Abstract
Cooperative breeding is comparatively rare among birds in the mainly temperate and boreal Northern Hemisphere. Here we test if the distribution of breeding systems reflects a response to latitude by means of a phylogenetic analysis using correlates with geographical range among the corvids (crows, jays, magpies and allied groups). The corvids trace their ancestry to the predominantly cooperative ‘Corvida’ branch of oscine passerines from the Australo-Papuan region on the ancient Gondwanaland supercontinent, but we could not confirm the ancestral state of the breeding system within the family, while family cohesion may be ancestral. Initial diversification among pair-breeding taxa that are basal in the corvid phylogeny, represented by genera such as Pyrrhocorax and Dendrocitta, indicates that the corvid family in its current form could have evolved from pair-breeding ancestors only after they had escaped the Australo-Papuan shield. Within the family, cooperative breeding (alloparental care/family cohesion) is strongly correlated to latitude and its predominance in species maintaining a southerly distribution indicates a secondary evolution of cooperative breeding in the lineage leading away from the basal corvids. Multiple transitions show plasticity in the breeding system, indicating a response to latitude rather than evolutionary inertia. The evolutionary background to the loss of cooperative breeding among species with a northerly distribution is complex and differs between species, indicating a response to a variety of selection forces. Family cohesion where the offspring provide alloparental care is a main route to cooperatively breeding groups among corvids. Some corvid species lost only alloparental care, while maintaining coherent family groups. Other species lost family cohesion and, as a corollary, they also lost the behaviour where retained offspring provide alloparental care.
Keywords: corvids, delayed, dispersal, cooperative, breeding, phylogeny
1. Introduction
Recent estimates point to the fact that cooperative breeding involving contribution of more than two birds, either as co-breeders or non-breeding extra birds, is more common than previously recognized. For instance, as many as one-quarter of all oscine passerines are currently estimated to reproduce cooperatively (Cockburn 2003). Phylogenetic analyses have recognized a strong role of history predisposing species to breed cooperatively, which is identified as the ancestral state of the breeding system in several avian lineages (Russell 1989; Edwards & Naeem 1993; Cockburn 1996; Nicholls et al. 2000; Ligon & Burt 2004). In contrast, ecological context, design and life-history traits conducive to cooperative breeding remain less well understood, with contradictory results for the role of factors like environmental unpredictability (Ford et al. 1988; Du Plessis et al. 1995) and longevity (Arnold & Owens 1998; Cockburn 2003).
Here we explore the evolutionary history of the breeding system and social behaviour among crows, jays, magpies and allied groups during their range expansion out of an area of origin in the Southern Hemisphere. A historic perspective offers an opportunity to integrate ecological factors and design features that commit extant species to cooperative breeding. Apart from identifying evolutionary stasis or revealing the direction of evolutionary changes, such an analysis may provide clues to the role of ecological conditions driving evolutionary change. Broad-scale comparative analyses can reveal general patterns in covariation between behavioural traits, such as the breeding system and ecological conditions. However, extant species differ not only in the ecological conditions of their environment. As a result of separate evolutionary histories and diversification, lineages differ also in design and associated life-history traits (Ridley 1983; Harvey & Pagel 1991; Winkler 2000). The role of ecology is therefore best seen in clades, which are more homogenous as the result of a more recent shared history. The corvids (Corvini sensu Sibley & Monroe 1990; Corvidae sensu Dickinson 2003) are a monophyletic group within the oscine passerines, presumably with a relatively Late or Middle Tertiary origin (Feduccia 1995). The relatively recent origin coupled with a homogenous design within the group makes it suitable for a comparative test.
A main feature of avian cooperative breeding is its relative paucity on the temperate and boreal landmasses of the Northern Hemisphere, reflecting the current geographical distribution within the species-rich Passerida branch of the oscine passerines (Cockburn 2003). Corvids trace their ancestry to the other main branch of the oscine passerine called ‘Corvida’ by Sibley & Monroe (1990), consisting of several highly cooperative lineages (Cockburn 1996; Nicholls et al. 2000; Ligon & Burt 2004) of Gondwanan origin and currently found mainly in the Australo-Papuan region (Barker et al. 2002; Ericson et al. 2002). An ancestry among cooperative lineages coupled with a Southern Hemisphere origin offers the opportunity for a comparative analysis of the response in breeding system to latitude. Corvids are suited for such a test in being exceptional among Corvida in the sense that they have dispersed extensively beyond the Australo-Papuan region, while cooperatively breeding birds are normally characterized by a limited dispersal capacity (Cockburn 2003). The corvid family itself may have evolved out of more dispersal-prone pair-breeding representatives among cooperative clades (Cockburn 2003) somewhere in Southeast Asia, only after their shrike-corvid ancestor had escaped Australo-Papua proper (Barker et al. 2004; Ericson et al. 2005). Yet, the group is well known for its complex social behaviour, which has attracted much attention and is comparatively well studied (for instance, Brown 1963a,b, 1970, 1974; Verbeek & Butler 1981; Woolfenden & Fitzpatrick 1984; Severinghaus 1987; Skutch 1987; Brown & Brown 1990; Marzluff & Balda 1990; Richner 1990; Ekman et al. 1994; Baglione et al. 2002a).
2. Material and methods
The characters of species do not represent evolutionary independent events, but species' similarity due to common descent, as reflected in a phylogenetic tree, has to be controlled for (Harvey & Pagel 1991; Harvey & Purvis 1991). Our analysis focuses on two traits: family cohesion and alloparental care by retained offspring. Both these characters are bivariate, and the Discrete v. 1.0.1b software, which provides maximum-likelihood reconstruction of ancestral states and correlated character evolution for discrete characters on a bifurcate phylogeny, is designed to analyse such characters (Pagel 1994, 1997, 1999a,b). A main advantage of the Discrete algorithm is that the analysis of correlated character evolution is not dependent upon the reconstruction of ancestral states, while the maximization process takes its beginning at random points in the phylogeny. The reconstruction of the ancestral distribution of traits in the Discrete program can either be ‘global’, assigning a state to all nodes in the phylogeny, or it can be ‘local’ and find the values for specific nodes (Pagel 1999b). A special case of the local reconstruction is to find the states at the roots.
