Abstract
Müllerian mimicry, in which toxic species gain mutual protection from shared warning signals, is poorly understood in vertebrates, reflecting a paucity of examples. Indirect evidence for mimicry is found if monophyletic species or clades show parallel geographic variation in warning patterns. Here, we evaluate a hypothesis of Müllerian mimicry for the pitvipers in Southeast Asia using a phylogeny derived from DNA sequences from four combined mitochondrial regions. Mantel matrix correlation tests show that conspicuous red colour pattern elements are significantly associated with sympatric and parapatric populations in four genera. To our knowledge, this represents the first evidence of a Müllerian mimetic radiation in vipers. The putative mimetic patterns are rarely found in females. This appears paradoxical in light of the Müllerian prediction of monomorphism, but may be explained by divergent selection pressures on the sexes, which have different behaviours. We suggest that biased predation on active males causes selection for protective warning coloration, whereas crypsis is favoured in relatively sedentary females.
Keywords: Müllerian mimicry, sex-limited, mitochondrial DNA phylogeny, green pitviper, Trimeresurus, hypothesis testing
1. Introduction
Müller's (1879) hypothesis that toxic species should converge on the same aposematic (warning) pattern due to mutual protection from predators is a key paradigm of natural selection. The shared benefit of similar warning signals is supported by field experiments demonstrating increased survival of morphs that match locally abundant species (Kapan 2001) and laboratory simulations showing reduced per capita attack rate on models at high density (Alatalo & Mappes 1996; Lindström et al. 2001). Purifying selection against novel variants is predicted to cause interspecific monomorphism in a given geographic area (Müller 1879; Joron & Mallet 1998).
Müllerian ‘mimicry rings’ are typified by insects, particularly neotropical butterflies (e.g. Joron & Mallet 1998; Kapan 2001). Studies of alternative models are lacking and numerous aspects of Müllerian mimicry remain paradoxical and the subject of intense debate (Brower 1996; Joron & Mallet 1998; Mallet 1999; Speed & Turner 1999; Skelhorn & Rowe 2005). In particular, there is a paucity of examples of Müllerian mimicry in vertebrates (Pough 1988). Putative cases occur in coral snakes (Greene & McDiarmid 1981) and salamanders (Brandon et al. 1979), and good evidence exists for a Müllerian radiation in dendrobatid frogs (Symula et al. 2001).
Among snakes, warning signals and mimicry are typically associated with actively foraging, diurnal or crepuscular species (Greene & McDiarmid 2005). This includes the highly venomous neotropical coral snakes (Micrurus, Micruroides) and their Batesian mimics, whose predominantly red colour patterns have been experimentally determined to function aposematically with respect to avian predators (Brodie 1993). Pitvipers (Serpentes: Viperidae: Crotalinae) are also venomous, but are largely nocturnal and generally display cryptic colour patterns associated with their relatively sedentary lifestyles and ambush mode of predation (Greene 1994; but see Wüster et al. 2004). The arboreal green pitvipers of the Trimeresurus radiation are widespread in Asia, where they are a conspicuous component of most reptilian faunas. Recent molecular systematic revisions have uncovered high levels of cryptic diversity within Trimeresurus group species (e.g. Malhotra & Thorpe 2000, 2004a; Giannasi et al. 2001; Sanders et al. 2004, in press). Molecular analysis of the Trimeresurus radiation has recently resulted in the proposal of a new taxonomy consisting of seven genera (Malhotra & Thorpe 2004b), all of which contain green species. In parts of Thailand and adjacent areas, several green species overlap in range and here they display unusual, conspicuous red colour pattern elements and striking phenotypic convergence that is suggestive of mimicry. Similarly, in the Indomalayan and Philippine archipelagos, a cluster of species belonging to the genus Parias display a basically green colour pattern which is heavily overlaid with black banding or other coloration.
