Abstract
We study the interplay of ecological and evolutionary dynamics in communities composed of populations with contrasting time-scales. In such communities, genetic variation of individual traits can cause population transitions between stationary and cyclic ecological regimes, hence abrupt variations in fitness. Such abrupt variations raise ridges in the adaptive landscape, where the populations are poised between equilibrium and cyclic coexistence and along which evolutionary trajectories can remain sliding for long times or halt at special points called evolutionary pseudo-equilibria. These novel phenomena should be generic to all systems in which ecological interactions cause fitness to vary discontinuously. They are demonstrated by the analysis of a predator–prey community, with one adaptive trait for each population. The eco-evolutionary dynamics of the system show a number of other distinctive features, including evolutionary extinction and two forms of Red Queen dynamics. One of them is characterized by intermittent bouts of cyclic oscillations of the two populations.
Keywords: slow–fast population dynamics, eco-evolutionary dynamics, Red Queen dynamics, evolutionary sliding and pseudo-equilibria, adaptive ridge, predator–prey coevolution
1. Introduction
Understanding the determinants of population dynamics is an important theme throughout biology, from human health to conservation. In studying population dynamics, much research has addressed how ecological interactions affect population stability yet ignoring the genetic diversity and ensuing evolvability of populations. Ford (1949) was perhaps the first to document that evolutionary change and population dynamics can occur interdependently; Pimentel (1968), Stenseth et al. (1984) and Metz et al. (1992), subsequently, conceptualized the notion of the ecological and evolutionary dynamics of a population being entangled in a feedback loop. The dynamical interplay of ecology and evolution prompts three general questions (May & Anderson 1983; Ferrière & Gatto 1993, 1995; Abrams 2000): (i) how does evolution of adaptive traits affect the ecological stability of a community? (ii) Under which conditions are ecological interactions expected to beget fluctuations in a population's genetic state? (iii) How do eco-evolutionary dynamics respond to environmental change?
Although, a significant number of studies have dealt with some aspects of these three questions, there has been so far no attempt to address them simultaneously in a unified framework. Moreover, most models of the adaptive evolution of traits related to inter- and/or intra-specific interactions have assumed stable ecological equilibria for all trait values in the relevant trait space. This study aims at developing a unified analysis of eco-evolutionary dynamics in communities containing ‘slow’ and ‘fast’ populations, which allows us to relax the ecological equilibrium assumption.
Slow–fast systems are composed of populations whose ecological fluctuations develop on contrasting time-scales. Predator–prey communities offer many instances of contrasting ecological time-scales. Prey is often smaller than predator, hence faster in growing and reproducing. In the plankton food chain, the turnover of algae is faster than that of most zooplankton species which, in turn, grow faster than fish (Scheffer 1998). The Boreal forest is also rich in examples: plants (forbs and grasses) have fast dynamics in comparison with most herbivores (hares, squirrels and small rodents) which reproduce faster than their predators (lynx, coyote and red fox) (Stenseth et al. 1997). The opposite case, namely that of slow prey and fast predator, is also frequently observed in nature—spruce budworm (Ludwig et al. 1978) and larch budmoth (Baltensweiler 1971) provide typical examples among plant–insect interactions. Hereafter, we investigate theoretically the coevolution of a slow predator and fast prey. This analysis will serve to demonstrate the general features of eco-evolutionary dynamics of slow–fast populations that we first outline qualitatively in §2.
2. Coevolutionary dynamics of slow–fast populations: general results
Eco-evolutionary processes, generally, assume two main ingredients: genetically based variation of individual traits generated through reproduction, and selection on this variation resulting from ecological interactions. This is a complex process because individual traits under consideration may affect both the birth process and the ecological interactions. The assumption of rare mutations of small effects allows one to approximate the dynamics of population densities and trait distributions with deterministic models.
In the limit of rare mutations of small effects, the rate of change of an adaptive trait over evolutionary time is proportional to the resident population birth output per unit time (proportional to the probability that a mutation occurs within a small time interval), and to the derivative, with respect to mutant's trait, of the per-capita rate of increase of a mutant population per generation (when positive, proportional to the probability of mutant non-extinction; Metz et al. 1996). In the general situation of a trait z, resident population density n at equilibrium, per-capita birth rate β (i.e. 1/β is the expected time between birth), mutant's trait z′ and instantaneous per-capita rate of increase S, this translates into the so-called canonical equation of adaptive dynamics (Dieckmann & Law 1996):
| 2.1 |
where is called selection derivative, and k is a parameter proportional to the probability that an offspring is a mutant and to the variance of mutation. Since, the resident population density is at equilibrium, this equation simplifies as both β cancel out.