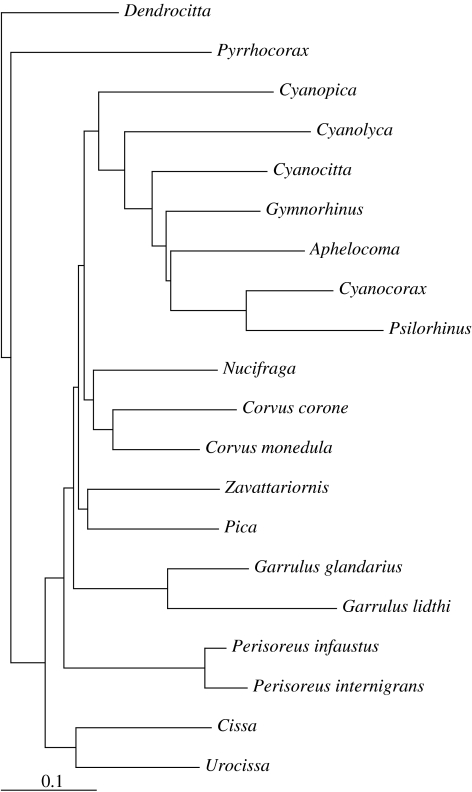
The ancestral state reconstruction and analyses of correlated character evolution were run, including branch lengths calculated from cytochrome b using PAUP v. 4.0b10 (Swofford 1998). The phylogeny we used is the hypothesis of Ericson et al. (2005) reconstructed from the combined data of one mitochondrial (cytochrome b) and two nuclear (rag-1 and myoglobin) genes (figure 1). Branch lengths in our analysis are estimated from cytochrome b data. There was no support for the position of Cyanopica in the analysis of Ericson et al. (2005) and a tree based on the cytochrome b gene alone gave it a different position in the tree used here. Species included in the analysis are listed in appendix A. Based on molecular data, two monospecific genera (Platylophus, Pseudopodoces) traditionally considered to be corvids are excluded here (James et al. 2002; Ericson et al. 2005), leaving 119 species from 23 genera in the group (Madge & Burn 1994). There are 2n possible assignments of ancestral states in a phylogeny with n nodes, and computations therefore grow fast with the number of species included. Taxa on the same branch and sharing traits contribute only insignificantly to the result in the Discrete algorithm (Pagel 1994), and computations could thus be reduced by collapsing nodes for such taxa. Species could be clustered and used as terminal taxa for genera with no known variation in trait combinations. Hence, we clustered congeneric species within the genera Nucifraga, Cissa, Urocissa, Dendrocitta, Pyrrhocorax and Pica. For the same reason it was possible to cluster genera on the same branch sharing trait combinations (Zavattariornis/Ptilostomus and Cyanocorax/Psilorhinus).
Figure 1.
Dataset with branch lengths. Species used in reconstruction of phylogeny: Dendrocitta formosae, Pyrrhocorax pyrrhocorax, Cyanopica cyana, Cyanolyca viridicyana, Cyanocitta cristata, Gymnorhinus cyanocephala, Aphelocoma coerulescens, Cyanocorax chrysops, Psilorhinus morio, Nucifraga caryocataces, Corvus corone, Corvus monedula, Zavattariornis stresemanni, Pica pica, Garrulus glandarius, Garrulus lidthi, Perisoreus infaustus, Perisoreus internigrans, Cissa chinensis, Urocissa erythrorhyncha.
A first test run included within-genus variation in trait combinations among three genera, for which we have data on branch length on species level (Garrulus, Corvus, Perisoreus; figure 1). In an extended dataset of a second run, we further included variation on species level within another four genera (Aphelocoma, Cyanocorax, Cyanolyca, Cyanocitta), although we did not have data on branch length for species as terminal taxa. For this run we included the following species in addition to the taxa in figure 1: Aphelocoma californica, Cyanocorax yncas (Texas population), Cyanolyca nana and Cyanocitta stelleri (test for family cohesion only). Species within these four genera were treated as sister groups to their congeners, with the nodes of branches leading to these species assumed to be located half way down the branch leading to their congeners. Branch length is taken into account in the Discrete algorithm and the role of branch length can be tested through the kappa parameter. A value approaching zero signifies a punctuational mode of evolution, where branch length contributes only insignificantly to the solution, while a kappa value of one represents a uniform rate of evolution (Pagel 1997). With a kappa value of 0.00054, for the corvids the branch length should be of minor significance for the Discrete solution, justifying the inclusion of species providing additional information on trait values despite the lack of data on branch length.
The Discrete algorithm is based on forward (α) and backward (β) transition rates of a bivariate trait within the phylogeny. These transition rates for traits can be either different (two-parameter model) or identical (one-parameter model). A two-parameter model can generate paradoxical results and is justified only if it produces a better fit to the data than a one-parameter model. Else the one-parameter model should be chosen as default (Mooers & Schluter 1999; Pagel 1999b). Here there was no support for a better fit of the corvid data on alloparental care by a two-parameter model (likelihood=−24.6045; one-parameter model likelihood=−24.7036; likelihood ratio=0.0991, p=0.75). For the data on family cohesion the two-parameter model (likelihood=−20.7971) produced a fit that nearly, but not fully, significantly improved the fit from a one-parameter model (likelihood=−22.7094; likelihood ratio=3.8246, p=0.0505). The ancestral distributions and correlated character evolution were therefore analysed with a one-parameter model (α=β). Correlated evolution of characters was tested by comparing the likelihood of a model, assuming the traits to be independent (H0) to one where the evolution of the traits is linked (H1). The likelihood ratio statistics (calculated as −2((likelihood H0)−(likelihood H1))) can be assumed to be χ2-distributed, and the probability of H1 can be estimated from testing it against a null hypothesis based on transitions rates generated from resampling of the original distribution using the Discrete software (Pagel 1997).