To provide evidence of mimicry, it is necessary to demonstrate parallel evolution of warning patterns in sympatric or parapatric monophyletic groups (Pough 1988). Such comparative analysis of the Asian pitvipers has until recently been hindered by systematic uncertainties and the lack of a well-corroborated phylogenetic framework. In this paper, we evaluate a hypothesis of mimicry for the green pitvipers in Thailand and adjacent areas using a recently published phylogeny based on four combined mitochondrial DNA sequences (Malhotra & Thorpe 2004b). We also provide a test of the null hypothesis of no association between colour pattern types and their relative distribution, taking phylogenetic relationships into account. Taken together, our analyses provide support for conspicuous colour pattern elements being mimetic in nature. A mimetic radiation of green pitvipers is likely to be Müllerian, as despite considerable variation all species possess potent haemorrhagic and necrotizing venom that could pose a substantial risk for mammalian and avian predators.
2. Material and methods
We based our analysis on a robust published phylogeny for the Asian pitvipers from Malhotra & Thorpe (2004b, 2005), which used 2403 base pairs from four mitochondrial gene regions (cytochrome b, NADH dehydrogenase subunit 4, 12S small subunit and 16S large subunit ribosomal RNA) and included only specimens from known localities and in which species identity could be verified. Both maximum parsimony and Bayesian MCMC methods gave very similar trees, and for this analysis we used the strict consensus of the three most parsimonious trees. The topology of this tree differs from the Bayesian tree only at two nodes. First, a subclade of Cryptelytrops (Cryptelytrops macrops, Cryptelytrops venustus and Cryptelytrops kanburiensis) is shown as basal to other Cryptelytrops species in the MP tree but is in a trichotomy with these and Viridovipera in the Bayesian tree. However, the monophyly of Cryptelytrops (i.e. the MP tree) is supported by taxonomically informative morphological characters (Malhotra & Thorpe 2004b). Second, the branching order of Cryptelytrops andersoni and Cryptelytrops cantori is unresolved in the MP tree, but the Bayesian tree supports the divergence of C. andersoni before C. cantori. Prior to further analysis, clades consisting of multiple conspecific populations that are monomorphic with respect to colour pattern type were trimmed to a single tip, in order to avoid biasing the analysis through uneven sampling across the phylogeny. In addition, we renamed the tips in the Popeia popeiorum complex, which comprises three separate lineages that have subsequently been recognized as full species (Sanders et al. in press).
The colour pattern types and distributions of the populations discussed in this paper were based on Gumprecht et al. (2004), which is consistent with our detailed morphometric studies (Malhotra & Thorpe 2004b,c; Sanders et al. in press) and unpublished data (A. Malhotra & R. S. Thorpe 2005). This information is summarized in table 1. Only records in which species identity could be verified for specimens of known geographic origin are included. MacClade 4.03 (Maddison & Maddison 2001) was used to trace the evolution of the colour pattern types on the phylogeny described above using linear parsimony. The accelerated transformation option was used to optimize the characters on the tree, and the effect of the uncertainty at the unresolved nodes in the strict consensus tree was explored by examining the reconstruction on all possible fully resolved trees.
Table 1.
A summary of colour pattern type, mtDNA lineage and distribution for species and populations included in this study. (C, central; N, north; S, south; W, west; E, east; NE, northeast; NW, northwest; SE, southeast; SW, southwest. The same populations/species may appear more than once if they show ontogenetic changes in colour pattern, or variation within populations. However, sexual dimorphism has been ignored for the purposes of this analysis (see text for further discussion).)
