
Figure 3.
Scanning electron micrographs of NHM fossils showing diagnostic characters. (a, b) R54844, France, Mid-Eocene, ‘latistella group’ slender branch with appressed tubular radial corallites (r) with costate coenosteum (c) and rounded openings (o) scales 1 mm and 500 μm, respectively. (c) R18265 Auvers, France, lower Bartonian, ‘hyacinthus group’ one of five vertical short branchlets with axial corallite indicating tabular colony (a) and radial corallites, scale 500 μm. (d) R54837 Paris, Eocene, ‘muricata group’ evenly sized and shaped radial corallites with costate walls and reticulate coenosteum (rc), scale 1 mm. (e) R54847 ‘aspera group’ with two distinct sizes of radial corallites, costate walls (cc) and reticulate coenosteum (rc), scale 1 mm.
Figure 4.
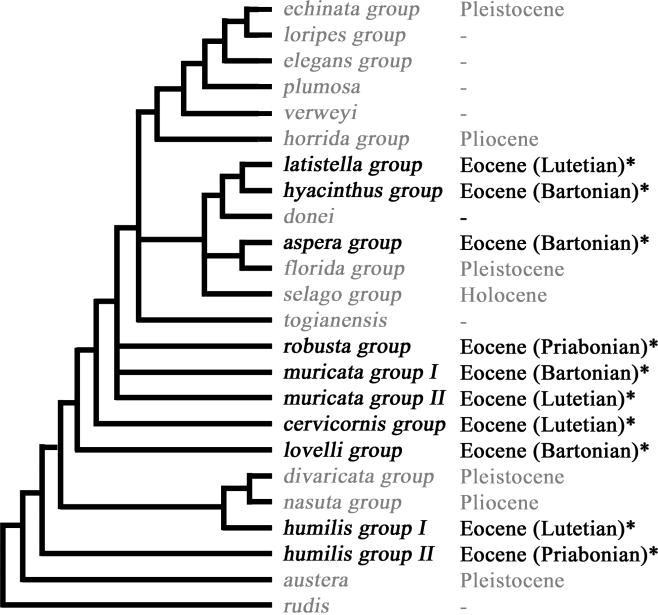
Morphology-based cladogram of species group relationships within genus Acropora updated from Wallace (1999), indicating earliest fossil occurrences: those from the present study indicated with an asterisk.
We found that morphologies identifiable to more than one-third of the currently recognized species groups of Acropora were present before the end of the Eocene epoch (figure 4). The greatest number of fossil forms (seven from six species groups) was recognized from the Bartonian samples (41.4–37.0 Myr) from Barton in England and Auvers-sur-Oise in France, with four forms from four species groups found before this in the Lutetian of France (49.0–41.3 Myr) and three forms (three groups) later in the Priabonian of England (36.0–34.2 Myr) (figure 5a).
Figure 5.
The fossil record of Acropora. (a) Occurrence of identified Acropora species groups in Eocene strata studied. Quotation marks denote poor precision of locality or age. (b) Occurrence of Acropora in major world regions over time. Dark bars indicate land barriers; earliest known fossil localities after Wilson & Rosen (1998), with subsequent time ranges interpolated from records in Rosen (1999), Wallace (1999), and our unpublished compilations.
4. Discussion
Our results, together with the existing knowledge of fossil Acropora summarized in Wallace (1999, p. 20), show that the genus was diverse (figure 5a), distributed in the Tethys seaway across three major world regions (figure 5b), and beginning to contribute to reef frameworks (Schuster 2002) ahead of the isolation of the Indo-Pacific from Europe. The earliest record, from Late Paleocene deposits in Berbera, Somalia (figure 1; Carbone et al. 1994), can be reasonably extrapolated to the western Indian Ocean, possibly to the then still open Tethyan seaway between Southwest Asia and Arabia.
Acropora had disappeared from the European record by the Mid-Miocene (Rosen 1999), by which time it was established throughout much of its Indo-Pacific range (figure 5b; Wallace 2002). While the genus is known from the Indo-Australian Arc in the Late Oligocene (Wilson & Rosen 1998), no Acropora material of Eocene or Early Oligocene age has been reported from the Pacific or central Indo-Pacific (figure 5b). This suggests that the varied and widespread Indo-Pacific Acropora of Late Oligocene to Miocene age were derived from a European–western Indian Ocean origin.