3. Characters
(a) Breeding system and family cohesion
The wealth of information on breeding system and social behaviour among corvids is summarized by Brown (1987), Skutch (1987), Madge & Burn (1994) and Cockburn (2003). Primary sources for the data used here are listed in appendix A. Cooperative breeding is conventionally identified from the presence of extra birds helping to breed (alloparenting), and is as such a bivariate trait in the form required by the Discrete program. Delayed offspring dispersal is the main route to become an alloparenting extra bird among corvids and family cohesion is thus a prerequisite for cooperative breeding (Skutch 1987). We therefore also tested for environmental correlates to family cohesion. As an operative definition, dispersal is conventionally considered to be delayed if mature offspring remain past a breeding season, and identification of retained offspring normally requires colour banding. Such data on family cohesion are lacking for a couple of less studied genera with tropical and subtropical distribution (Cissa, Dendrocitta) reported to live in family groups while breeding as pairs (Madge & Burn 1994). We tentatively treated Cissa and Dendrocitta as having delayed dispersal in the test for family cohesion including cooperatively breeding species and corvid species, where colour banding identified coherent family groups in the absence of alloparental care. In-depth studies of a number of other corvid species have revealed that unassisted pair breeding is not an uncommon breeding system within coherent families (Verbeek & Butler 1981; Gayou 1986; Eden 1987; Strickland 1991; Ekman et al. 1994; Caffrey 2000). We further included Steller's jay (C. stelleri). In this species the offspring maintain a prolonged association with their parent that lasts at least into autumn and winter of their first year (Brown 1963b). To test for the effect of using this more inclusive definition of delayed dispersal, we also ran analyses without Cissa, Dendrocitta and C. stelleri.
(b) Distribution
To analyse for any responses in the breeding system to the expansion out of their area of origin in the Australo-Papuan region (Sibley & Ahlquist 1990; Barker et al. 2002; Ericson et al. 2002, 2005), the latitudinal distribution of corvids was dichotomized. Species were assigned to the two categories based on the northernmost point of distribution, taken to characterize the conditions limiting the expansion northwards. Distributions were taken from Madge & Burn (1994) and references therein. Few terminal taxa had the northern limit of their distribution in the range between around 55 and 60° N, which were used as cut-off points to characterize species with a northern and a southern distribution in our analysis. One group of seven terminal taxa had their distribution limit around 60° N or further north, while the distribution did not reach further north than around 55° N for another group of 15 terminal taxa. This criterion correlated strongly to the midpoint of distribution limits for each species (r=0.92, p<0.0001).
(c) Habitat
The corvids expanded out of the Australo-Papuan region in Mid to Late Tertiary, while it was covered with rain forest (White 1987). The evolutionary history and changes in the breeding system among extant corvids was therefore also tested against habitat. The habitat was represented by forest structure categorized as closed or open, which includes habitats ranging from forest edges to clearings, parklands, open brush land and desert.
4. Results
Alloparental care is known for 27 (32%) corvid species out of 84 with known breeding system. With the exception of Corvus caurinus, where help is rare (Verbeek & Butler 1981), and the cooperative Covus corax (Christensen & Grünkorn 1997), this list is identical to Cockburn (2003). The mating system of cooperatively breeding corvids ranges from monogamous pairs (for instance, Woolfenden & Fitzpatrick 1984) to colonial breeders (Brown 1990; Marzluff & Balda 1990). In addition, 26 solitary breeding species are recorded to live in family groups, which for some of these species was confirmed by in-depth studies of colour-banded individuals, while another 24 species are recorded to live solitarily or in pairs. Thus, social cohesion is strong among corvids, with the majority of species (69%; 53 out of 77 species with known social system) living in cohesive family groups.
(a) Latitude effects
There is a strong association between latitudinal distribution and alloparental care (table 1). A global model without any restriction on the states at the root and based on the data in table 1 returned a strong association between absence of alloparental care and a northern distribution (likelihood ratio statistics=12.5652, p=0.001; for test procedure see §2). This result was robust to the inclusion of C. yncas (Texas population), C. nana and A. californica (likelihood ratio=8.5816, p=0.001; for test procedure see §2), for which we lack data on branch length. Inclusion of these three solitary breeders with a southern distribution is conservative, while supporting the null hypothesis that lack of alloparental care is not linked to a northerly distribution.
Table 1.
Latitudinal distribution of delayed dispersal and alloparental care within genera or for species to represent within-genera variation.
| species distribution | breeding system | ||
|---|---|---|---|
| unassisted biparental care | delayed dispersal, no alloparental care | alloparental care | |
| Northern | Cyanocitta cristata | Cyanocitta stelleri | |
| Corvus monedula | Pica pica | ||
| Nucifraga caryocatactes | Perisoreus infaustus | ||
| Garrulus glandarius | |||
| Southern | Cyanolyca nana | Cissa chinensis | Cyanocorax chrysops |
| Dendrocitta formosae | Cyanolyca viridicyanea | ||
| Cyanocorax yncas | Corvus corone (Spain) | ||
| Pyrrhocorax? | Cyanopica cyana | ||
| Garrulus lidthi | |||
| Zavattariornis stresemanni | |||
| Perisoreus internigrans | |||
| Urocissa erythrorhyncha | |||
Likewise, a global model without restrictions on states at the root, also including C. yncas (Texas population), C. nana and A. californica, showed a strong association between latitudinal distribution and family cohesion (likelihood ratio statistics=15.7766, p=0.001; for test procedure see §2). The relationship between latitude and delayed dispersal remained significant (likelihood ratio=12.8166, p=0.001; for test procedure see §2) when we included C. stelleri, in which the offspring retain a prolonged family cohesion (Brown 1963b) unlike in its congener Cyanocitta cristata. Classifying the Steller jay as having delayed dispersal would be conservative, while it rather supports the null hypothesis that the ancestral state of natal philopatry has been retained at high latitudes. The analysis was robust to reduction in the tree by collapsing nodes for poorly studied species (Cissa, Dendrocitta) and consistently returned an association between latitude and family cohesion.