| colour pattern type | mtDNA lineage | distribution |
|---|---|---|
| green+red | C. albolabris 1 | C Vietnam |
| C. albolabris 2 | N and NE Thailand | |
| C. insularis | E Java and Nusa Tenggara, Indonesia | |
| P. malcolmi | Sabah, E Malaysia | |
| P. popeiorum 1 | N Thailand; SW Myanmar; NE India; N Laos | |
| P. popeiorum 2 | W Thailand | |
| P. sabahi 1 | Sabah, E Malaysia | |
| P. sabahi 2 | W Malaysia (excluding Cameron Highlands) | |
| Viridovipera gumprechti | N Myanmar; NE Thailand; NW Vietnam Laos/Vietnam border; Hainan, SW China | |
| V. medoensis | NE India; N Myanmar; SW China | |
| V. stejnegeri | NE India; N Vietnam; Hainan, SE China; Taiwan | |
| V. vogeli | SE Thailand; S Laos; SW Cambodia; C Vietnam | |
| not green | C. andersoni | Nicobar and Andaman Islands, India |
| C. cantori | Nicobar Islands, India | |
| C. purpureomaculatus 1 | S Myanmar | |
| C. purpureomaculatus 2 | S Thailand; W Malaysia; Singapore;W, S and N Sumatra | |
| H. tibetanus | Nepal; Tibet | |
| P. flavomaculatus 1 | N Philippines | |
| Parias mcgregori | N Philippines | |
| Trimeresurus borneensis | S Thailand; West Malaysia; W and N Sumatra; Borneo | |
| T. gramineus | S India | |
| T. malabaricus | S India | |
| T. puniceus | Java; W and S Sumatra; Mentawai Islands | |
| T. trigonocephalus | Sri Lanka | |
| green only | C. albolabris lineage 3 | W Java; S, SE, C and W Thailand; Myanmar; Hainan, S China; SW, Cambodia; Laos; S and N Vietnam |
| C. erythrurus | Bangladesh; NE India, W Myanmar; | |
| C. macrops | Thailand; Cambodia; S Laos; S Vietnam | |
| C. purpureomaculatus 3 | S Myanmar | |
| C. septentrionalis | Nepal, NW India | |
| P. flavomaculatus 1 | N Philippines | |
| P. hageni | S Thailand; W Malaysia; Singapore, Sumatra; Nias and Mentawai Islands | |
| P. nebularis | Cameron Highlands, W Malaysia | |
| P. sabahi 3 | W and S Sumatra | |
| green+banding | C. kanburiensis | W Thailand |
| C. venustus | S Thailand | |
| P. flavomaculatus 1 | N Philippines | |
| P. sabahi 4 | S Thailand | |
| green+other | H. tibetanus | Nepal; SW China |
| P. hageni | S Thailand; W Malaysia; W, S and N Sumatra; Nias and Mentawi Islands | |
| P. flavomaculatus 2 | S Philippines | |
| P. malcolmi | Sabah, E Malaysia | |
| P. schultzei | Palawan, Philippines | |
| P. sumatranus | W and S Sumatra; Borneo | |
| T. gramineus | S India | |
| T. trigonocephalus | Sri Lanka |
In order to test the hypothesis of no association between colour pattern and relative distribution, distance matrices of colour pattern type (based on the five categories shown in table 1, for each colour pattern type separately as well as a matrix representing similarity in all colour pattern types) and relative distribution (where 1 represented parapatry or sympatry and 0 represented allopatry) were constructed. The correlation between them was determined using a Mantel matrix correlation test (Manly 1991). Since colour pattern type may have a phylogenetic component (e.g. the ‘green plus other’ type is found in most Parias species and several Trimeresurus sensu stricto species), this has to be factored out. This was done by using a partial Mantel test, in which a patristic distance matrix (recalculated for the reduced tree using PAUP* v. 4b10) is first regressed against the colour pattern matrix, and the residuals from this regression are then compared to the relative distribution matrix. The freeware program zt (Bonnet & Van de Peer 2002) was used, with permutation of the residuals rather than raw values following the recommendation of Legendre (2000).
3. Results
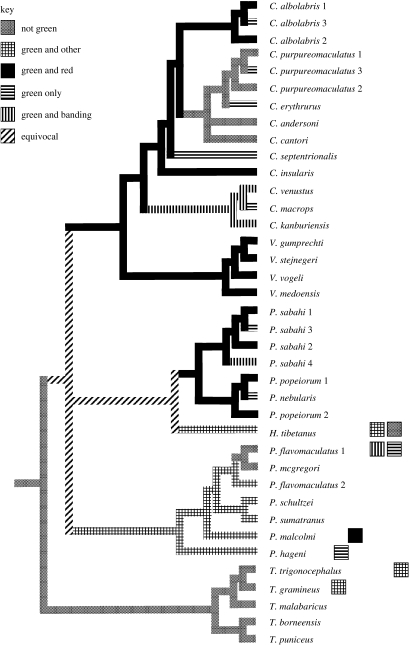
Figure 1 shows the phylogeny from fig. 1 in Malhotra & Thorpe (2004b), together with the distribution of the various colour pattern types as mapped by MacClade. Alternative resolutions of C. andersoni and C. cantori do not affect the reconstruction, so only the reconstructions involving the trichotomy between Parias, (Popeiorum+Himalayophis) and (Viridovipera+Cryptelytrops) are shown. There are six most parsimonious reconstructions involving 14 steps in each case, with a rescaled consistency index of 0.07 and a retention index of 0.4. In all possible reconstructions, the ancestral colour pattern type is ‘not green’, Although the number of times that the different colour pattern types evolve does not change between alternative reconstructions (see figure 1), the colour pattern type reconstructed for the nodes in question (i.e. the sequence of events) does differ. It is apparent that there have been multiple losses and gains of different colour pattern types in the radiation of this group of pitvipers.
