Abstract
Many birds exhibit short-term, reversible adjustments in basal metabolic rate (BMR), but the overall contribution of phenotypic plasticity to avian metabolic diversity remains unclear. The available BMR data include estimates from birds living in natural environments and captive-raised birds in more homogenous, artificial environments. All previous analyses of interspecific variation in BMR have pooled these data. We hypothesized that phenotypic plasticity is an important contributor to interspecific variation in avian BMR, and that captive-raised populations exhibit general differences in BMR compared to wild-caught populations. We tested this hypothesis by fitting general linear models to BMR data for 231 bird species, using the generalized least-squares approach to correct for phylogenetic relatedness when necessary. The scaling exponent relating BMR to body mass in captive-raised birds (0.670) was significantly shallower than in wild-caught birds (0.744). The differences in metabolic scaling between captive-raised and wild-caught birds persisted when migratory tendency and habitat aridity were controlled for. Our results reveal that phenotypic plasticity is a major contributor to avian interspecific metabolic variation. The finding that metabolic scaling in birds is partly determined by environmental factors provides further support for models that predict variation in scaling exponents, such as the allometric cascade model.
Keywords: metabolic scaling, captivity, allometry, basal metabolic rate
1. Introduction
Physiological diversity arises from several fundamental sources of phenotypic variation. Most analyses of metabolic diversity in endotherms have focused on the allometric scaling of metabolic traits with body mass (Mb; Lasiewski & Dawson 1967; McNab 1988; Lovegrove 2000; White & Seymour 2003; Savage et al. 2004) and/or metabolic adaptation to physical and/or biotic characteristics of environments (Scholander et al. 1950; McNab 1986; Lovegrove 2000, 2003; Tieleman & Williams 2000; Mueller & Diamond 2001; Wikelski et al. 2003; Anderson & Jetz 2005). Recently, however, there has been increasing interest in phenotypic plasticity as an additional source of metabolic variation. Phenotypic plasticity may involve phenotypic flexibility (short-term, reversible changes within an individual) and/or developmental plasticity (irreversible changes that result from developmental processes; Piersma & Drent 2003). In birds, there is increasing evidence for considerable phenotypic flexibility in metabolic rates (Tieleman et al. 2003; Swanson in press) and in several digestive and thermoregulatory traits (Martinez del Rio et al. 1995; Karasov 1996; Levey et al. 1999; Tieleman & Williams 2002; McKechnie & Wolf 2004b). Avian metabolic flexibility appears to be most pronounced in long-distance migrants and year-round residents at high latitudes (Piersma et al. 1995; Dawson & O'Conner 1996; Swanson & Olmstead 1999; Battley et al. 2001; Lindström & Klaassen 2003; Swanson in press), but is also evident in non-migrant species from the tropics and sub-tropics (Ambrose & Bradshaw 1988; Tieleman et al. 2003). Many mammals exhibit quantitatively similar phenotypic flexibility, with the seasonal direction of metabolic adjustments varying with Mb (Lovegrove 2005).
Most comparative analyses of avian metabolic rate have examined variation in basal metabolic rate (BMR), the lower limit of avian metabolic heat production (e.g. Weathers 1979; Daan et al. 1990; Rezende et al. 2002). BMR is a standardized baseline metabolic parameter and represents maintenance energy demand in the absence of increases in metabolism associated with thermoregulation, digestion, activity or circadian rhythms (McNab 1997; Swanson in press). Although there is evidence that at least some free-ranging birds adjust their BMR over relatively short time-scales, these phenotypic adjustments have been investigated in too few species to reveal the nature of BMR reaction norms (sensu Schlichting & Pigliucci 1998). Quantitative variation in the capacity for phenotypic adjustments among taxa remains unclear, as do the ways in which natural selection acts on the capacity to adjust BMR in response to fluctuating environmental conditions (Tieleman et al. 2003). The realization that phenotypic plasticity greatly complicates the identification of metabolic adaptation has led to some authors employing common-garden experimental designs. Wikelski et al. (2003), for instance, recently partitioned intraspecific BMR variation in stonechats (Saxicola torquata) into phenotypic plasticity and genotypic adaptation by raising birds from geographically distinct populations under identical conditions before comparing metabolic and behavioural parameters among populations.