(b) Ancestral states
The correlation between breeding systems among corvids indicates a loss of alloparental care and delayed dispersal as they expanded their range northwards from the ancestral states (Cockburn 1996, 2003; Nicholls et al. 2000; Ligon & Burt 2004). Indeed, a global model without root restrictions and including C. yncas (Texas population), C. nana and A. californica, in addition to the terminal taxa in figure 1, returned a reconstruction of the ancestral distribution with alloparental care at all internal nodes except for the one leading to the Perisoreus genus (figure 2, likelihood=−27.7255). Yet with a likelihood ratio statistics≪1 in a local model, the character states at the root (alloparental care/no alloparental care) were not identified with significance. The reconstruction was however robust and returned alloparental care as the ancestral state also when nodes were collapsed or data removed for less-well-studied genera (e.g. Cissa). A corresponding analysis including C. stelleri identified family cohesion (delayed dispersal) as the ancestral state at all internal nodes (figure 3, likelihood=−28.4550) and as the state at the root in a local model (likelihood ratio=5.1932, d.f.=1, p=0.05). The reconstruction was robust to removing C. stelleri, Cissa and Dendrocitta and collapsing nodes close to the root.
Figure 2.
The reconstruction of the ancestral distribution of alloparental care by the Discrete software. Dark, alloparental care; light, no alloparental care; striped, breeding system uncertain.
Figure 3.
The ancestral distribution of family cohesion (delayed dispersal) by the Discrete software. Dark, family cohesion (delayed dispersal); light, no family cohesion among mature birds.
(c) Correlated evolution and the uncertainty of ancestral states
To test for the uncertainty in assignment of the ancestral states of characters, the probability of alloparental care and delayed dispersal being correlated to latitudinal distribution was investigated further for all four possible combinations of character states at the roots (table 2). Latitude showed a significant association to both delayed dispersal and alloparental care for all combinations of character states at the root, verifying that the association is robust to the uncertainty in assignment of ancestral states.
Table 2.
Test for correlation between latitude and delayed dispersal (family cohesion; top) and alloparental care by retained offspring (bottom) for different states of latitude and trait value at the root. (Log likelihood given as absolute values.)
| root latitude (0=south; 1=north) | root delayed dispersal (0=no; 1=yes) | independent evolution log likelihood | dependent evolution log likelihood | likelihood ratio | p-value (chi-square test) |
|---|---|---|---|---|---|
| 0 | 0 | 26.7601 | 22.2532 | 9.0136 | 0.01 |
| 0 | 1 | 26.7362 | 22.6439 | 8.1846 | 0.01 |
| 1 | 0 | 26.3002 | 21.9852 | 8.6300 | 0.02 |
| 1 | 1 | 26.2763 | 22.2880 | 7.9764 | 0.02 |
| root latitude (0=south; 1=north) | root alloparental care (0=no; 1=yes) | independent evolution log likelihood | dependent evolution log likelihood | likelihood ratio | p-value (chi-square test) |
|---|---|---|---|---|---|
| 0 | 0 | 27.3419 | 24.8211 | 5.0416 | 0.02 |
| 0 | 1 | 27.3399 | 24.8450 | 4.9898 | 0.02 |
| 1 | 0 | 27.4979 | 24.3450 | 6.2878 | 0.02 |
| 1 | 1 | 27.4959 | 24.8538 | 5.2840 | 0.02 |
(d) Habitat
The analysis could not confirm a forest origin. A global model without root restrictions is consistent with the hypothesis that the ancestral habitat of the corvids was closed forest (likelihood=−36.4608). A local model could not reconstruct the states at the root with any significance, but both habitat states (closed forest/open forest) are equally likely.
5. Discussion
We analysed cooperative breeding as a two-stage process, where family cohesion (delayed dispersal) is the permissive condition for alloparental care. There would be several caveats in confining the test for environmental correlates to cooperative breeding to species with alloparental care alone. Alloparental care could be selected against in cohesive families, which in the absence of alloparental care would be excluded from cooperative breeders and combined with species that do not organize into family groups. This procedure would fail to account for the fact that the same conditions promoting family cohesion also promote cooperative breeding by families (Brown 1987), and these would thus be misrepresented in an analysis. It would be more reasonable to see the breeding system and the role of extra-birds in cohesive families as the outcome of a parent/offspring conflict, where a solution with the offspring providing no care is just an endpoint to a continuum of different levels of alloparental care (Pruett-Jones 2004). The absence of alloparental care among species living in cohesive family units may hence be as informative as its presence to selection forces shaping cooperative breeding (Ekman et al. 2001).
Cooperative breeding is a more frequent breeding system among bird species with a distribution in tropical and subtropical environments on the Southern Hemisphere than in temperate and boreal regions of the Northern Hemisphere (Rowley 1968, 1976; Fry 1977; Brown 1987; Russell 1989). This latitudinal distribution with its inherent correlation between climatic regions and breeding systems has been a source of ideas for linking environmental factors to the evolution of cooperative breeding (Verbeek 1973; Brown 1974; Ford et al. 1988; Ekman & Rosander 1992; Du Plessis et al. 1995; Russell et al. 2004). Yet, so far the current evidence has identified this pattern as the result of an evolutionary inertia among the species-rich Passerida branch of the oscine passerines and their current mainly-Northern Hemisphere distribution (Cockburn 2003). This conclusion was based on the view that the Passerida conserved their ancestral pair-breeding system as they escaped from their Gondwanan origin via the Australo-Papuan region. This view has been challenged by the discovery of a deep divergence in the Passerida lineage revealing a second escape route out of Gondwanaland over South-Africa, suggesting older history in Africa than previously assumed (Barker et al. 2004; Beresford et al. 2005). Among taxa representing the deep divergence of the African Passerida lineage there are cooperative species (e.g. rockjumpers genus Chaetops), calling into question the route which the Passerida reached the Northern Hemisphere, their ancestral breeding system and to what extent the Northern Hemisphere predominance of pair breeding represents evolutionary inertia. Among corvids there could be several explanations to the latitude correlation to breeding system; all of which are consistent with a response to latitude, where cooperation is selected against with a northerly distribution either because it is lost or it has not evolved secondarily as in congeners with a more southerly distribution.