Figure 1.
Evolution of colour pattern type reconstructed on a mtDNA phylogeny (based on 2403 base pairs from four regions) from Malhotra & Thorpe (2004b). The tree illustrated is the reduced version of the strict consensus of three most parsimonious trees; see text for further details. Only the topology is shown. For convenience the branches are shaded/patterned according to the state at the node/tip to which they lead. ‘Not green’ is the ancestral type shown by the outgroups (not shown), ‘green and red’ evolved at least twice, ‘green and banding’ at least three times, ‘green and other’ at least four times, and ‘green only’ at least nine times (these figures include the derived conditions known to be present within polymorphic species, shown by the squares adjacent to the relevant tip). For locality information of the numbered populations, refer to table 1.
Partial Mantel tests results are summarized in table 2. The null hypothesis of no association between relative distribution and colour pattern type, once the effect of phylogenetic correlation had been removed, could be rejected for ‘green only’, ‘green plus red’, ‘green plus other’ and for all colour pattern types together, but could not be rejected for ‘not green’, or for ‘green plus banding’.
Table 2.
(a) Results of a simple pairwise Mantel test testing the null hypothesis of no association between colour pattern type and relative distribution and genealogical relationships respectively; (b) results of a partial Mantel test testing the null hypothesis of no association between colour pattern type and relative distribution while taking into account the effect of genealogical relationships. Figures represent either Pearson correlation coefficients (pairwise test) or partial correlation coefficients (partial tests). *p<0.05, **p<0.01 (one-tailed).
| colour pattern type | relative distribution | genealogical relationships |
|---|---|---|
| (a) pairwise test | ||
| all types together | −0.094907* | 0.142661** |
| not green | −0.085090* | 0.170449** |
| green only | 0.108507* | −0.024213 |
| green+red | −0.076894 | −0.013094 |
| green+banding | −0.013873 | −0.013774 |
| green+other | −0.121432* | 0.095342 |
| (b) partial test | ||
| all types together | −0.069674* | |
| not green | −0.054357 | |
| green only | 0.105863* | |
| green+red | −0.080883* | |
| green+banding | −0.016811 | |
| green+other | −0.105658* |
4. Discussion
The lack of correlation between ‘not green’ colour pattern types and relative distribution is expected as this is the ancestral colour pattern typical of most Asian pitvipers. ‘Green only’ species (which typically display a reddish tail tip in both sexes and a white lateral, and sometimes postocular, stripe in males), include all Cryptelytrops albolabris populations except those listed as ‘green plus red’ in table 1 (i.e. stretching from southern Thailand and West Java through southeast Asia and into southern China), Cryptelytrops erythrurus from Bangaldesh and Myanmar, and C. macrops in Thailand, Cambodia, Laos and southern Vietnam. Other ‘green only’ populations include Popeia sabahi from Sumatra, Popeia nebularis in the Cameron Highlands of West Malaysia, and this colour pattern is also found in some individuals of Parias flavomaculatus from Luzon and Himalayophis tibetanus from Nepal. ‘Green plus other’ colour patterns are found predominantly in species of the genus Parias (Parias hageni, Parias sumatranus, Parias malcolmi adults, Parias schultzei, P. flavomaculatus from Mindanao) in the Indomalayan and Philippine archipelagos, but also in Trimeresurus gramineus and Trimeresurus trigonocephalus from the Indian subcontinent. The association between ‘green only’ and ‘green plus other’ colour patterns and relative distribution may perhaps be better explained by habitat-driven selection pressures involving camouflage and thermoregulation (Sanders et al. 2004), but this needs further testing.