Here, we need an extension of equation (2.1) to the case of more general resident population attractors. The rigorous derivation of such an extension is a hard mathematical exercise that lies beyond the scope of this paper (see Dieckmann & Law (1996), for a heuristic discussion of the problem). However, as explained below, averaging the mutation term (βn), and selection term (s/β), over the attractor is appropriate in the case considered in this paper. In formulas, this results in
| 2.2 |
where brackets indicate temporal averaging over the resident population attractor.
At this point, the analysis of eco-evolutionary dynamics through equation (2.2) would remain problematic because, in general, the resident population attractor is not known analytically in closed form. Slow–fast systems represent a significant exception to this predicament. Indeed, any slow–fast population attractor can be approximated with the so-called singular attractor corresponding to completely separated time-scales (Rinaldi & Scheffer 2000), and this permits explicit calculation of the averages in equation (2.2). The case of slow-predator–fast-prey limit cycles is particularly favourable because the cycle can be easily identified. Moreover, such cycles are characterized by long phases of slow motion of both populations alternating with fast phases of significant prey variation. Thus, slow–fast cycles are very long, so that mutant populations experience little variation in the resident state during their initial phase of growth or decline (with the only exception of particular mutations occurring during the short episodes of fast variation of the prey). This supports the use of equation (2.2), which indeed takes the expectation of (βn) and (s/β) over all possible resident states at the time of mutant arising.
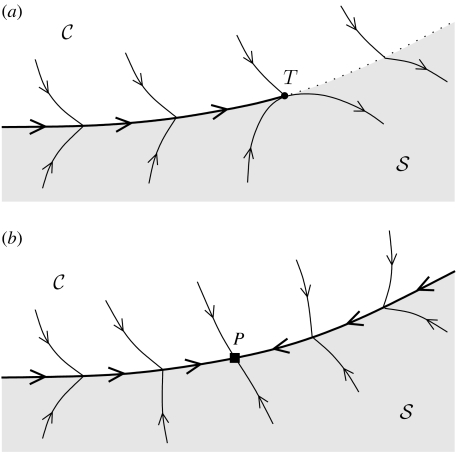
A key evolutionary consequence of slow–fast ecological interactions is that the selection pressure becomes discontinuous across the trait space when stationary and cyclic coexistence are possible for different combinations of the traits. This is so because the transition from an equilibrium to a singular cycle is discontinuous (Rinaldi & Scheffer 2000). General implications for eco-evolutionary dynamics can be outlined by focusing on two populations, e.g. a prey and a predator, coevolving in a two-dimensional trait space. Predator–prey interactions have long been known for their potential to generate a whole spectrum of ecological dynamics in response to variation in individual trait values, from extinction to stable equilibria and cycles. Thus, the trait space splits in three regions: extinction of at least one population in , stationary coexistence in , and cyclic coexistence in . The selection pressure driving the dynamics of the two traits is continuous inside region and region , but it is discontinuous at the boundary separating and . Two different evolutionary gradients are associated with each point of the boundary: one is the vector tangent to the evolutionary trajectory driven by the selection pressure operating in region and the other is the vector tangent to the evolutionary trajectory driven by the selection pressure operating in region (see figure 1 for an example). If the transversal components of these two vectors with respect to the discontinuity boundary have the same sign, as in figure 1a (dotted part of the discontinuity boundary), the trajectory crosses the boundary and the populations switch from cyclic to stationary (or vice versa). On the contrary, if the transversal components of the two vectors are of opposite sign, i.e. if the two evolutionary gradients are ‘pushing’ in opposite directions (solid part of the discontinuity boundary in figure 1), the traits are forced to remain on the boundary and ‘slide’ on it. In other words, the boundary separating the two possible ecological regimes can raise an attractive ridge in the adaptive landscape, along which evolutionary trajectories from various ancestral conditions are canalized. The evolution on the ridge can be temporary, as in figure 1a, where the sliding motion terminates at point T, or permanent, when the sliding motion halts at a so-called pseudo-equilibrium, namely at a point P on the boundary (see figure 1b), where the two evolutionary gradients align. A pseudo-equilibrium has all the properties of an equilibrium (in particular, it can be an attractor, a saddle or a repellor) even if the selection pressures do not vanish at that point.
Figure 1.
Evolution of the traits in the neighbourhood of the boundary separating stationary coexistence (region ) from cyclic coexistence (region ). (a) Evolutionary sliding toward T (solid boundary) and crossing (dotted boundary). (b) Evolutionary sliding toward the pseudo-equilibrium P.