The corvid family in its current form is likely to have originated outside Australo-Papua (Barker et al. 2004; Ericson et al. 2005), and low dispersal proneness among cooperative breeders indicates that the initial escape of the corvids lineage from the Australo-Papuan region may well have been by pair-breeding representatives from cooperative clades (Cockburn 2003). The initial diversification among the corvids would then have involved more dispersal-prone pair-breeding taxa represented by genera such as Dendrocitta and Pyrrhocorax. This initial diversification would then have been followed by a secondary evolution of alloparental care in the lineage, leading away from these basal branches. Our global reconstructions could not identify the ancestral state of the breeding system in the corvid family, although they trace their ancestry to highly cooperative Corvida lineages (Cockburn 1996, 2003; Nicholls et al. 2000; Ligon & Burt 2004). Our difficulties in identifying the ancestral state reflect a labile breeding system among the Corvida, where cooperative breeding has been lost and gained several times (Ligon & Burt 2004).
The reconstruction of ancestral states shows that cooperative breeding among corvids is a highly plastic trait with multiple transitions (figures 2 and 3), but it also indicates a diversity of selection forces involved in the response of the breeding system to latitude. The Discrete program did identify family cohesion as ancestral and the absence of cooperation is associated with loss of family cohesion in several taxa (C. cristata, C. nana, A. californica, genus Nucifraga, Garrulus glandarius/lanceolatus, and numerous species within genus Corvus). In other species, the offspring do not provide alloparental care despite maintaining family cohesion (Cissa, Dendrocitta, C. yncas—Texas population, Persisoreus infaustus/canadensis, Pica pica, C. stelleri). These species maintain a social system that would be permissive to selection for alloparental care. Yet, they do not breed cooperatively. The most likely explanation for this absence of alloparental care among coherent families is that cooperative breeding is selected against. The prevalence of cooperative breeding among congeners with a southern distribution coupled to multiple transitions seems to eliminate evolutionary inertia among an ancestrally non-cooperative breeding system as an explanation for its geographical pattern. Multiple transitions in breeding and social system among corvids reflect a highly plastic behaviour, and our results are consistent with an analysis of cooperative breeding in the acrocephaline warblers, where the breeding system was found to be equally labile with links to food abundance and habitat (Leisler et al. 2002). This sensitivity of breeding systems to environmental conditions is consistent with family cohesion being responsive to manipulation of feeding conditions as well as the social environment (Komdeur 1992; Baglione et al. 2002b; Ekman & Griesser 2002; Covas et al. 2004), and raises the question as to whether a consistently cooperative breeding system within entire genera and over their entire ranges should be taken as evidence for evolutionary inertia or a response to selection (Edwards & Naeem 1993).
The corvids evolved in an environment of rain forest (Feduccia 1995), but we found no support for the hypothesis that shifts in breeding system were correlated to an expansion of the distribution into more open habitats. Apart from entering more open habitats, the corvids encountered seasonal environments with low ambient temperatures and short days in winter at the expansion of their range into the Northern Hemisphere. While the offspring may delay dispersal for lack of independent breeding opportunities in a saturated environment (Brown 1969; Komdeur 1992), food-limited survival during temperate and boreal region winters (Jansson et al. 1981; Brittingham & Temple 1988) may reduce numbers to an extent that will lift constraints on independent breeding and dispersal (Verbeek 1973; Brown 1974). This can, however, not be the full explanation for the paucity of cooperative breeding while there is a non-breeding surplus in many Northern Hemisphere populations and still the offspring do not postpone dispersal (Brown 1969). A non-random seasonal timing of natal dispersal in the Northern Hemisphere, where the offspring in species without delayed dispersal almost invariably leave before the energetically challenging winter (Russell et al. 2004), rather indicates avoidance of within-family competition driving the offspring to leave, thus precluding delayed dispersal. Indeed, when dispersal is delayed it is also associated with relaxed aggression within families as seen in that parents share food with retained offspring in winter, while they deny unrelated group members such unhindered access to food (Scott 1981; Barkan et al. 1986; Ekman et al. 1994; Pravosudova et al. 2000; Dickinson & McGowan 2005). Such a joint one-way effect of adverse climatic conditions on population saturation and parent behaviour would explain why cooperatively breeding corvids responded with loss of family cohesion (and hence alloparental care) to relaxed population pressure coupled to within-family competition in temperate and boreal climates, while the same conditions would have allowed non-cooperative taxa to retain their ancestral breeding system of unassisted pair breeding in the absence of family cohesion.
Acknowledgments
We gratefully acknowledge the constructive comments of the reviewers on this manuscript. This research was financially supported by the Swedish Research Council (grant no. 621-2004-2913 to P.E. and grant no. 621-2002-5076 to J.E.). This study was carried out while J.E. was working as a distinguished research fellow at the Institute of Advanced Study (IAS), La Trobe University, Melbourne.
Appendix A
Appendix A.