The inability to reject the null hypothesis for ‘green plus banding’ may be due to the small number of species that show this colour pattern type. Male and juvenile P. sabahi and all C. venustus in southern Thailand have distinct deep red or purplish cross-bands on the dorsal surface of the body and tail, and yellow or grey eyes. A third banded species, C. kanburiensis, which is closely related to C. venustus, is also found in Thailand, but is presently known only from the western province of Kanchanaburi, and has a less saturated coloration (Gumprecht et al. 2004; Malhotra & Thorpe 2004a). Finally, some colour morphs of the polymorphic species P. flavomaculatus (endemic to the Philippine islands) have a similar banded appearance. Since this species is highly polymorphic within populations, and allopatric to any other banded pitvipers, it is apparent that Müllerian mimicry is not an explanation for the presence of a banded colour pattern in this species, but nevertheless it may be for geographically close populations of P. sabahi and C. venustus in southern Thailand. A new species, recently described as Trimeresurus truongsonensis, also has purplish-brown dorsal cross-bands but is only known to occur in the karst region of central Vietnam (Orlov et al. 2004). Its phylogenetic position in relation to the other banded species is presently unknown, and therefore, it has not been included in the analysis.
The putatively mimetic ‘green plus red’ coloration is mirrored in four genera. P. popeiorum occurs in Northeast India, West and North Thailand, Laos and Myanmar, and here its range closely approaches that of V. gumprechti. In both species, males display vivid red and white postocular and lateral stripes that extend to the vent, and develop red eyes as they mature. Females of both species lack lateral red markings, although female P. popeiorum have red eyes. Viridovipera medoensis approaches the ranges of P. popeiorum and V. gumprechti in Myanmar and northeast India and is known to coexist with the former in at least one locality (A. Captain 2002, personal communication); both sexes of this species display bold red and white lateral stripes, but lack postocular markings and red eyes. Viridovipera stejnegeri occurs in North Vietnam (where it approaches the range of V. gumprechti), China and Taiwan; most males and some females have red and white lateral stripes. Males of V. stejnegeri usually also have red or orange eyes and occasionally display red postocular stripes. It is interesting that the red colour pattern elements are considerably reduced (no red in eyes or tail, and only a faint red lateral stripe present in some males) in the closely related Viridovipera vogeli which overlaps largely with ‘green-only’ species. In parts of north and northeastern Thailand, where C. albolabris overlaps in altitudinal range with P. popeiorum and V. gumprechti, this species also has vivid red or orange eyes. In other parts of its range, this widespread species normally has yellow eyes, although red-eyed forms of C. albolabris may also be found in parts of Vietnam. In Sabah, east Malaysia, both juvenile male P. malcolmi and male P. sabahi possess red colour pattern elements. However, species with this colour pattern type can also be found in allopatric situations. For example, some populations of P. sabahi in west Malaysia have red lateral stripes, and Cryptelytrops insularis in east Java and the Nusa Tenggara islands typically have dark red eyes (while some island populations of this species are known to show unusual body colour variants, e.g. cream, yellow, turquoise rather than green, this is clearly a derived condition possibly related to limited genetic diversity associated with a recent founder effect, and has been ignored in this analysis as it is unique to this species). Colour photographs of representatives of all these colour pattern types are shown in the electronic supplementary material.
Our study provides compelling evidence for a Müllerian mimetic radiation in Asian green pitvipers. However, experimental work on this system is required before mimicry can be established conclusively. In particular, it will be necessary to demonstrate an aposematic function of the putatively mimetic red colour pattern elements and selection against rare morphs in specific localities. Natural selection experiments using plasticine replicas have provided direct evidence of mimicry in coral snakes (Brodie 1993) and will be a priority for future study. It will also be important to identify the agents (predators) that may be causing selection to favour mimicry in this system. Canopy and understorey birds are possible candidates; they are successful snake predators and are highly perceptive of colour and pattern (Pough 1988).