The rest of the paper focuses on a specific predator–prey model to demonstrate patterns of evolutionary sliding and pseudo-equilibria, and to recast them among other distinctive features of predator–prey coevolution. Such features include enhancement of Red Queen dynamics through the increase of genetic variation of the prey, generic occurrence of evolutionary extinction in the predator, and coevolution acting against ecological destabilization resulting from environmental enrichment.
3. A model of predator–prey eco-evolutionary dynamics
Our presentation of a specific model of slow–fast populations and their eco-evolutionary dynamics focuses on the main features of the model, while details on mathematical derivations and approximations are relegated into the electronic supplementary material.
The predator–prey model we consider is the so-called Rosenzweig–MacArthur model (Rosenzweig & MacArthur 1963) composed of a logistic prey and a Holling type II predator
| 3.1 |
| 3.2 |
where x(t) and y(t) are prey and predator population densities at time t. In the absence of predator, the prey population grows logistically (with intrinsic growth rate r and carrying capacity K), while in the absence of prey the predator population decays exponentially (the intrinsic birth rate b is smaller than the death rate d but the maximum birth rate (b+ea) is greater than d). The maximum predation rate is a, the functional response half-saturation constant is h, and the extra natality resulting from predation is proportional to the predation rate through an efficiency coefficient e. The presence of a saturating functional response makes stationary and cyclic coexistence possible for different parameter settings (§A1 in electronic supplementary material). The limit cycle is not known analytically, yet if prey grow at a much faster rate than predators, it can be approximated by the singular limit cycle (derived algebraically in §A1 in electronic supplementary material).
Let u and v denote the adaptive traits for the prey and predator, respectively. Assume that the prey has density- and trait-independent birth rate, while its death rate has a density-dependent component controlled by u. Thus, in equation (3.1) r is constant, while K depends on u. We further assume that K peaks at u0, for which the prey is most effective. Similarly, the predator intrinsic birth rate b is constant, while its death rate d depends upon v and is minimum at v0, at which predators are best adapted to their environment. The predation rate is a function of both traits, and predator (prey) benefit (lose) most from the interaction when traits are balanced, i.e. when u and v are in a suitable relationship, which defines a ‘bidirectional axis of prey vulnerability’ (Abrams 2000). This mechanism is present if, for example, the searching effectiveness of the predator depends upon both traits but with a certain degree of plasticity, so that the same effectiveness can be achieved for a continuum of pairs (u, v). Since, the half-saturation constant h is inversely related to searching effectiveness, h(u, v) must be minimum when u and v are balanced, i.e. u=v provided both traits are measured on an appropriate scale. These are standard assumptions for predator–prey community modelling (Abrams 2000), which have the advantage of involving the minimum possible number of demographic parameters. In our analyses we use (§A3 in electronic supplementary material):
| 3.3 |
The trait space (u, v) can be partitioned into the three regions , and previously described (§A4 in electronic supplementary material). At the boundary between and all quantities associated with the asymptotic regime of the slow–fast system are discontinuous.
In conclusion, taking into account that the prey birth rate is density-independent, the canonical equation (2.2) can be specified for the two populations as follows:
| 3.4 |
| 3.5 |
where superscripts u and v are used to indicate the corresponding species. Of course, the selection derivatives su and sv must be explicitly computed (§A5 in electronic supplementary material) and the time averages must be performed in order to transform equations (3.4) and (3.5) into standard ordinary differential equations. Moreover, since the time averages are different in the coexistence regions and of trait space, this operation must be performed twice (§§A6 and A7 in electronic supplementary material).
4. Results and discussion
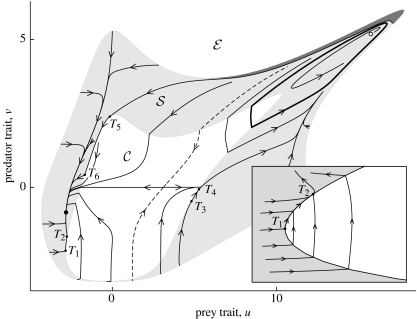
State portraits for the eco-evolutionary model (3.4) and (3.5) can be constructed by numerical simulations. A typical example is shown in figure 2. It contains a small region (dark), where the predator undergoes evolutionary extinction (Matsuda & Abrams 1994a; Ferrière 2000; Dieckmann & Ferrière 2004), and two evolutionary attractors: an equilibrium with low trait values and a cycle characterized by high trait values. The two basins of attraction are separated by the stable manifold of the saddle lying in region . If the ancestral conditions are on the left of this manifold, the traits converge to the equilibrium so that, after evolutionary transients, the populations coexist at ecological equilibrium, since the evolutionary equilibrium is in region . However, for some ancestral conditions, one piece of the evolutionary orbit lies in region : this means that during the corresponding period of time the populations oscillate along an ecological cycle that slowly drifts on the evolutionary time-scale. At evolutionary equilibrium prey evolutionary branching (Geritz et al. 1997, 1998; Dercole et al. 2003) may occur, but will not be investigated here.