Information on social behaviour.
| genus/species | |
|---|---|
| Dendrocitta formosae | Ali & Ripley (1972) |
| Pyrrhocorax pyrrhocorax | Holyoak (1972) |
| Urocissa erythrorhyncha | Severinghaus (1987) |
| Cissa chinensis | Ali & Ripley (1972) |
| Perisoreus infaustus | Ekman et al. (1994), Lillandt et al. (2003) |
| Perisoreus internigrans | Y.-H. Sun 2003, personal communication |
| Garrulus lidhti | Bruce (1979) |
| Garrulus glandarius | Cramp & Perrins (1994) |
| Pica pica | Eden (1987) |
| Zavattariornis stresemanni | Fry et al. (2000) |
| Ptilostomus afer | C. Spottiswoode 2003, personal communication |
| Nucifraga caryocatactes | Bent (1946), Rolando (1996), Rolando & Caristo (2003) |
| Corvus monedula | Cramp & Perrins (1994) |
| Corvus corone (Spain) | Baglione et al. (2002a) |
| Cyanopica cyana | Hosono (1983), Canario et al. (2004) |
| Cyanolyca viridicyanea | A. Cockburn 2003, personal communication |
| Cyanolyca nana | Hardy (1971) |
| Cyanocitta stelleri | Brown (1963b) |
| Cyanocitta cristata | Bent (1946) |
| Psilorhinus morio | Skutch (1960) |
| Cyanocorax yncas | Bent (1946); Gayou (1986) |
| Cyanocorax chrysops | Brown (1974) |
| Aphelocoma californica | Carmen (2004) |
| Aphelocoma coerulescens | Woolfenden & Fitzpatrick (1984) |
| Gymnorhinus cyanocephalus | Ligon (1971) |
References
- Ali S, Ripley S.D. Handbook of the birds of India and Pakistan. vol. 5. Oxford University Press; Bombay: 1972. [Google Scholar]
- Arnold K.E, Owens I.P.F. Cooperative breeding in birds: a comparative test of the life history hypothesis. Proc. R. Soc. B. 1998;265:739–745. 10.1098/rspb.1998.0355 [Google Scholar]
- Baglione V, Marcos J.M, Canestrari D. Cooperative breeding groups of Carrion crow (Corvus corone corone) in northern Spain. Auk. 2002a;119:790–799. [Google Scholar]
- Baglione V, Canestrari D, Marcos J.M, Griesser M, Ekman J. History environment and social behaviour, experimentally induced cooperative breeding in the carrion crow. Proc. R. Soc. B. 2002b;269:1247–1251. doi: 10.1098/rspb.2002.2016. 10.1098/rspb.2002.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan C.P.L, Craig J.L, Strahl S.D, Stewart A.M, Brown J.L. Social dominance in communal Mexican jays Aphelocoma ultramarina. Anim. Behav. 1986;34:175–187. 10.1016/0003-3472(86)90021-7 [Google Scholar]
- Barker K.F, Barrowclough G.F, Groth J.G. A phylogenetic analysis for passerine birds: taxonomic and biogeographic implications of an analysis of nuclear DNA sequence data. Proc. R. Soc. B. 2002;269:295–308. doi: 10.1098/rspb.2001.1883. 10.1098/rspb.2001.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker K.F, Cibois A, Schikler P, Feinstein J, Cracraft J. Phylogeny and diversification of the largest avian radiation. Proc. Natl Acad. Sci. 2004;101:1040–1045. doi: 10.1073/pnas.0401892101. 10.1073/pnas.0401892101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent A.C. Life histories of North American jays, crows and titmice. Bull. US Natl Mus. 1946:191. [Google Scholar]
- Beresford P, Barker F.K, Ryan P.G, Crowe T.M. African endemics span the tree of songbirds (Passeri): molecular systematics of several evolutionary enigmas. Proc. R. Soc. B. 2005;272:849–858. doi: 10.1098/rspb.2004.2997. 10.1098/rspb.2004.2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittingham M.C, Temple S.T. Impact of supplemental feeding of survival rates of black-capped chickadees. Ecology. 1988;69:581–589. [Google Scholar]
- Brown J.L. Social behaviour of the Mexican jay. Condor. 1963a;65:126–153. [Google Scholar]
- Brown J.L. Aggressiveness, dominance, and social organization in the Steller jay. Condor. 1963b;65:460–484. [Google Scholar]
- Brown J.L. Territorial behaviour and population regulation in birds, a review and re-evaluation. Wilson Bull. 1969;81:293–329. [Google Scholar]
- Brown J.L. Cooperative breeding and altruistic behaviour in the Mexican jay, Aphelocoma ultramarina. Anim. Behav. 1970;18:366–378. 10.1016/S0003-3472(70)80050-1 [Google Scholar]
- Brown J.L. Alternate routes to sociality in jays—with a theory for the evolution of altruism and cooperative breeding. Am. Zool. 1974;14:63–80. [Google Scholar]
- Brown J.L. Princeton University Press; Princeton, NJ: 1987. Helping and communal breeding in birds. [Google Scholar]
- Brown J.L, Brown E.R. Mexican jays: uncooperative breeding. In: Stacey P.B, Koenig W.D, editors. Cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 1990. pp. 267–288. [Google Scholar]
- Bruce M.D. Notes on the status, vocalization and behaviour of Lidth's jay Garrulus lidthi. Le Gerfaut. 1979;69:353–356. [Google Scholar]
- Caffrey C. Correlates of reproductive success in cooperatively breeding western American crows: if helpers help, it's not by much. Condor. 2000;102:333–341. [Google Scholar]
- Canario F, Mators S, Soler M. Environmental constraints and cooperative breeding in the Azure-winged magpie. Condor. 2004;106:608–617. [Google Scholar]
- Carmen W.J. Studies in Avian Biology, no. 28. Cooper Ornithological Society; Camarillo, CA: 2004. Noncooperative breeding in the California scrub-jay. [Google Scholar]
- Christensen H, Grünkorn T. Nesthilfe beim Kolkraben (Corvus corax L.) nachgewiesen. Corax. 1997;17:66–67. [Google Scholar]