The biased occurrence of the putative mimetic patterns in males indicates a sex or size limited form of mimicry (males are smaller in all species). Female-limited mimicry occurs in butterflies that display Batesian mimicry (Ohsaki 1995). Some authors have attributed this to sexual selection against males (Krebs & West 1988; Lederhouse & Scriber 1996). Intraspecific communication in snakes rarely involves visual displays and is unlikely to be important in the evolution of colour patterns (Carpenter 1977; Madsen & Shine 1992). Alternative explanations implicate differences in predation pressure between the sexes, relating to reduced agility (Ohsaki 1995) or, in a rare case of male-limited mimicry, increased diurnal activity (Vane-Wright 1975).
Sexual and ontogenetic differences in selection pressures are well documented for snakes. Small body size is associated with increased foraging activity and consequently higher predation pressure (Peters 1983). Males are more active over a broader range of habitats while searching for mates during the breeding season (A. Malhotra & R. S. Thorpe 1998, personal observation; Shine et al. 2003), which may increase their risk of predation (see Bonnet et al. 1998). Due to these differences in behaviour, it is reasonable to expect that males and juveniles would gain more from aposematism and mimicry than adult females. This argument is consistent with Wüster et al.'s (2004) observation that the aposematic zigzag patterns of European vipers are most conspicuous in males during the breeding season (see also Shine & Madsen 1994). Sex and size biased mimicry presents something of a paradox in a Müllerian system. Examples of sex-limited mimicry are currently restricted to Batesian mimics, which are more highly protected when rare (Ohsaki 1995). In Müllerian systems, it is expected that purifying selection in favour of the common pattern should cause all individuals in a population to be mimetic (Mallet & Joron 1999). It is possible that female pitvipers rarely become mimics because their sedentary lifestyles cause crypsis to be favoured over warning coloration. However, this remains speculative at present.
Clearly, there is much more to learn about this putatively mimetic radiation of Asian pitvipers. Field studies are likely to generate new insights into the dynamics of mimicry in a novel model organism and could provide a valuable test of the many generalizations concerning Müllerian mimicry.
Acknowledgments
We acknowledge the Natural Environment Research Council (NER/S/A2000/03695), the Leverhulme Trust (F174/I and F174/0) and the Wellcome Trust (057257/Z/99/Z and 060384/Z/00/Z) for major funding. Andreas Gumprecht, Ashok Captain and Darrell Shoub are thanked for supplying information and photographic material that contributed to the conclusions drawn in this paper.
Supplementary Material
Representative forms of the five colour pattern categories recognised in this study
References
- Alatalo R.V, Mappes J. Tracking the evolution of warning signals. Nature. 1996;382:708–710. 10.1038/382708a0 [Google Scholar]
- Bonnet E, Van de Peer Y. zt: a software tool for simple and partial Mantel tests. J. Stat. Soft. 2002;7:1–12. [Google Scholar]
- Bonnet X, Naulleau G, Shine R. The dangers of leaving home: dispersal and mortality in snakes. Biol. Conserv. 1998;89:39–50. 10.1016/S0006-3207(98)00140-2 [Google Scholar]
- Brandon R, Labanick G, Huheey J. Relative palatability, defensive behaviour, and mimetic relationships of red salamanders, mud salamanders, and red efs. Herpetologica. 1979;35:289–303. [Google Scholar]
- Brodie E.D. Differential avoidance of coral snake banded patterns by free-ranging avian predators. Evolution. 1993;47:227–235. doi: 10.1111/j.1558-5646.1993.tb01212.x. [DOI] [PubMed] [Google Scholar]