It has been argued that all extant Indo-Pacific Acropora species diversified from a single Pliocene ancestor, any other fossil lines having become extinct by the Mid-Miocene (Veron 1995; Fukami et al. 2000). The morphological similarity of the present collection of Eocene fossils to modern representatives of such a large number of groups (a finding noted by Duncan (1866), but disregarded since) supports an alternative proposal: that several species group lineages already present in the Europe–western Indian Ocean region during the Eocene survived to contribute to Indo-Pacific diversification. Evolutionary persistence of several groups, rather than origin from a single species ancestor, would be more compatible with widespread Indo-Pacific distribution of Acropora, including at least three modern species by the Miocene and continuation of Acropora as a reef dominant with many modern species widely distributed throughout the Plio–Pleistocene, in some cases more so than today (Wallace 2001; Wallace 2002).
There is insufficient stratigraphic or sedimentological documentation from which to infer the original autochthonous coral facies to which the fossils belonged and, thus, the actual conditions in which they lived. The specimens, being fragmentary, suggest a taphonomic history of colony break-up, followed by at least some transportation with subsequent detrital deposition and possibly re-working. The geology of the sedimentary basins which yielded our present samples has been intensively studied in an extensive literature going back over two centuries (see references table 1), but so far we have been unable to find any work showing that our samples could have been derived from reefal formations. However, Acropora today is not restricted to reefal biotopes (Wallace 1999; Veron 2000).
Typical colony shapes of the most similar modern species in the identified species groups include digitate, tabular, corymbose and arborescent forms (Wallace 1999). Missing are flat-branched and hispidose (‘bottle brush’) forms, found in the echinata, loripes, elegans and horrida species groups. The colony shapes present give useful clues to habitat type: in modern coral assemblages, they occur mostly on shallow parts of reefs, including intertidal reef flats, reef and rock edges, upper slopes and shallow shoals (usually less than 12 m depth, Wallace 1999). We interpret the fossil assemblages as occurring in shallow patches in embayments, similar to those presently found in Moreton Bay, Australia (27° S), which has seven Acropora species groups represented, including five of those identified from the European Eocene localities (C. C. Wallace, I. Fellegara & P. L. Harrison, unpublished data). Although high latitude daylight levels are sub-optimal for coral growth (Guinotte et al. 2003), this could be mitigated where the corals occur at very shallow depths (Harriott & Banks 2002) as in Moreton Bay (Johnson & Neil 1998) and in the Eocene localities discussed here.
Episodes of elevated sea surface temperatures (SSTs) have recently placed severe stress on modern tropical reefs (Lough 2000), and, in combination with increased atmospheric CO2, these may reduce the capacity of many tropical reefs to sustain carbonate accretion in the event of persistent global warming (Guinotte et al. 2003). Models of the interaction of aragonite saturation, SSTs and atmospheric CO2 indicate that it is unlikely that this will be compensated by new reef development in higher latitudes. Nevertheless, our demonstration of a diversity of Acropora existing in Northern Hemisphere Eocene assemblages provides a precedent for the survival of coral species at high latitude in warmer global temperatures, possibly contributing to preservation of genetic diversity in the event of depletion of species from lower latitudes.
Our findings support the proposal (Wilson & Rosen 1998) that the central Indo-Pacific, rather than being the locality of a global centre of origin for numerous coral genera and all Indo-Pacific species groups of Acropora as previously interpreted (Stehli & Wells 1971), was the locality of only a regional subset of speciation events. Pending future discovery of pre-Chattian Acropora in Southeast Asia, the Paleogene gap (Wilson & Rosen 1998) indicates that within this region, new species of Acropora could only have appeared from the Chattian (28–23 Myr) onwards. We propose that the existence of several species group lines, originating in the Eocene outside the central Indo-Pacific, subsequently allowed both speciation events within existing species groups and evolution of new groups, and therefore facilitated rapid speciation in the Indo-Pacific. This would be consistent with the observed non-concentric distribution of modern Acropora species ranges around the Southeast Asian centre of diversity (Wallace 2001; Wallace 2002), and with cladistic biogeographical interpretations made by Wallace et al. (1991) and Pandolfi (1992).