- Cockburn A. Why do so many Australian birds cooperate: social evolution in the Corvida? In: Floyd R.B, Sheppard A.W, De Barro P.J, editors. Frontiers of population ecology. CSIRO; East Melbourne: 1996. pp. 451–472. [Google Scholar]
- Cockburn A. Cooperative breeding in oscine passerines: does sociality inhibit speciation. Proc. R. Soc. B. 2003;270:2207–2214. doi: 10.1098/rspb.2003.2503. 10.1098/rspb.2003.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covas R.C, Doutrelant C, du Plessis M.A. Experimental evidence of a link between breeding conditions and the decision to breed or to help in a colonial breeding bird. Proc. R. Soc. B. 2004;271:827–832. doi: 10.1098/rspb.2003.2652. 10.1098/rspb.2003.2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramp S, Perrins C.M. Oxford University Press; Oxford: 1994. The birds of the western Palearctic, vol. VIII. Crows to finches. [Google Scholar]
- Dickinson E.C, editor. The Howard and Moore complete checklist of the birds of the world. 2003. Revised and enlarged 3rd edn. London. 1040 pp. [Google Scholar]
- Dickinson J.L, McGowan A. Winter resource wealth drives delayed dispersal and family-group living in western bluebirds. Proc. R. Soc. B. 2005;272:2423–2428. doi: 10.1098/rspb.2005.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Plessis M.A, Siegfried W.R, Armstrong A.J. Ecology and life history correlates of cooperative breeding in South-African birds. Oecologia. 1995;102:180–188. doi: 10.1007/BF00333250. 10.1007/BF00333250 [DOI] [PubMed] [Google Scholar]
- Eden S.F. Natal philopatry of the magpie Pica pica. Ibis. 1987;129:477–490. [Google Scholar]
- Edwards S.V, Naeem S. The phylogenetic component of cooperative breeding in perching birds. Am. Nat. 1993;141:754–789. doi: 10.1086/285504. 10.1086/285504 [DOI] [PubMed] [Google Scholar]
- Ekman J, Griesser M. Why offspring delay dispersal: experimental evidence for a role of parental tolerance. Proc. R. Soc. B. 2002;269:1709–1713. doi: 10.1098/rspb.2002.2082. 10.1098/rspb.2002.2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman J, Rosander B. Survival enhancement through food sharing: a means for parental control of natal dispersal. Theor. Popul. Biol. 1992;42:117–129. doi: 10.1016/0040-5809(92)90008-h. 10.1016/0040-5809(92)90008-H [DOI] [PubMed] [Google Scholar]
- Ekman J, Sklepkovych B, Tegelström H. Offspring retention in the Siberian jay (Perisoreus infaustus): the prolonged brood care hypothesis. Behav. Ecol. 1994;5:245–253. [Google Scholar]
- Ekman J, Baglione V, Eggers S, Griesser M. Delayed dispersal: living under the reign of nepotistic parents. Auk. 2001;118:1–10. [Google Scholar]
- Ericson P.G.P, Christidis L, Cooper A, Irestedt M, Jackson J, Johansson U.S, Norman J.A. A Gondwanan origin of the passerine birds supported by DNA sequences of the endemic New Zealand wrens. Proc. R. Soc. B. 2002;269:435–441. doi: 10.1098/rspb.2001.1877. 10.1098/rspb.2001.1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson P.G.P, Jansén A.-L, Johansson U.S, Ekman J. Inter-generic relationships of crows, jays, magpies and allied groups (Aves: Corvidae) based on nucleotide sequence data. J. Avian Biol. 2005;36:222–234. 10.1111/j.0908-8857.2001.03409.x [Google Scholar]
- Feduccia A. Explosive evolution in Tertiary birds. Science. 1995;267:637–638. doi: 10.1126/science.267.5198.637. [DOI] [PubMed] [Google Scholar]
- Ford H.A, Bell H, Nias R, Noske R. The relationship between ecology and the incidence of cooperative breeding in Australian birds. Behav. Ecol. Sociobiol. 1988;22:239–250. 10.1007/BF00299838 [Google Scholar]
- Fry C.H. The evolutionary significance of co-operative breeding in birds. In: Stonehouse B, Perrins C, editors. Evolutionary ecology. University Park Press; Baltimore, MD: 1977. pp. 127–135. [Google Scholar]
- Fry C.H, Keith S, Urban E.K. The birds of Africa. vol. VI. Academic Press; London: 2000. [Google Scholar]
- Gayou D.C. The social system of the Texas green jay. Auk. 1986;103:540–547. [Google Scholar]
- Hardy J.W. Habitat and habits of the dwarf jay Aphelocoma nana. Wilson Bull. 1971;83:5–30. [Google Scholar]
- Harvey P.H, Pagel M.D. Oxford university press; Oxford: 1991. The comparative method in evolutionary ecology. [Google Scholar]
- Harvey P.H, Purvis A. Comparative methods for explaining adaptations. Nature. 1991;351:619–624. doi: 10.1038/351619a0. 10.1038/351619a0 [DOI] [PubMed] [Google Scholar]
- Holyoak D.T. Behaviour and ecology of the Chough and Alpine Chough. Bird Study. 1972;19:215–227. [Google Scholar]
- Hosono T. A study of the life history of the Blue Magpie. II. Breeding helpers and nest-parasitism by cuckoos. J. Yamashina Inst. Ornithol. 1983;15:63–71. [Google Scholar]
- James H.F, Ericson P.G.P, Slikas B, Lei F.-M, Gill F.B, Olson S.L. Pseudopodoces humilis, a misclassified terrestrial tit (Aves: Paridae) of the Tibetan Plateau: evolutionary consequences of shifting adaptive zones. Ibis. 2002;145:185–202. 10.1046/j.1474-919X.2003.00170.x [Google Scholar]
- Jansson C, Ekman J, von Brömssen A. Winter mortality and food supply in tits Parus spp. Oikos. 1981;37:313–322. [Google Scholar]