The short-term adjustments in BMR exhibited by many birds (e.g. Piersma et al. 1995; Tieleman et al. 2003; Klaassen et al. 2004) raise the possibility that scaling relationships between BMR and Mb are influenced by phenotypic flexibility and/or developmental plasticity in the individuals used for metabolic measurements. Although short-term adjustments in BMR have been examined in relatively few species, the signal of phenotypic plasticity can potentially be identified in avian BMR data sets by accounting for the origins of populations used for metabolic measurements. In some species, BMR was measured in individuals that had recently been caught from free-ranging populations, but in other cases BMR estimates were obtained from individuals that had been bred and/or raised in captivity. Hence, the available avian BMR data permit a comparative analysis of the BMR of birds from natural environments and that of captive-raised birds. Compared to populations in natural environments, birds raised in captivity can be viewed as belonging to experimental populations maintained under conditions of reduced thermal variability, greater quantitative and qualitative homogeneity of energy, nutrient and water resources and reduced levels of activity. We hypothesized that captive-raised birds exhibit general metabolic differences to individuals from natural environments, manifested either as differences in the allometric scaling coefficients for BMR, or as Mb-independent differences in BMR magnitude. Because several factors associated with captive conditions could potentially lead to metabolic adjustments, it is difficult to make specific predictions concerning the direction of differences in BMR between wild-caught and captive-raised birds. For instance, the available evidence suggests that increased food intake can be expected to lead to increases in the mass of metabolically active organs such as the stomach, liver and intestines, and hence elevated BMR (Karasov 1996; Mueller & Diamond 2001). In contrast, more moderate minimum environmental temperatures in captivity can be expected to result in reduced BMR (Tieleman et al. 2003; Swanson in press).
2. Material and methods
(a) Basal metabolic rate data
We obtained BMR (W) and Mb (g) data for 231 avian species from the literature (Appendix 1, electronic supplementary material). Observed metabolic rates were included if they were measured during the rest phase of the circadian cycle at thermoneutral air temperatures in resting individuals that could reasonably be assumed to be postabsorptive (i.e. data that met inclusion criteria 1–3 in McKechnie & Wolf 2004a). We opted to include data irrespective of sample size, since this approach approximately doubled the size of our data set. Although some of the data we included represented means for only one or two individuals, the BMR of an individual is equally likely to be above or below the population mean. Hence, it is improbable that our conclusions were significantly affected by the inclusion of these data. All Mb and BMR data were log-transformed prior to analysis. For each datum, we consulted the original source and classified the population in which BMR was measured as wild-caught (birds obtained from wild populations, and which typically spent periods ranging from several days to weeks in captivity before BMR measurements) or captive-raised (birds that hatched in captivity and/or spent most of their lives in captivity). We excluded several taxa with large Mb that were represented in only the wild-caught category, namely the ostrich (Struthio camelus) and members of the Procellaridae, Laridae and Spheniscidae.
(b) Data analyses
We constructed a phylogeny (Appendix 2, electronic supplementary material) based primarily on Sibley & Ahlquist's (1990) DNA-hybridization data, using the phylogenies in Reynolds & Lee (1996), Tieleman & Williams (2000), McKechnie (2001), Schleucher (2002), Tieleman et al. (2002) and McKechnie & Wolf (2004a). We used a generalized least-squares (GLS) approach, using the phylogeny to account for covariance among the species (Pagel 1994; Martins & Hansen 1997; Pagel 1999; Freckleton et al. 2002). We assessed the need for phylogenetic correction by calculating the parameter λ for each model (Pagel 1999; Freckleton et al. 2002). The λ-statistic adjusts the phylogenetic correction according to the degree of phylogenetic dependence in the residuals of the linear model. A λ-value of zero indicates that the residuals show no phylogenetic structure, whilst a λ-value of one indicates that there is close phylogenetic dependence. We estimated λ using a maximum likelihood approach and used this value in the analysis. We then fitted general linear models to the data, using BMR as the continuous dependent variable and successively adding the following independent variables: Mb (continuous), passerine/non-passerine (categorical), population origin (categorical: wild-caught or captive-raised), migratory tendency (categorical: migrant or non-migrant) and habitat aridity (categorical: desert or non-desert). We added the latter two variables to control for potential bias in the data since several migratory species are known to exhibit large seasonal changes in BMR (Piersma et al. 1995; Battley et al. 2001; Lindström & Klaassen 2003), and birds inhabiting arid habitats have significantly lower BMR than their mesic counterparts (Tieleman & Williams 2000). We included a categorical variable for passerine or non-passerine membership for statistical reasons since there are significant differences in Mb (Garland & Ives 2000) and potential grade-shifts between the two clades. After adding each independent variable, we checked for changes in the Akaike Information Criterion (Sakamoto et al. 1986) and adjusted r2-values, and tested for interaction terms indicating heterogeneity of slopes.