Fossil evidence of species group lines in Acropora offers the potential of further data points for calibration of a molecular clock for this exemplar coral group. Presently, calibration points for species evolution in corals are based only on time of first occurrence of the genus or higher level taxon, and this may have contributed to various dilemmas in accounting for apparent rapid sympatric speciation and widespread species distributions (Fukami et al. 2000; van Oppen et al. 2001). We can now offer minimum dates for the first appearances of nine species groups. This demonstrates the potential value of the fossil record in understanding evolutionary origins of modern biodiversity patterns.
Acknowledgments
The authors thank: P. Muir for assistance with all aspects of manuscript preparation; A. Jorre, Z. Richards and B. Chick for further assistance; P. Wilson, P. L. Harrison, R. P. M. Bak, J. Wolstenholme, A. Beltran-Torres, K. Johnson and Z. Dinesen for manuscript advice; M. Huber, J. Golonka, and F. Rögl for access to maps and simulations; A. Gale and C. Perrin for geological advice; C. Daniels for surveying the NHM Cenozoic collections; J. Darrell (NHM) for specimen loans, and NHM, MTQ and ARC Discovery grant to T.P. Hughes for funding support.
Supplementary Material
References
- Berggren W.A, Kent D.V, Swisher C.C, Aubry M.-P. SEPM; Oklahoma, Tulsa: 1995. Geochronology, time scales and global stratigraphic correlation. Special Publication No. 54. [Google Scholar]
- Budd A.F. Neogene paleontology in the northern Dominican Republic. The family Faviidae (Anthozoa: Scleractinia). Part I. The genera Montastraea and Solenastrea. Bull. Am. Paleontol. 1991;101:1–83. [Google Scholar]
- Budd A. Diversity and extinction in the Cenozoic history of Caribbean reefs. Coral Reefs. 2000;19:25–35. 10.1007/s003380050222 [Google Scholar]
- Carbone F, Matteucci R, Pignatti J.S, Russo A. Facies analysis and biostratigraphy of the Auradu limestone formation in the Berbera-Sheikh area, north-western Somalia. Geol. Romana. 1994;29:213–235. [Google Scholar]
- Cavelier C, Labourguigne J, Mégnien C, Mégnien F, Pomerol C, Wyn R. Eocène superieur. In: Mégnien C, Mégnien F, editors. Synthèse géologique du Bassin de Paris. Stratigraphie et Paléogeographie, vol. I. Mémoires du Bureau de Recherches Géologiques et Minières, mémoire. Vol. 101. 1980. 379–399. [Google Scholar]
- Davis A.G. The Brockenhurst beds at Victoria Tilery, Brockenhurst, Hampshire. Proc. Geol. Assoc. 1952;63:215–219. [Google Scholar]
- Debrand-Passard, S. (ed.) 1980 Synthèse géologique du Bassin de Paris Atlas, vol. II. Mémoires du Bureau de Recherches Géologiques et Minières, mémoire 102.
- Duncan P.M. Palaeontolographical Society; London: 1866. A monograph of the British fossil corals. Second series. Part 1. Introduction; corals from the Tertiary formations. [Google Scholar]
- Forey P.L, Fortey R.A, Kenrick P, Smith A.B. Taxonomy and fossils: a critical appraisal. Phil. Trans. R. Soc. B. 2004;359:639–653. doi: 10.1098/rstb.2003.1453. 10.1098/rstb.2003.1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami H, Omori M, Hatta M. Phylogenetic relationships in the coral family Acroporidae, reassessed by inference from the mitochondrial genes. Zool. Sci. 2000;17:689–696. doi: 10.2108/zsj.17.689. 10.2108/zsj.17.689 [DOI] [PubMed] [Google Scholar]
- Gale A.S, Jeffery P.A, Huggett J.M, Connolly P. Eocene inversion history of the Sandown Pericline, Isle of Wight, southern England. J. Geol. Soc. Lond. 1999;156:327–339. [Google Scholar]
- Golonka J. Uniwersytetu Jagiellońskiego; Kraków: 2000. Cambrian–Neogene: plate tectonic maps. [Google Scholar]