- Komdeur J. Importance of habitat saturation and territory quality for evolution of cooperative breeding in Seychelles Warbler. Nature. 1992;358:493–495. 10.1038/358493a0 [Google Scholar]
- Leisler B, Winkler H, Wink M. Evolution of breeding systems in acrocephaline warblers. Auk. 2002;119:379–390. [Google Scholar]
- Ligon J.D. Late summer-autumnal breeding of the Pinon jay in New Mexico. Condor. 1971;73:147–153. [Google Scholar]
- Ligon, Burt . Evolutionary origins. In: Koenig W.D, Dickinson J.L, editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004. pp. 5–34. [Google Scholar]
- Lillandt B.G, Bensch S, von Schantz T. Family structure in the Siberian jay as revealed by microsatellite analyses. Condor. 2003;105:505–514. [Google Scholar]
- Madge S, Burn H. Christopher Helm; London: 1994. Crows and jays. A guide to the crows, jays and magpie of the world. [Google Scholar]
- Marzluff J.M, Balda R.P. Pinyon jays: making the best of a bad situation by helping. In: Stacey P.B, Koenig W.D, editors. Cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 1990. pp. 197–238. [Google Scholar]
- Mooers A.ø, Schluter D. Reconstructing ancestor states with maximum likelihood: support for one- and two-rate models. Syst. Biol. 1999;48:623–633. 10.1080/106351599260193 [Google Scholar]
- Nicholls J.A, Double M.C, Rowell D.M, Magrath R.D. The evolution of cooperative and pair-breeding in the thornbill Acanthiza (Pardalotidae) J. Avian Biol. 2000;31:165–176. 10.1034/j.1600-048X.2000.310208.x [Google Scholar]
- Pagel M. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. B. 1994;255:37–45. [Google Scholar]
- Pagel M. Inferring evolutionary processes from phylogenies. Zool. Scripta. 1997;26:331–348. 10.1111/j.1463-6409.1997.tb00423.x [Google Scholar]
- Pagel M. Inferring the historical pattern of biological evolution. Nature. 1999a;401:877–884. doi: 10.1038/44766. 10.1038/44766 [DOI] [PubMed] [Google Scholar]
- Pagel M. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol. 1999b;48:612–622. 10.1080/106351599260184 [Google Scholar]
- Pravosudova E.V, Grubb T.C, Parker P.G. The influence of kinship on nutritional conditions and aggression levels in winter social groups of Tufted Titmouse. Condor. 2000;103:821–828. [Google Scholar]
- Pruett-Jones S. Summary. In: Koenig W.D, Dickinson J.L, editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004. pp. 228–238. [Google Scholar]
- Richner H. Helpers at the nest in Carrion crows Corvus corone corone. Ibis. 1990;132:105–108. [Google Scholar]
- Ridley M. Oxford university press; Oxford: 1983. The explanation of organic diversity: the comparative method and adaptations for mating. [Google Scholar]
- Rolando A. Home range and habitat selection by the Nutcracker (Nucifraga caryocatactes) during autumn in the Alps. Ibis. 1996;138:384–390. [Google Scholar]
- Rolando A, Carisio L. Non-territorial system in corvids: the case for the Nutcracker (Nucifraga caryocatactes) in the Alps. J. Ornithol. 2003;144:69–80. [Google Scholar]
- Rowley I. Communal species of Australian birds. Bonn. Zool. Betr. 1968;19:362–368. [Google Scholar]
- Rowley I. Cooperative breeding in Australian birds. Proc. Int. Ornithol. Congr. 1976;16:657–666. [Google Scholar]
- Russell E.M. Co-operative breeding: a Gondwanan perspective. Emu. 1989;89:61–62. [Google Scholar]
- Russell E.M, Yom-Tov Y, Geffen E. Extended parental care and delayed dispersal: northern, tropical and southern passerines compared. Behav. Ecol. 2004;15:831–838. 10.1093/beheco/arh088 [Google Scholar]
- Scott D.K. Functional aspects of prolonged parental care in Bewick's swans. Anim. Behav. 1981;28:938–952. [Google Scholar]
- Severinghaus L.L. Flocking and cooperative breeding of Formosan Blue Magpie. Bull. Inst. Zool. Acad. Sinica. 1987;26:27–37. [Google Scholar]
- Sibley C.G, Ahlquist J.E. Yale University Press; New Haven, CT: 1990. Phylogeny and classification of birds. [Google Scholar]
- Sibley C.G, Monroe B.L., Jr . Yale University Press; New Haven, CT: 1990. Distribution and taxonomy of birds of the world. [Google Scholar]
- Skutch A.F. Life histories of Central American Birds. Pac. Coast Avif. 1960;34:1–593. [Google Scholar]
- Skutch A.F. Iowa University Press; Iowa: 1987. Helpers at birds' nests. [Google Scholar]
- Strickland D. Juvenile dispersal in gray jays: dominant brood member expels siblings from natal territory. Can. J. Zool. 1991;69:2935–2945. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, MA: 1998. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10. [Google Scholar]
- Verbeek N.A.M. The exploitation system of the yellow-billed magpie. Univ. Calif. Publ. Zool. 1973;99:1–58. [Google Scholar]
- Verbeek N.A.M, Butler R.W.W. Cooperative breeding of the Northwestern Crow Corvus caurinus in British Columbia. Ibis. 1981;123:183–189. [Google Scholar]
- White M.E. Reed Books; Frenchs Forest, Australia: 1987. Greening of Gondwana. [Google Scholar]
- Winkler D.W. The phylogenetic approach to avian life histories: an important complement to within-population studies. Condor. 2000;102:52–59. [Google Scholar]
- Woolfenden G.E, Fitzpatrick J.W. Princeton University Press; Princeton, NJ: 1984. The Florida Scrub jay: demography of a cooperative breeding bird. [Google Scholar]