- Brower A.V.Z. Parallel race formation and the evolution of mimicry in Heliconius butterflies: a phylogenetic hypothesis from mitochondrial DNA sequences. Evolution. 1996;50:195–221. doi: 10.1111/j.1558-5646.1996.tb04486.x. [DOI] [PubMed] [Google Scholar]
- Carpenter C.C. Communication and displays of snakes. Am. Zool. 1977;17:217–223. [Google Scholar]
- Giannasi N.C, Thorpe R.S, Malhotra A. The use of amplified fragment length polymorphism (AFLP) in determining species trees at fine taxonomic levels: analysis of a medically important snake, Trimeresurus albolabris. Mol. Ecol. 2001;10:419–426. doi: 10.1046/j.1365-294x.2001.01220.x. [DOI] [PubMed] [Google Scholar]
- Greene H.W. University of California Press; Berkeley, CA: 1994. Snakes: the evolution of mystery in nature. [Google Scholar]
- Greene H, McDiarmid R. Coral snake mimicry: does it occur? Science. 1981;213:1207–1212. doi: 10.1126/science.213.4513.1207. [DOI] [PubMed] [Google Scholar]
- Greene H.W, McDiarmid R.W. Wallace and savage: heroes, theories, and venomous snake mimicry. In: Donnelly M.A, Crother B.I, Guyer C, Wake M.H, White M.E, editors. Ecology and evolution in the tropics: a herpetological perspective. University of Chicago Press; Chicago, IL: 2005. pp. 190–208. [Google Scholar]
- Gumprecht A, Tillack F, Orlov N.L, Captain A, Ryabov S. GeitjeBooks; Berlin, Germany: 2004. Asian pitvipers. [Google Scholar]
- Joron M, Mallet J. Diversity in mimicry: paradox or paradigm? Trends Ecol. Evol. 1998;13:461–466. doi: 10.1016/s0169-5347(98)01483-9. 10.1016/S0169-5347(98)01483-9 [DOI] [PubMed] [Google Scholar]
- Kapan D.D. Three-butterfly system provides a field test of Müllerian mimicry. Nature. 2001;409:338–340. doi: 10.1038/35053066. 10.1038/35053066 [DOI] [PubMed] [Google Scholar]
- Krebs R.A, West D.A. Female mate preference and the evolution of female-limited Batesian mimicry. Evolution. 1988;42:1101–1104. doi: 10.1111/j.1558-5646.1988.tb02530.x. [DOI] [PubMed] [Google Scholar]
- Lederhouse R.C, Scriber J.M. Intrasexual selection constrains the evolution of dorsal colour pattern of male black swallowtail butterflies, Papilio polyxenes. Evolution. 1996;50:717–722. doi: 10.1111/j.1558-5646.1996.tb03881.x. [DOI] [PubMed] [Google Scholar]
- Legendre P. Comparison of permutation methods for the partial correlation and partial mantel tests. J. Stat. Comput. Simul. 2000;67:37–73. [Google Scholar]
- Lindström L, Alatalo R.V, Lyytinen A, Mappes J. Strong antiapostatic selection against novel, rare, aposematic prey. Proc. Natl Acad. Sci. USA. 2001;98:9181–9184. doi: 10.1073/pnas.161071598. 10.1073/pnas.161071598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison W.P, Maddison D.R. Sinauer Associates; Sunderland, MA: 2001. MacClade 4: analysis of phylogeny and character evolution. Version 4.03. [Google Scholar]
- Madsen T, Shine R. A rapid, sexually-selected shift in mean body size in a population of snakes. Evolution. 1992;46:120–124. doi: 10.1111/j.1558-5646.1992.tb00630.x. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Thorpe R.S. A phylogeny of the Trimeresurus group of pit-vipers: new evidence from a mitochondrial gene tree. Mol. Phylogenet. Evol. 2000;16:199–211. doi: 10.1006/mpev.2000.0779. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Thorpe R.S. Reassessment of the validity and diagnosis of the pitviper Trimeresurus venustus (Vogel, 1991) Herpetol. J. 2004a;14:21. [Google Scholar]
- Malhotra A, Thorpe R.S. A phylogeny of four mitochondrial gene regions suggests a revised taxonomy for Asian pitvipers (Trimeresurus and Ovophis) Mol. Phylogenet. Evol. 2004b;32:83–100. doi: 10.1016/j.ympev.2004.02.008. 10.1016/j.ympev.2004.02.008 [DOI] [PubMed] [Google Scholar]
- Malhotra A, Thorpe R.S. Maximising information in systematic revisions: a combined molecular and morphological analysis of a cryptic green pitviper complex (Trimeresurus stejnegeri) Biol. J. Linn. Soc. 2004c;82:219–235. 10.1111/j.1095-8312.2004.00354.x [Google Scholar]