3. Results
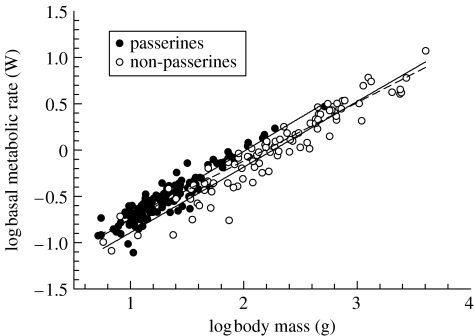
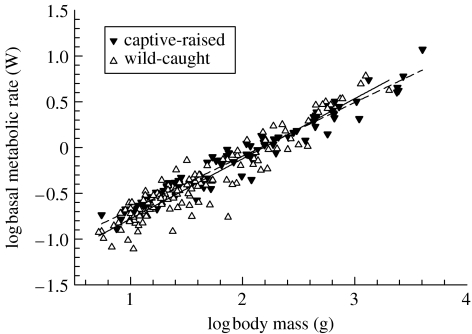
In the GLS framework accounting for phylogenetic relatedness, the overall relationship between BMR (W) and Mb (g) was best described by the linear regression log BMR=0.623 log Mb−1.371. However, the overall scaling exponent was affected by the inclusion of non-passerine species with larger Mb than any of the passerines, and the significantly higher absolute BMR of passerines compared to non-passerines (F1,230=183.04, p<0.001; figure 1). When we controlled for non-passerine/passerine membership, the scaling exponent shifted to 0.707, reflecting the slopes calculated separately for passerines (0.717, 95% CI: 0.047) and non-passerines (0.699, 95% CI: 0.045; table 1). Next we tested for a difference in BMR between wild-caught (N=137) and captive-raised (N=94) birds. There was a significant effect of population origin on the slope of the Mb-dependence of BMR (table 1). Captive-raised birds showed a shallower scaling coefficient (0.670, 95% CI: 0.035) than wild-caught birds (0.744, 95% CI: 0.047) after passerine/non-passerine membership was controlled for (figure 2). The two regression lines intersect at log Mb=2.284 g and log BMR=0.029 W. Interaction terms for (i) population origin and non-passerine/passerine membership and (ii) population origin, body mass and non-passerine/passerine membership were non-significant. The differences in scaling coefficients between wild-caught and captive-raised birds remained significant after migratory tendency and habitat aridity were controlled for (table 1).
Figure 1.
The relationship between basal metabolic rate (BMR) and body mass (Mb) in passerine birds (upper solid line), non-passerine birds (lower solid line) and across both clades (dashed line). The lines are generalized least-squares regressions controlling for phylogeny (see table 1). The inclusion of non-passerine species with Mb greater than the range of passerine Mb affects the overall slope.
Table 1.
Generalized least-squares model for variation in avian basic metabolic rate (BMR). (Model results denote the intercept, the effect of body mass, passerine/non-passerine membership and the origin of populations used for BMR measurements, i.e. captive-raised or wild-caught (origin). For each variable, the parameter estimate (b) is provided. We also tested for the effects of migratory tendency (migrant or non-migrant) and habitat aridity (desert or non-desert environment). The parameter λ provides a measure of the phylogenetic dependence of BMR (see Freckleton et al. 2002), and can vary from zero to one, with higher values indicating an increasing degree of phylogenetic dependence. Statistical significance is indicated with asterices: statistical significance at *p<0.05, **p<0.01 and ***p<0.001.)
| variable | λ | b | F | r2 (adj) |
|---|---|---|---|---|
| intercept | 0.130*** | −1.371 | 0.916 | |
| body mass | 0.623 | 2504.15*** | ||
| intercept | 0.158*** | −1.604 | 0.932 | |
| body mass | 0.707 | 1993.27*** | ||
| passerine/non-passerine | 0.158 | 57.10*** | ||
| intercept | 0.000 | −1.676 | 0.939 | |
| body mass | 0.743 | 1489.80*** | ||
| passerine/non-passerine | 0.160 | 61.69*** | ||
| origin | 0.120 | 8.33*** | ||
| origin×body mass | −0.070 | 9.97*** | ||
| intercept | 0.000 | −1.665 | 0.944 | |
| body mass | 0.727 | 1495.37*** | ||
| passerine/non-passerine | 0.136 | 44.68*** | ||
| migratory tendency | 0.067 | 20.54*** | ||
| origin | 0.119 | 8.33** | ||
| origin×body mass | −0.050 | 5.29* | ||
| intercept | 0.000 | −1.706 | 0.940 | |
| body mass | 0.741 | 1499.62*** | ||
| passerine/non-passerine | 0.153 | 55.15*** | ||
| habitat aridity | 0.045 | 2.07* | ||
| origin | 0.167 | 16.32*** | ||
| origin×body mass | −0.071 | 10.47** |
Figure 2.
The scaling relationship between basal metabolic rate (BMR) and body mass (Mb) differs between birds from wild-caught and captive-raised populations. The dashed line is the scaling relationship (log BMR=0.670 log Mb−1.501 (N=94)) for captive-raised birds calculated from a generalized least-squares model, which accounts for phylogenetic relatedness and Mb differences between passerines and non-passerines. The solid line is the corresponding relationship for wild-caught birds (log BMR=0.744 log Mb−1.670 (N=137)).
The relationships between BMR and Mb and passerine/non-passerine membership showed significant phylogenetic dependences, with λ-values significantly different from zero (table 1). However, phylogenetic dependence decreased with the inclusion of additional independent variables, and λ-values reached zero after population origin, migratory tendency and desert/non-desert were each added to the model (table 1), indicating that identical parameter values would be estimated in a conventional analysis. Our analysis suggests that the phylogenetic dependence in BMR arose mainly as a consequence of phylogenetic dependence in the predictor variables, and that once these had been accounted for the residuals exhibited phylogenetic independence. The observation that the position of a species in a BMR–Mb relationship does not depend directly on phylogeny accords with the hypothesis that phenotypic plasticity is an important contributor to metabolic diversity.