- Guinotte J.M, Buddemeier R.W, Kleypas J.A. Future coral reef habitat marginality: temporal and spatial effects of climate change in the Pacific basin. Coral Reefs. 2003;22:551–558. 10.1007/s00338-003-0331-4 [Google Scholar]
- Harriott V.J, Banks S.A. Latitudinal variation in coral communities in eastern Australia: a qualitative biophysical model of factors regulating coral reefs. Coral Reefs. 2002;21:83–94. [Google Scholar]
- Harris G.F, Burrows H.W. The Geologists' Association; London: 1891. The Eocene & Oligocene beds of the Paris Basin. [Google Scholar]
- Huber M, Caballero R. Eocene El Niño: evidence for robust tropical dynamics in the “Hothouse”. Science. 2003;299:877–881. doi: 10.1126/science.1078766. 10.1126/science.1078766 [DOI] [PubMed] [Google Scholar]
- Insole A, Daley B. A revision of the lithostratigraphical nomenclature of the Late Eocene and Early Oligocene strata of the Hampshire Basin, Southern England. Tertiary Res. 1985;7:67–100. [Google Scholar]
- Johnson P.R, Neil D.T. The corals of Moreton Bay: living with extremes. In: Tibbets I.R, Hall N.J, Dennison W.C, editors. Moreton Bay and catchment. School of Marine Science, University of Queensland; Brisbane: 1998. pp. 503–524. [Google Scholar]
- Kleypas J.A, Buddemeier R.W, Gattuso J.P, Langdon C, Opdyke B.N. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science. 1999;284:118–120. doi: 10.1126/science.284.5411.118. 10.1126/science.284.5411.118 [DOI] [PubMed] [Google Scholar]
- Kleypas J.A, Buddemeier R.W, Gattuso J.-P. The future of coral reefs in an age of global change. Earth Sci. (Geol. Rundsch.) 2001;90:426–437. 10.1007/s005310000125 [Google Scholar]
- Lough J. 1997–98: unprecedented thermal stress to coral reefs? Geophys. Res. Lett. 2000;27:3901–3904. 10.1029/2000GL011715 [Google Scholar]
- McCall J, Rosen B.R, Darrell J.G. Carbonate deposition in accretionary prism settings: Early Miocene coral limestones and corals of the Makran mountain range in southern Iran. Facies. 1994;31:141–178. [Google Scholar]
- Mégnien, F. (ed.) 1980 Synthèse géologique du Bassin de Paris. Lexique des noms de formation, vol III. Mémoires du Bureau de Recherches Géologiques et Minières, mémoire 103.
- Mégnien, C. & Mégnien, F. (eds) 1980 Synthèse géologique du Bassin de Paris. Stratigraphie et paléogéographie, vol. I. Mémoires du Bureau de Recherches Géologiques et Minières, mémoire 101.
- Melville R.V, Freshney E.C. 4th edn. Institute of Geological Sciences, Her Majesty's Stationery Office; London: 1982. British regional geology. The Hampshire Basin and adjoining areas. [Google Scholar]
- Oken L. Steinkorallen. Lehrbuch Naturgesch. 1815;3:59–74. [Google Scholar]
- Opdyke B.N, Wilkinson B.H. Carbonate mineral saturation state and cratonic limestone accumulation. Am. J. Sci. 1993;293:217–234. [Google Scholar]
- Pandolfi J.M. Successive isolation rather than evolutionary centres for the origination of Indo-Pacific reef corals. J. Biogeogr. 1992;19:593–609. [Google Scholar]
- Pomerol C. Ellis Horwood Limited; Chichester: 1982. The Paleogene in France [Transl. D. W. Humphries & E. Humphries] [Google Scholar]
- Potts D.C. Sea-level fluctuations and speciation in Scleractinia. In: Hamelin V, Salvat B, editors. Proc. 5th Int. Coral Reef Congr. vol. 4. International Society of Reef Studies; Tahiti: 1985. pp. 127–132. [Google Scholar]
- Rosen B.R. Palaeoclimatic implications of the energy hypothesis from Neogene corals of the Mediterranean region. In: Agusti J, Rook L, Andrews P.J, editors. Hominoid evolution and climatic change in Europe. Cambridge University Press; Cambridge, UK: 1999. pp. 309–327. [Google Scholar]