Figure 2.
A state portrait of the eco-evolutionary model (3.4) and (3.5). There are three equilibria (a stable node (filled dot) and an unstable focus (empty dot) in region and a saddle in region ) and one limit cycle (partly in region and partly in region ). There are two attractors, the node and the cycle, and their basins of attraction are separated by the stable manifold of the saddle. There are three sliding segments, one stable (T1T2, stretched and magnified in the lower right panel) and two unstable (T3T4 and T5T6). Predator evolutionary extinction occurs in the dark region. Parameter values: ku=0.1, kv=1, r=1, K0=1, h0=0.02, h1=0.02, d0=0.01, b=0.001, e=0.1, a=5, u0=1, v0=3.
Population dynamics associated with evolutionary trajectories in the other basin of attraction are radically different. Indeed, long periods of time characterized by slowly varying populations recurrently alternate with long periods of time during which populations fluctuate on an ecological cycle, as a consequence of the attracting evolutionary cycle being partly in regions and .
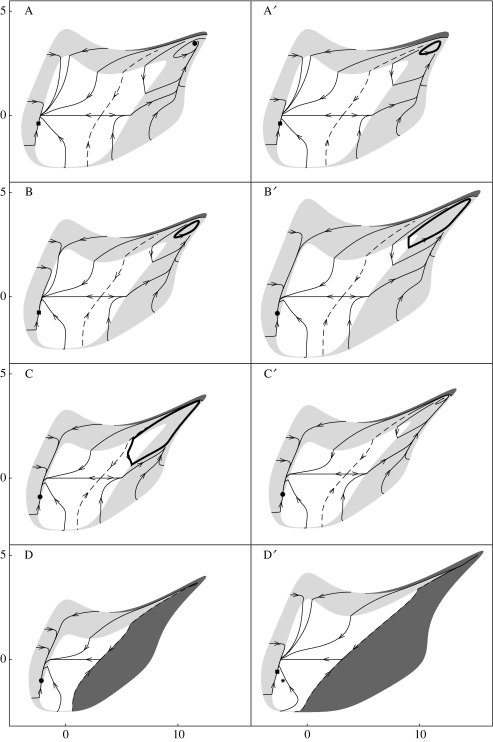
Figure 2 highlights the possibility of coevolution along adaptive ridges, which are segments of the boundary separating cyclic from stationary coexistence. Sliding is a novel type of evolutionary dynamics with important ecological implications: when traits are sliding along an adaptive ridge, prey and predator are poised between stationary and cyclic coexistence, i.e. coevolution drives the populations toward and maintain them at the onset of their most complex dynamic behaviour. How sensitive these phenomena are to parameters is investigated by means of a thorough bifurcation analysis (§A8 in electronic supplementary material). Synthesizing the results yields a series of eight statements (the first three are general, while the others are specific of predator–prey systems) that we list hereafter and illustrate with selected state portraits (figure 3).
Evolutionary sliding and pseudo-equilibria. Evolutionary sliding along the boundary separating stationary from cyclic coexistence occurs for many parameter settings. The evolutionary sliding can be temporary (sliding segment) or halt at an evolutionary pseudo-equilibrium. When the adaptive traits are sliding, or resting at a pseudo-equilibrium, the populations are in critically stable ecological states and their mean characteristics (densities, density-dependent parameters) can vary abruptly for small changes of individual traits.
Evolutionary extinction. There is always a subregion (dark in all state portraits), where the orbits tend toward the boundary of region . This causes the predator to go extinct in the long run, a phenomenon that is not predictable on the basis of purely ecological arguments. Evolution to extinction had been noted in predator–prey and competition models by Matsuda & Abrams (1994a,b) and Dieckmann et al. (1995); and is also known to occur in models of mutualistic (Ferrière et al. 2002) and cannibalistic (Dercole & Rinaldi 2002; Dercole 2003) interactions. These examples highlight a common mechanism. Adaptive evolution is driven by the ‘marginal’ benefit of performing better in interactions (predation, mutualism) than other conspecifics; yet the ‘direct’, physiological cost to the individual can become so great that eventually the population growth rate becomes negative, causing extinction.