4. Discussion
By accounting for the historical origins of bird populations used for metabolic measurements, we have extracted a signal of phenotypic plasticity from a large avian BMR data set, and revealed interactions between two of the fundamental sources of avian metabolic variation. To the best of our knowledge, this is the first study to verify that phenotypic plasticity is a major contributor to Mb-dependent interspecific variation in an endotherm physiological trait.
Our results support the hypothesis that phenotypic adjustments to artificial environments lead to general metabolic differences between captive-raised birds and their wild-caught counterparts. Almost no data exist on the effects of captivity on avian metabolic parameters within species. In the only such study of which we are aware, the BMR of freshly caught apapanes (Himatione sanguinea) was similar to that of individuals that had been in captivity for one year (Weathers et al. 1983). For a few species, BMR estimates are available for captive-raised as well as wild-caught populations. For instance, the BMR of wild-caught speckled mousebirds (Colius striatus) was 0.236 W (McKechnie & Lovegrove 2001a), equivalent to approximately 75% of that of second- and third-generation captives in an earlier study (Bartholomew & Trost 1970). The shallower scaling exponent for captive-raised birds reflects the fact that metabolic adjustments to artificial environments vary with Mb (figure 2). In general, small species upregulate BMR in captivity, whereas large species downregulate BMR (figure 2). The non-significance of interactions between population origin and non-passerine/passerine membership, and population origin, body mass and non-passerine/passerine membership, indicates that metabolic responses to captivity do not differ in a consistent way between passerines and non-passerines.
Metabolic adjustments to captive conditions are potentially driven by several fundamental differences between natural and artificial environments. One major category of differences concerns the availability and/or quality of food provided in captivity. The digestive tract morphology of many species is highly plastic, with large changes in organ masses occurring in response to variation in food intake and/or quality (Karasov 1996; Starck 1999; Dekinga et al. 2001). For instance, several species rapidly adjust gizzard mass in response to the fibre content of their diet (Starck 1999; Dekinga et al. 2001). The observation that the caeca and small intestines of red grouse (Lagopus lagopus) decreased in length when fed an artificial diet in captivity (Moss 1972) suggests that switches from natural to artificial diets may be accompanied by major changes in digestive tract morphology. In view of the phenotypic flexibility in body composition exhibited by many birds, it is likely that adjustments of organ masses are responsible for at least some of the metabolic variation observed in this study. Besides changes in organ masses, limited exercise in captivity could lead to reductions in the mass and/or metabolic intensity of flight muscles. Phenotypic flexibility in avian body composition, and the BMR variation that arises from changes in organ and/or muscle masses, results in the slopes of intraspecific BMR allometries generally being steeper than those of interspecific scaling relationships (Kvist & Lindström 2001; Battley et al. 2001).
The Mb-dependence of metabolic responses to captivity and the consequent differences in metabolic scaling between birds from wild-caught and captive-raised populations reveal that the origins of study populations need to be taken into account when testing hypotheses concerning metabolic adaptation. Analyses testing for metabolic divergence among taxa may produce misleading results if the BMR of wild-caught populations is compared to that of captive-raised populations, or vice versa. The assumption that the BMR of captive-raised individuals is representative of wild individuals is widespread in comparative studies, and may be explicit (e.g. Schleucher & Withers 2002) or implicit (e.g. McNab 2001).
Our results call into question whether adaptive variation in endotherm metabolic traits reflects adaptation through natural selection, phenotypic plasticity, or some combination of these two sources of phenotypic variation. Many studies have implicitly assumed that inter- and/or intraspecific metabolic variation constitutes adaptation, both in phylogenetically independent and conventional analyses (Lovegrove 2000, 2003; Merola-Zwartjes & Ligon 2000; McNab 2003). In the case of avian BMR, this assumption is unjustified, since phenotypic adjustments contribute significantly to phylogenetically independent variation. In view of our results, we suggest that attempts to correlate avian BMR with ecological factors should focus on the BMR of wild-caught birds. However, caution needs to be exercised in inferring adaptation, unless inter- and/or intraspecific data are collected under carefully controlled common garden conditions (Wikelski et al. 2003; Broggi et al. 2005). Another approach to identifying physiological adaptation while explicitly accounting for phenotypic plasticity has been employed by Tieleman et al. (2003), who hypothesized that larks (Alaudidae) inhabiting unpredictable arid habitats have evolved greater phenotypic flexibility in BMR than species in mesic habitats. These authors tested their hypothesis by comparing BMR adjustments in response to thermal acclimation among four species of larks (Tieleman et al. 2003).
We have assumed that the differences in BMR between wild-caught and captive-raised birds reflect phenotypic adjustments, and not genotypic divergence. Captive populations presumably experience some degree of artificial selection favouring individuals that breed under captive conditions, and/or selection for particular traits such as plumage colour. However, we consider it unlikely that captive-raised birds experience strong enough selection on metabolic traits to cause the fundamental differences in the scaling of BMR demonstrated here. Metabolic adjustments to captive conditions could potentially involve developmental plasticity and/or phenotypic flexibility, but our analyses do not allow us to distinguish between these two forms of phenotypic plasticity. Avian BMR can be significantly adjusted over time-scales of days to weeks (Lindström 1997; Battley et al. 2001; Tieleman et al. 2003), and the fact that many of the wild-caught populations included in this analysis spent several weeks in captivity before BMR measurements (e.g. McKechnie & Lovegrove 2001b, 2003) suggests that the differences between wild-caught and captive-raised birds partly reflect developmental effects.