- Schuster F. Oligocene and Miocene examples of Acropora dominated palaeo-environments: Mesohellenic Basin (NW Greece) and northern Gulf of Suez (Egypt) In: Moosa M.K, et al., editors. Proc. 9th Int. Coral Reef Symp. vol. 1. International Society of Reef Studies; Bali: 2002. pp. 199–204. [Google Scholar]
- Stehli F.G, Wells J.W. Diversity and age patterns in hermatypic corals. Syst. Zool. 1971;20:115–126. [Google Scholar]
- van Oppen M, McDonald B, Willis B, Miller D. The evolutionary history of the coral genus Acropora (Scleractinia, Cnidaria) based on a mitochondrial and a nuclear marker: reticulation, incomplete lineage sorting or morphological convergence? Mol. Biol. Evol. 2001;18:1315–1329. doi: 10.1093/oxfordjournals.molbev.a003916. [DOI] [PubMed] [Google Scholar]
- Veron J.E.N. University of New South Wales Press; Sydney: 1995. Corals in space and time: biogeography and evolution of the Scleractinia. [Google Scholar]
- Veron J.E.N. Australian Institute of Marine Science; Townsville: 2000. Corals of the world. [Google Scholar]
- Veron J.E.N. Australian Institute of Marine Science; Townsville: 2003. New species described in corals of the world. [Google Scholar]
- Veron J.E.N, Wallace C.C. Australian Institute of Marine Science Monograph Series; Townsville: 1984. Scleractinia of Eastern Australia. Part V. Family Acroporidae. [Google Scholar]
- Volmer S.V, Palumbi S.R. Hybridization and the evolution of reef coral diversity. Science. 2002;296:2023–2025. doi: 10.1126/science.1069524. 10.1126/science.1069524 [DOI] [PubMed] [Google Scholar]
- Wallace C.C. CSIRO Publishing; Melbourne: 1999. Staghorn corals of the world: a revision of the coral genus Acropora (Scleractinia; Astrocoeniina; Acroporidae) worldwide, with emphasis on morphology, phylogeny and biogeography. [Google Scholar]
- Wallace C.C. Wallace's line and marine organisms: the distribution of staghorn corals (Acropora) in Indonesia. In: Metcalf I, editor. Faunal and floral migrations and evolution in SE Asia–Australasia. Balkema; Rotterdam: 2001. pp. 168–178. [Google Scholar]
- Wallace C. Journey to the heart of the centre—origins of high faunal diversity in the central Indo-Pacific from the perspective of an acropologist. In: Moosa M.K, et al., editors. Proc. 9th Int. Coral Reef Symp. vol. 1. International Society of Reef Studies; Bali: 2002. pp. 33–39. [Google Scholar]
- Wallace C.C, Willis B.L. Systematics of the coral genus Acropora: implications of new biological findings for species concepts. Annu. Rev. Ecol. Syst. 1994;25:237–262. [Google Scholar]
- Wallace C.C, Pandolfi J.M, Young A, Wolstenholme J. Indo-Pacific coral biogeography: a case study from the Acropora selago group. Aust. Syst. Bot. 1991;4:199–210. 10.1071/SB9910199 [Google Scholar]
- Wilson M.E.J. Cenozoic carbonates in Southeast Asia: implications for equatorial carbonate development. Sediment. Geol. 2002;147:295–428. 10.1016/S0037-0738(01)00228-7 [Google Scholar]
- Wilson M.E.J, Rosen B.R. Implications of paucity of corals in the Paleogene of SE Asia: plate tectonics or centre of origin? In: Hall R, Holloway J.D, editors. Biogeography and geological evolution of SE Asia. Backbuys Publishers; Leiden: 1998. pp. 165–195. [Google Scholar]
- Wolstenholme J.K. Temporal reproductive isolation and gametic compatibility are evolutionary mechanisms in the Acropora humilis species group (Cnidaria; Scleractinia) Mar. Biol. 2003;144:567–582. 10.1007/s00227-003-1209-2 [Google Scholar]
- Wolstenholme J.K, Wallace C.C, Chen C.A. Species boundaries within the Acropora humilis species group (Cnidaria; Scleractinia): a morphological and molecular interpretation of evolution. Coral Reefs. 2003;22:155–166. 10.1007/s00338-003-0299-0 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.