Multiple evolutionary equilibria. The eco-evolutionary system most often possesses several equilibria: attractors, repellors and saddles, in addition to predator evolutionary extinction. Two general implications can be drawn. First, in the long run, the same populations can reach different evolutionary states and develop different ecological dynamics due to ancient differences in their genotypic state. Experimental evolution in Escherichia coli provides strong empirical support to this prediction (Travisano et al. 1995). Second, the co-occurrence of predator evolutionary extinction and other viable evolutionary attractors provides a firm mathematical basis for the notion of evolutionary trapping suggested from empirical observations (Colas et al. 1997; Schlaepfler et al. 2002): under given environmental conditions, the predator population can be trapped on an evolutionary trajectory heading to extinction whereas alternative, ecologically safe evolutionary attractors could have been reached. Schlaepfler et al. (2002) and Ferrière et al. (2004) have discussed the implications of evolutionary trapping in a conservation perspective.
Two forms of Red Queen dynamics. The first one (evolutionary cycle in region , see portraits B and A′) corresponds to slow periodic variations of the traits entraining slow population cycles. This form is well-known from Lotka–Volterra models that did not allow for other forms of Red Queen dynamics (Abrams 2000). The second form (evolutionary cycle in region and , see portraits C and B′) corresponds to slow periodic variations of the traits accompanied by recurrent and long bouts of ecological oscillations. This complex pattern was predicted by Khibnik & Kondrashov (1997), who have named it ‘eco-genetically driven Red Queen dynamics’.
Factors enhancing Red Queen dynamics. Our study confirms that a bidirectional axis of prey vulnerability is a potent mechanism for generating evolutionary cycles (Abrams 2000). By increasing the impact of the traits on vulnerability the evolutionary attractor changes in a typical sequence (see portraits A, B, C): first an equilibrium associated with steady populations, then an evolutionary cycle with entrained population oscillations, and finally an evolutionary cycle associated with recurrent bouts of ecological oscillations. For a further increase of the vulnerability mechanism Red Queen dynamics suddenly disappear (see portrait D), a phenomenon that has gone unnoticed in previous studies. Increasing the probability of prey mutation, the variance of the distribution of prey mutational effects, and the maximum predation rate can also trigger and enhance Red Queen dynamics (see portraits (A, A′) and (B, B′)).
The predator chases the prey. All evolutionary cycles we have detected are anticlockwise. Thus, the predator trait increases when the prey trait is large and decreases in the opposite case. This results from the bidirectional axis of prey vulnerability, and is, indeed, present in all studies where the prey has a most vulnerable phenotype depending upon predator's trait (see Marrow et al. 1992; Dieckmann et al. 1995; Dieckmann & Law 1996; Abrams & Matsuda 1997; Khibnik & Kondrashov 1997).
Evolution toward ecological stability: the paradox of enrichment. Ecological theory predicts that predator–prey interactions should cause large amplitude cycles in rich environments (Rosenzweig 1971). The ‘paradox of enrichment’ emphasizes that this does not occur in nature (e.g. Murdoch et al. 1998). Abrams & Walters (1996) found an ecological solution to the paradox for certain types of predator–prey communities, later confirmed by experimental findings (McCauley et al. 1999). Rosenzweig & Schaffer (1978) took a general, evolutionary approach to the problem, arguing that evolution should tend to restore ecological stability lost through enrichment. Evolution may actually play such a significant role in light of, e.g. Yoshida et al.'s (2003) findings on rapid evolutionary change in predator–prey systems. Our work substantiates, refines, and broadens Rosenzweig & Schaffer's view in the case of slow predator and fast prey. In fact, if the system is at its evolutionary equilibrium in region (portrait D), should evolutionary processes be absent (i.e. u and v being kept frozen), significant enrichment would destabilize the populations. This is clearly recognizable from portrait D′ where the point * in region is the copy of the evolutionary equilibrium of portrait D. Interestingly, after enrichment the evolutionary processes act in the opposite direction and the final result (portrait D′) is that the traits tend to an evolutionary pseudo-equilibrium. In other words, the full destabilization of the populations triggered by enrichment is opposed by the counteracting forces of evolution.