The fractal geometry and allometric cascade models proposed by West et al. (1997) and Darveau et al. (2002), respectively, have rekindled the long-standing debate surrounding the processes underlying allometric scaling patterns, and specifically the exponent(s) relating metabolic rate to body size (Banavar et al. 2002; West et al. 2003; White & Seymour 2003; Savage et al. 2004). Our finding that avian BMR scales differently depending on the origins of populations used for metabolic measurements has important implications for this debate, since it reveals (i) that BMR–Mb relationships in birds cannot be described by a single, universal scaling exponent, and (ii) that scaling exponents are environment-dependent. The scaling model of West et al. is based on general physical properties of resource distribution networks and, under the commonly used assumptions and optimal conditions, predicts an exponent of 0.75 for the scaling of whole-organism metabolic rate (West et al. 1997; Savage et al. 2004). Banavar et al. (2002) proposed a similar model that predicts limited variation in scaling exponents around 0.75, arising from inefficiencies in resource distribution networks or compensating physiological mechanisms. The allometric cascade model of Darveau et al. (2002) also predicts variation in scaling exponents, but on the basis of differences in the respective contributions and coefficients of multiple rate-limiting steps in energy demand and supply pathways. In an expansion of the original formulation of the allometric cascade model, Hochachka et al. (2003) identified numerous potential intrinsic and extrinsic contributors to overall metabolic scaling. Although not specifically predicted by Darveau et al. (2002) or Hochachka et al. (2003), many of the factors that contribute to metabolic scaling can a priori be expected to differ between natural environments and captive conditions. Factors characteristic of captive conditions, such as increased food intake, reduced exercise, and different patterns of time and energy allocation, likely affect several of the contributors to overall control of metabolic scaling (see fig. 1 of Hochachka et al. 2003). Our finding that scaling exponents for avian BMR are not fixed, but determined in part by environmental factors, argues against the existence of a universal exponent of 0.75 and lends further support to allometric scaling models that explicitly predict variation in scaling exponents.
In summary, we have identified a signal of phenotypic plasticity in avian metabolic scaling, manifested as different scaling coefficients for BMR in captive-raised and wild-caught birds. The environmental factors and physiological mechanisms underlying these differences remain to be identified, and the relative contributions of developmental plasticity and phenotypic flexibility are unclear. Phenotypic plasticity in traits such as BMR significantly complicates the process of identifying physiological adaptation. Increasingly sophisticated approaches to correcting for the phylogenetic non-independence of comparative data (Felsenstein 1985; Garland et al. 1992, 1993; Pagel 1999; Garland & Ives 2000; Martins 2000; Freckleton et al. 2002; Martins et al. 2002) have yielded new answers to long-standing questions in ecological and evolutionary physiology. Our results highlight a major new challenge: the partitioning of variation in physiological traits into genetic adaptation and phenotypic plasticity. Our results also call into question whether phylogenetically independent interspecific variation in metabolic traits represents true adaptation arising from genetic divergence through natural selection, or environment-mediated phenotypic adjustments.
Acknowledgments
We thank Van Savage (Harvard University) for insightful comments. A.E.M. received funding from the National Research Foundation, the University of the Witwatersrand and the DST/NRF Centre of Excellence at the Percy FitzPatrick Institute. R.P.F. is a Royal Society University Research Fellow. W.J. was funded by the Emmy Noether Program of the Deutsche Forschungsgesellschaft (DFG), and would like to thank J. H. Brown (University of New Mexico) for support. The study was facilitated by an Astor Travel Grant from the University of Oxford to R.P.F. and W.J.