- Malhotra A, Thorpe R.S. Erratum to “A phylogeny of four mitochondrial gene regions suggests a revised taxonomy for Asian pit vipers (Trimeresurus and Ovophis)”. Mol. Phylogenet. Evol. 2005;34:680–681. doi: 10.1016/j.ympev.2004.02.008. 10.1016/j.ympev.2004.12.012 [Erratum in Mol. Phylogenet. Evol. 2004 32, 83–100.] [DOI] [PubMed] [Google Scholar]
- Mallet J. Causes and consequences of a lack of coevolution in Müllerian mimicry. Evol. Ecol. 1999;13:777–806. 10.1023/A:1011060330515 [Google Scholar]
- Mallet J, Joron M. Evolution of diversity in warning colour and mimicry: polymorphisms, shifting balance, and speciation. Annu. Rev. Ecol. Syst. 1999;30:201–233. 10.1146/annurev.ecolsys.30.1.201 [Google Scholar]
- Manly B.J.F. 2nd edn. Chapman & Hall; London, UK: 1991. Randomization, Bootstrap and Monte Carlo methods in biology. [Google Scholar]
- Müller F. Ituna and Thyridia; a remarkable case of mimicry in butterflies. Trans. Entomol. Soc. Lond. 1879;1879:xx–xxix. [Google Scholar]
- Ohsaki N. Preferential predation of female butterflies and the evolution of Batesian mimicry. Nature. 1995;378:173–175. 10.1038/378173a0 [Google Scholar]
- Orlov N.L, Ryabov S.A, Thanh B.N, Cuc H.T. A new species of Trimeresurus (Ophidia: Viperidae: Crotalinae) from karst region in Central Vietnam. Russ. J. Herpetol. 2004;11:139–149. [Google Scholar]
- Peters R.H. Cambridge University Press; Cambridge, UK: 1983. The ecological implications of body size. [Google Scholar]
- Pough F.H. Mimicry of vertebrates: are the rules different? Am. Nat. 1988;131:67–102. 10.1086/284767 [Google Scholar]
- Sanders K.L, Malhotra A, Thorpe R.S. Ecological diversification in a group of Indomalayan pitvipers (Trimeresurus): convergence in taxonomically important traits has implications for species identification. J. Evol. Biol. 2004;17:721–731. doi: 10.1111/j.1420-9101.2004.00735.x. 10.1111/j.1420-9101.2004.00735.x [DOI] [PubMed] [Google Scholar]
- Sanders, K. L., Malhotra, A. & Thorpe, R. S. In press. Combining molecular, morphological and ecological data to infer species boundaries in a cryptic tropical pitviper. Biol. J. Linn. Soc
- Shine R, Madsen T. Sexual dichromatism in snakes of the genus Vipera: a review and a new evolutionary hypothesis. J. Herpetol. 1994;28:114–117. [Google Scholar]
- Shine R, Shine T, Shine B. Intraspecific habitat partitioning by the sea snake Emydocephalus annulatus (Serpentes, Hydrophiidae): the effects of sex, body size, and colour pattern. Biol. J. Linn. Soc. 2003;80:1–10. 10.1046/j.1095-8312.2003.00213.x [Google Scholar]
- Skelhorn J, Rowe C. Tasting the difference: do multiple defence chemicals interact in Müllerian mimicry? Proc. R. Soc. B. 2005;272:1471–2954. doi: 10.1098/rspb.2004.2953. 10.1098/rspb.2004.2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed M, Turner J. Learning and memory in mimicry. Do we understand the mimicry spectrum. Biol. J. Linn. Soc. 1999;67:281–312. 10.1006/bijl.1998.0310 [Google Scholar]
- Symula R, Schulte R, Summers K. Molecular evidence for a mimetic radiation in Peruvian poison frogs supports a Müllerian mimicry hypothesis. Proc. R. Soc. B. 2001;268:2415–2421. doi: 10.1098/rspb.2001.1812. 10.1098/rspb.2001.1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane-Wright R.I. An integrated classification for polymorphism and sexual dimorphism in butterflies. J. Zool. 1975;177:329–337. [Google Scholar]
- Wüster W, et al. Do aposematism and Batesian mimicry require bright colours? A test, using European viper markings. Proc. R. Soc. B. 2004;271:2495–2499. doi: 10.1098/rspb.2004.2894. 10.1098/rspb.2004.2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative forms of the five colour pattern categories recognised in this study