Evolution opposes permanent ecological oscillations. There seems to be no realistic environmental conditions under which an evolutionary attractor is entirely in region , although evolutionary trajectories are often trapped on the boundary between and (see statement (i)). Only if the predation rate is almost independent of prey and predator traits (i.e. if h1 is of the order of 10−3), there is an evolutionary equilibrium in (notice that for h1=0, point (u0, v0) is a stable evolutionary equilibrium in ). Thus, evolution seems to oppose permanent ecological oscillations.
Figure 3.
Eight state portraits of the eco-evolutionary model (3.4) and (3.5). In the first row, the parameters are ku=0.1, kv=1, r=1, K0=1, h0=0.02, d0=0.01, b=0.001, e=0.1, a=1.5, u0=1, v0=3, and h1=0.01 in A, h1=0.017 in B, h1=0.05 in C, h1=0.05 in D. State portrait A′ is obtained from A by increasing ku from 0.1 to 0.145. State portrait B′ is obtained from B by increasing a from 1.5 to 3. State portrait C′ is obtained from C by increasing v0 from 3 to 3.3. State portrait D′ is obtained from D by increasing K0 from 1 to 5. All dark regions correspond to predator evolutionary extinction. Evolutionary sliding is present in all panels, while pseudo-equilibria (squared points) are present in panels A, A′, B, D′.
5. Concluding comments
Our analysis unravels novel evolutionary phenomena whose scope extends beyond predator–prey coevolution. This includes the possibility that coevolution guides the traits along adaptive ridges formed by segments of the boundary between the regions of stationary and cyclic coexistence (evolutionary sliding), or comes to a halt at special points of that boundary (evolutionary pseudo-equilibria).
Our conclusions for predator–prey coevolution are likely to be influenced by the specific model chosen to describe the interaction. The analysis, however, should in principle be repeatable for any ecological model involving slow–fast dynamics. Considering the dual case of slow prey (e.g. plants) and fast predator (e.g. insects), and how common recurrent insect–pest outbreaks are in natural or exploited forests, coevolution might well in this case have just the opposite effect on ecological dynamics, namely that of favouring cyclic coexistence.
Finally, the conjecture formulated by Ellner & Turchin (1995) on the basis of their analyses of population time-series, namely that ‘ecosystems might evolve toward the edge of chaos’, finds some support in the present study. Indeed, our findings suggest that ecosystems might evolve toward the edge of their most complex dynamic regime, which in the case of predator–prey models, is indeed cyclic coexistence. But the support could become even stronger once the present analysis is extended to tritrophic food chains with potentially chaotic ecological dynamics. This should be feasible since singular cycles and singular bifurcations responsible for chaotic dynamics have already been found in such communities (De Feo & Rinaldi 1998). Bifurcations leading to chaos are a likely cause of discontinuity in fitness across trait space. Complex Red Queen dynamics involving intermittent bouts of chaotic fluctuations of the populations would then rank among the expected outcomes, as predicted by Ellner & Turchin (1995).
Acknowledgements
The authors are grateful to Peter Abrams and to an anonymous reviewer for their comments and constructive criticisms on earlier drafts of this manuscript. F.D., A.G. and S.R. acknowledge funding support from MIUR (under project FIRB-RBNE01CW3M). R.F. acknowledges funding support from the French National Programmes ‘New Science Interfaces with Mathematics’ and ‘Biological Invasions’, and from the European Research and Training Network ‘ModLife’ (HPRN-CT-2000-00051).