Supplementary Material
References
- Ambrose S.J, Bradshaw S.D. Seasonal changes in standard metabolic rates in the white-browed scrubwren Sericornis frontalis (Acanthizidae) from arid, semi-arid and mesic environments. Comp. Biochem. Physiol. A. 1988;89:79–83. 10.1016/0300-9629(88)91142-5 [Google Scholar]
- Anderson K.J, Jetz W. The broad-scale ecology of energy expenditure of endotherms. Ecol. Lett. 2005;8:310–318. 10.1111/j.1461-0248.2005.00723.x [Google Scholar]
- Banavar J.R, Damuth J, Maritan A, Rinaldo A. Supply–demand balance and metabolic scaling. Proc. Natl Acad. Sci. USA. 2002;99:1056–1059. doi: 10.1073/pnas.162216899. 10.1073/pnas.162216899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew G.A, Trost C.H. Temperature regulation in the speckled mousebird, Colius striatus. Condor. 1970;72:141–146. [Google Scholar]
- Battley P.F, Dekinga A, Dietz M.W, Piersma T, Tang S, Hulsman K. Basal metabolic rate declines during long-distance migratory flight in great knots. Condor. 2001;103:838–845. [Google Scholar]
- Broggi J, Hohtola E, Orell M, Nilsson J.-Å. Local adaptation to winter conditions in a passerine spreading north: a common-garden approach. Evolution. 2005;59:1600–1603. [PubMed] [Google Scholar]
- Daan S, Masman D, Groenewold A. Avian basal metabolic rates: their association with body compostition and energy expenditure in nature. Am. J. Physiol. 1990;R333:R340. doi: 10.1152/ajpregu.1990.259.2.R333. [DOI] [PubMed] [Google Scholar]
- Darveau C.-A, Suarez R.K, Andrews R.D, Hochachka P.W. Allometric cascade as a unifying principle of body mass effects on metabolism. Nature. 2002;417:166–170. doi: 10.1038/417166a. 10.1038/417166a [DOI] [PubMed] [Google Scholar]
- Dawson W.R, O'Conner T.P. Energetic features of avian thermoregulatory responses. In: Carey C, editor. Avian energetics and nutritional ecology. Chapman & Hall; New York: 1996. pp. 85–124. [Google Scholar]
- Dekinga A, Dietz M.W, Koolhaas A, Piersma T. Time course and reversability of changes in the gizzards of red knots alternately eating hard and soft food. J. Exp. Biol. 2001;204:2167–2173. doi: 10.1242/jeb.204.12.2167. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. 10.1086/284325 [DOI] [PubMed] [Google Scholar]
- Freckleton R.P, Harvey P.H, Pagel M. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 2002;160:712–726. doi: 10.1086/343873. 10.1086/343873 [DOI] [PubMed] [Google Scholar]
- Garland T, Ives A.R. Using the past to predict the present: confidence intervals for regression equations in phylogenetic comparative methods. Am. Nat. 2000;155:346–364. doi: 10.1086/303327. 10.1086/303327 [DOI] [PubMed] [Google Scholar]
- Garland T, Harvey P.H, Ives A.R. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 1992;41:18–32. [Google Scholar]
- Garland T, Dickerman A.W, Janis C.M, Jones J.A. Phylogenetic analysis of covariance by computer simulation. Syst. Biol. 1993;42:265–292. [Google Scholar]
- Hochachka P.W, Darveau C.-A, Andrews R.D, Suarez R.K. Allometric cascade: a model for resolving body mass effects on metabolism. Comp. Biochem. Physiol. A. 2003;134:675–691. doi: 10.1016/s1095-6433(02)00364-1. 10.1016/S1095-6433(02)00364-1 [DOI] [PubMed] [Google Scholar]
- Karasov W.H. Digestive plasticity in avian energetics and feeding ecology. In: Carey C, editor. Avian energetics and nutritional ecology. Chapman & Hall; New York, NY: 1996. pp. 61–84. [Google Scholar]
- Klaassen M, Oltrogge M, Trost L. Basal metabolic rate, food intake, and body mass in cold- and warm-acclimated Garden Warblers. Comp. Biochem. Physiol. A. 2004;137:639–647. doi: 10.1016/j.cbpb.2003.12.004. 10.1016/j.cbpb.2003.12.004 [DOI] [PubMed] [Google Scholar]
- Kvist A, Lindström Å. Basal metabolic rate in migratory waders: intra-individual, intraspecific, interspecific and seasonal variation. Funct. Ecol. 2001;15:465–473. 10.1046/j.0269-8463.2001.00549.x [Google Scholar]
- Lasiewski R.C, Dawson W.R. A re-examination of the relation between standard metabolic rate and body weight in birds. Condor. 1967;69:13–23. [Google Scholar]
- Levey D.J, Place A.R, Rey P.J, Martinez del Rio C. An experimental test of dietary enzyme modulation in pine warblers Dendroica pinus. Physiol. Biochem. Zool. 1999;72:576–587. doi: 10.1086/316689. 10.1086/316689 [DOI] [PubMed] [Google Scholar]
- Lindström Å. Basal metabolic rates of migrating waders in the Eurasian Arctic. J. Avian Biol. 1997;28:87–92. [Google Scholar]
- Lindström Å, Klaassen M. High basal metabolic rates of shorebirds while in the Arctic: a circumpolar view. Condor. 2003;105:420–427. [Google Scholar]
- Lovegrove B.G. The zoogeography of mammalian basal metabolic rate. Am. Nat. 2000;156:201–219. doi: 10.1086/303383. 10.1086/303383 [DOI] [PubMed] [Google Scholar]
- Lovegrove B.G. The influence of climate on the basal metabolic rate of small mammals: a slow–fast metabolic continuum. J. Comp. Physiol. B. 2003;173:87–112. doi: 10.1007/s00360-002-0309-5. [DOI] [PubMed] [Google Scholar]
- Lovegrove B.G. Seasonal thermoregulatory responses in mammals. J. Comp. Physiol. B. 2005;175:231–247. doi: 10.1007/s00360-005-0477-1. 10.1007/s00360-005-0477-1 [DOI] [PubMed] [Google Scholar]
- Martinez del Rio C, Brugger K.W, Rios J.L, Vergara M.E, Witmer M.C. An experimental and comparative study of dietary modulation of intestinal enzymes in the European starling. Physiol. Zool. 1995;68:490–511. [Google Scholar]
- Martins E.P. Adaptation and the comparative method. Trends Ecol. Evol. 2000;15:296–299. doi: 10.1016/s0169-5347(00)01880-2. 10.1016/S0169-5347(00)01880-2 [DOI] [PubMed] [Google Scholar]
- Martins E.P, Hansen T.F. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 1997;149:646–667. 10.1086/286013 [Google Scholar]
- Martins E.P, Diniz-Filho J.A.F, Housworth E.A. Adaptive constraints and the phylogenetic comparative method: a computer simulation test. Evolution. 2002;56:1–13. [PubMed] [Google Scholar]
- McKechnie, A. E. 2001 Patterns, mechanisms and evolution of avian facultative hypothermic responses: a southern African perspective. Ph.D. thesis, University of Natal, Pietermaritzburg.