Supplementary Material
References
- Abrams P.A. The evolution of predator–prey interactions: theory and evidence. Annu. Rev. Ecol. Syst. 2000;31:79–105. 10.1146/annurev.ecolsys.31.1.79 [Google Scholar]
- Abrams P.A, Matsuda H. Prey evolution as a cause of predator–prey cycles. Evolution. 1997;51:1742–1750. doi: 10.1111/j.1558-5646.1997.tb05098.x. [DOI] [PubMed] [Google Scholar]
- Abrams P.A, Walters C.J. Invulnerable prey and the paradox of enrichment. Ecology. 1996;77:1125–1133. [Google Scholar]
- Baltensweiler W. The relevance of changes in the composition of larch bud moth populations for the dynamics of its number. In: den Boer P.J, Gradwell G.R, editors. Dynamics of populations. Center for Agricultural Publishing and Documentation; Wageningen, The Netherlands: 1971. pp. 208–219. [Google Scholar]
- Colas B, Olivieri I, Riba M. Centaurea corymbosa, a cliff-dwelling species tottering on the brink of extinction. A demographic and genetic study. Proc. Natl Acad. Sci. 1997;94:3471–3476. doi: 10.1073/pnas.94.7.3471. 10.1073/pnas.94.7.3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Feo O, Rinaldi S. Singular homoclinic bifurcations in tritrophic food chains. Math. Biosci. 1998;148:7–20. doi: 10.1016/s0025-5564(97)10001-3. 10.1016/S0025-5564(97)10001-3 [DOI] [PubMed] [Google Scholar]
- Dercole F. Remarks on branching-extinction evolutionary cycles. J. Math. Biol. 2003;47:569–580. doi: 10.1007/s00285-003-0236-4. 10.1007/s00285-003-0236-4 [DOI] [PubMed] [Google Scholar]
- Dercole F, Rinaldi S. Evolution of cannibalism: scenarios derived from adaptive dynamics. Theor. Popul. Biol. 2002;62:365–374. doi: 10.1016/s0040-5809(02)00008-4. 10.1016/S0040-5809(02)00008-4 [DOI] [PubMed] [Google Scholar]
- Dercole F, Irisson J.-O, Rinaldi S. Bifurcation analysis of a prey–predator coevolution model. SIAM J. App. Math. 2003;63:1378–1391. 10.1137/S0036139902411612 [Google Scholar]
- Dieckmann U, Ferrière R. Adaptive dynamics and evolving biodiversity. In: Ferrière R, Dieckmann U, Couvet D, editors. Evolutionary conservation biology. Cambridge University Press; Cambridge, UK: 2004. pp. 188–224. [Google Scholar]
- Dieckmann U, Law R. The dynamical theory of coevolution: a derivation from stochastic ecological processes. J. Math. Biol. 1996;34:579–612. doi: 10.1007/BF02409751. 10.1007/s002850050022 [DOI] [PubMed] [Google Scholar]
- Dieckmann U, Marrow U, Law R. Evolutionary cycling in predator–prey interactions: population dynamics and the Red Queen. J. Theor. Biol. 1995;176:91–102. doi: 10.1006/jtbi.1995.0179. 10.1006/jtbi.1995.0179 [DOI] [PubMed] [Google Scholar]
- Ellner S, Turchin P. Chaos in a noisy world: new methods and evidence from time series analysis. Am. Nat. 1995;145:343–375. 10.1086/285744 [Google Scholar]
- Ferrière R. Options 12–16. International Institute for Applied Systems Analysis; Laxenburg, Austria: 2000. Adaptive responses to environmental threats: evolutionary suicide, insurance and rescue. [Google Scholar]
- Ferrière R, Gatto M. Chaotic dynamics can result from natural selection. Proc. R. Soc. B. 1993;251:33–38. doi: 10.1098/rspb.1993.0005. [DOI] [PubMed] [Google Scholar]
- Ferrière R, Gatto M. Lyapunov exponents and the mathematics of invasion in oscillatory or chaotic populations. Theor. Popul. Biol. 1995;48:126–171. 10.1006/tpbi.1995.1024 [Google Scholar]
- Ferrière R, Bronstein J.L, Rinaldi S, Law R, Gauduchon M. Cheating and the evolutionary stability of mutualisms. Proc. R. Soc. B. 2002;269:773–780. doi: 10.1098/rspb.2001.1900. 10.1098/rspb.2001.1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrière R, Dieckmann U, Couvet D. Introduction. In: Ferrière R, Dieckmann U, Couvet D, editors. Evolutionary conservation biology. Cambridge University Press; Cambridge, UK: 2004. pp. 1–14. [Google Scholar]
- Ford, E. B. 1949. Mendelism and Evolution. London, UK: Methuen and Co.
- Geritz S.A.H, Metz J.A.J, Kisdi E, Meszéna G. The dynamics of adaptation and evolutionary branching. Phys. Rev. Lett. 1997;78:2024–2027. 10.1103/PhysRevLett.78.2024 [Google Scholar]
- Geritz S.A.H, Kisdi E, Meszéna G, Metz J.A.J. Evolutionarily singular strategies and the adaptive growth and branching of the evolutionary tree. Evol. Ecol. 1998;12:35–57. 10.1023/A:1006554906681 [Google Scholar]
- Khibnik A.I, Kondrashov A.S. Three mechanisms of Red Queen dynamics. Proc. R. Soc. B. 1997;264:1049–1056. 10.1098/rspb.1997.0145 [Google Scholar]
- Ludwig D, Jones D.D, Holling C.S. Qualitative analysis of insect outbreak systems: the spruce budworm and forest. J. Anim. Ecol. 1978;47:315–332. [Google Scholar]
- Marrow P, Law R, Cannings C. The coevolution of predator–prey interactions: ESSs and Red Queen dynamics. Proc. R. Soc. B. 1992;250:133–141. [Google Scholar]
- Matsuda H, Abrams P.A. Runaway evolution to self-extinction under asymmetrical competition. Evolution. 1994a;48:1764–1772. doi: 10.1111/j.1558-5646.1994.tb02212.x. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Abrams P.A. consumers: self extinction due to adaptive change in foraging and anti-predator effort. Theor. Popul. Biol. 1994b;45:76–91. 10.1006/tpbi.1994.1004 [Google Scholar]
- May R.M, Anderson R.M. Epidemiology and genetics in the coevolution of parasites and hosts. Proc. R. Soc. B. 1983;219:281–313. doi: 10.1098/rspb.1983.0075. [DOI] [PubMed] [Google Scholar]
- McCauley E, Nisbet R.M, Murdoch W.W, de Roos A.M, Gurney W.S.C. Large-amplitude cycles of Daphnia and its algal prey in enriched environments. Nature. 1999;402:653–656. 10.1038/45223 [Google Scholar]
- Metz J.A.J, Nisbet R.M, Geritz S.A.H. How should we define fitness for general ecological scenarios? Trends Ecol. Evol. 1992;7:198–202. doi: 10.1016/0169-5347(92)90073-K. 10.1016/0169-5347(92)90073-K [DOI] [PubMed] [Google Scholar]
- Metz J.A.J, Geritz S.A.H, Meszéna G, Jacobs F.J.A, van Heerwaarden J.S. Adaptive dynamics: a geometrical study of the consequences of nearly faithful reproduction. In: van Strien S.J, Verduyn Lunel S.M, editors. Stochastic and spatial structures of dynamical systems. Elsevier Science; North-Holland, Amsterdam: 1996. pp. 183–231. [Google Scholar]
- Murdoch W.W, Nisbet R.M, McCauley E, de Roos A.M, Gurney W.S.C. Plankton abundance and dynamics across nutrient levels: test of hypotheses. Ecology. 1998;79:1339–1356. [Google Scholar]
- Pimentel D. Population regulation and genetic feedback. Science. 1968;159:1432–1437. doi: 10.1126/science.159.3822.1432. [DOI] [PubMed] [Google Scholar]
- Rinaldi S, Scheffer M. Geometric analysis of ecological models with slow and fast processes. Ecosystems. 2000;3:507–521. 10.1007/s100210000045 [Google Scholar]
- Rosenzweig M.L. Paradox of enrichment: destabilization of exploitation ecosystems in ecological time. Science. 1971;171:385–387. doi: 10.1126/science.171.3969.385. [DOI] [PubMed] [Google Scholar]
- Rosenzweig M.L, MacArthur R.H. Graphical representation and stability conditions of predator–prey interactions. Am. Nat. 1963;97:209–223. 10.1086/282272 [Google Scholar]
- Rosenzweig M.L, Schaffer W.M. Homage to Red Queen II. Coevolutionary response to enrichment of exploitation ecosystems. Theor. Popul. Biol. 1978;14:158–163. doi: 10.1016/0040-5809(78)90009-6. 10.1016/0040-5809(78)90009-6 [DOI] [PubMed] [Google Scholar]
- Scheffer M. Chapman & Hall; London, UK: 1998. Ecology of shallow lakes. [Google Scholar]
- Schlaepfler M.A, Runge M.C, Sherman P.W. Ecological and evolutionary traps. Trends Ecol. Evol. 2002;17:474–479. 10.1016/S0169-5347(02)02580-6 [Google Scholar]
- Stenseth N.C, Maynard Smith J. Coevolution in ecosystems: Red Queen evolution or stasis. Evolution. 1984;38:870–880. doi: 10.1111/j.1558-5646.1984.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Stenseth N.C, Falck W, Bjornstad O.N, Krebs C.J. Population regulation in snowshoe hare and Canadian lynx: asymmetric food web configurations between hare and lynx. Proc. Natl Acad. Sci. 1997;94:5147–5152. doi: 10.1073/pnas.94.10.5147. 10.1073/pnas.94.10.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travisano M, Vasi F, Lenski R.E. Long-term experimental evolution in Escherichia coli. 3. Variation among replicate populations in correlated responses to novel environments. Evolution. 1995;49:189–200. doi: 10.1111/j.1558-5646.1995.tb05970.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Jones L.E, Ellner S.P, Fussmann G.F, Hairston N.G., Jr Rapid evolution drives ecological dynamics in a predator–prey system. Nature. 2003;424:303–306. doi: 10.1038/nature01767. 10.1038/nature01767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.