- McKechnie A.E, Lovegrove B.G. Heterothermic responses in the speckled mousebird (Colius striatus) J. Comp. Physiol. B. 2001a;171:507–518. doi: 10.1007/s003600100201. 10.1007/s003600100201 [DOI] [PubMed] [Google Scholar]
- McKechnie A.E, Lovegrove B.G. Thermoregulation and the energetic significance of clustering behavior in the white-backed mousebird (Colius colius) Physiol. Biochem. Zool. 2001b;74:238–249. doi: 10.1086/319669. 10.1086/319669 [DOI] [PubMed] [Google Scholar]
- McKechnie A.E, Lovegrove B.G. Facultative hypothermic responses in an Afrotropical arid-zone passerine, the red-headed finch (Amadina erythrocephala) J. Comp. Physiol. B. 2003;173:339–346. doi: 10.1007/s00360-003-0341-0. 10.1007/s00360-003-0341-0 [DOI] [PubMed] [Google Scholar]
- McKechnie A.E, Wolf B.O. The allometry of avian basal metabolic rate: good predictions need good data. Physiol. Biochem. Zool. 2004a;77:502–521. doi: 10.1086/383511. 10.1086/383511 [DOI] [PubMed] [Google Scholar]
- McKechnie A.E, Wolf B.O. Partitioning of evaporative water loss in white-winged doves: plasticity in response to short-term thermal acclimation. J. Exp. Biol. 2004b;207:203–210. doi: 10.1242/jeb.00757. 10.1242/jeb.00757 [DOI] [PubMed] [Google Scholar]
- McNab B.K. The influence of food habits on the energetics of eutherian mammals. Ecol. Monogr. 1986;56:1–19. [Google Scholar]
- McNab B.K. Complications inherent in scaling the basal rate of metabolism in mammals. Q. Rev. Biol. 1988;63:25–54. doi: 10.1086/415715. 10.1086/415715 [DOI] [PubMed] [Google Scholar]
- McNab B.K. On the utility of uniformity in the definition of basal rates of metabolism. Physiol. Zool. 1997;70:718–720. doi: 10.1086/515881. [DOI] [PubMed] [Google Scholar]
- McNab B.K. Energetics of toucans, barbets and a hornbill: implications for avian frugivory. Auk. 2001;118:916–933. [Google Scholar]
- McNab B.K. Ecology shapes bird bioenergetics. Nature. 2003;426:620–621. doi: 10.1038/426620b. 10.1038/426620b [DOI] [PubMed] [Google Scholar]
- Merola-Zwartjes M, Ligon J.D. Ecological energetics of the Puerto Rican tody: heterothermy, torpor and intra-island variation. Ecology. 2000;81:990–1002. [Google Scholar]
- Moss R. Effects of captivity on gut lengths in red grouse. J. Wildl. Manage. 1972;36:99–104. [Google Scholar]
- Mueller P, Diamond J. Metabolic rate and environmental productivity: well-provisioned animals evolved to run and idle fast. Proc. Natl Acad. Sci. USA. 2001;98:12 550–12 554. doi: 10.1073/pnas.221456698. 10.1073/pnas.221456698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel M. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. B. 1994;255:37–45. [Google Scholar]
- Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. 10.1038/44766 [DOI] [PubMed] [Google Scholar]
- Piersma T, Drent J. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 2003;18:228–233. 10.1016/S0169-5347(03)00036-3 [Google Scholar]
- Piersma T, Cadée N, Daan S. Seasonality in basal metabolic rate and thermal conductance in a long distance migrant shorebird, the knot (Calidris canutus) J. Comp. Physiol. 1995;165:37–45. [Google Scholar]
- Reynolds P.S, Lee R.M. Phylogenetic analysis of avian energetics: passerines and non-passerines do not differ. Am. Nat. 1996;147:735–759. 10.1086/285877 [Google Scholar]
- Rezende E.L, Swanson D.L, Novoa F.F, Bozinovic F. Passerines versus nonpasserines: so far, no statistical differences in the scaling of avian energetics. J. Exp. Biol. 2002;205:101–107. doi: 10.1242/jeb.205.1.101. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Ishiguro M, Kitagawa G. D. Reidel; Dordrecht, The Netherlands: 1986. Akaike information criterion statistics. [Google Scholar]
- Savage V.M, Gillooly J.F, Woodruff W.H, West G.B, Allen A.P, Enquist B.J, Brown J.H. The predominance of quarter-power scaling in biology. Funct. Ecol. 2004;18:257–282. 10.1111/j.0269-8463.2004.00856.x [Google Scholar]
- Schleucher E. Metabolism, body temperature and thermal conductance of fruit-doves (Aves: Columbidae, Treronidae) Comp. Biochem. Physiol. A. 2002;131:417–428. doi: 10.1016/s1095-6433(01)00499-8. 10.1016/S1095-6433(01)00499-8 [DOI] [PubMed] [Google Scholar]
- Schleucher E, Withers P.C. Metabolic and thermal physiology of doves and pigeons. Physiol. Biochem. Zool. 2002;75:439–450. doi: 10.1086/342803. 10.1086/342803 [DOI] [PubMed] [Google Scholar]
- Schlichting C.D, Pigliucci M. Sinauer Associates; Sunderland, MA: 1998. Phenotypic evolution: a reaction norm perspective. [Google Scholar]
- Scholander P.F, Hock R, Walters V, Irving L. Adaptation to cold in arctic and tropical mammals and birds in relation to body temperature, insulation and basal metabolic rate. Biol. Bull. 1950;99:259–271. doi: 10.2307/1538742. [DOI] [PubMed] [Google Scholar]
- Sibley C.G, Ahlquist J.E. Yale University Press; New Haven, CT: 1990. Phylogeny and classification of birds. [Google Scholar]
- Starck J.M. Phenotypic flexibility of the avian gizzard: rapid, reversible and repeated changes of organ size in response to changes in dietary fibre content. J. Exp. Biol. 1999;202:3171–3179. doi: 10.1242/jeb.202.22.3171. [DOI] [PubMed] [Google Scholar]
- Swanson, D. L. In press. Seasonal metabolic variation in birds: functional and mechanistic correlates. Curr. Ornithol 17.
- Swanson D.L, Olmstead K.L. Evidence for a proximate influence of winter temperatures on metabolism in passerine birds. Physiol. Biochem. Zool. 1999;72:566–575. doi: 10.1086/316696. 10.1086/316696 [DOI] [PubMed] [Google Scholar]
- Tieleman B.I, Williams J.B. The adjustment of avian metabolic rates and water fluxes to desert environments. Physiol. Biochem. Zool. 2000;73:461–479. doi: 10.1086/317740. 10.1086/317740 [DOI] [PubMed] [Google Scholar]
- Tieleman B.I, Williams J.B. Cutaneous and respiratory water loss in larks from arid and mesic environments. Physiol. Biochem. Zool. 2002;75:590–599. doi: 10.1086/344491. 10.1086/344491 [DOI] [PubMed] [Google Scholar]
- Tieleman B.I, Williams J.B, Buschur M.E. Physiological adjustments to arid and mesic environments in larks (Alaudidae) Physiol. Biochem. Zool. 2002;75:305–313. doi: 10.1086/341998. 10.1086/341998 [DOI] [PubMed] [Google Scholar]
- Tieleman B.I, Williams J.B, Buschur M.E, Brown C.R. Phenotypic variation of larks along an aridity gradient: are desert birds more flexible? Ecology. 2003;84:1800–1815. [Google Scholar]
- Weathers W.W. Climatic adaptation in avian standard metabolic rate. Oecologia. 1979;42:81–89. doi: 10.1007/BF00347620. [DOI] [PubMed] [Google Scholar]
- Weathers W.W, Weathers D.L, van Riper C. Basal metabolism of the apapane: comparison of freshly caught birds with long-term captives. Auk. 1983;100:977–978. [Google Scholar]
- West G.B, Brown J.H, Enquist B. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–126. doi: 10.1126/science.276.5309.122. 10.1126/science.276.5309.122 [DOI] [PubMed] [Google Scholar]
- West G.B, Savage V.M, Gillooly J.F, Enquist B, Woodruff W.H, Brown J.H. Why does metabolic rate scale with body size? Nature. 2003;421:713. doi: 10.1038/421713a. 10.1038/421713a [DOI] [PubMed] [Google Scholar]
- White C.R, Seymour R.S. Mammalian basal metabolic rate is proportional to body mass2/3. Proc. Natl Acad. Sci. USA. 2003;100:4046–4049. doi: 10.1073/pnas.0436428100. 10.1073/pnas.0436428100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikelski M, Spinney L, Schelsky W, Scheuerlein A, Gwinner E. Slow pace of life in tropical sedentary birds: a common-garden experiment on four stonechat populations from different latitudes. Proc. R. Soc. B. 2003;270:2383–2388. doi: 10.1098/rspb.2003.2500. 10.1098/rspb.2003.2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.