Abstract
Population-level laterality is generally considered to reflect functional brain specialization. Consequently, the strength of population-level laterality in manipulatory tasks is predicted to positively correlate with task complexity. This relationship has not been investigated in tool manufacture. Here, we report the correlation between strength of laterality and design complexity in the manufacture of New Caledonian crows' three pandanus tool designs: wide, narrow and stepped designs. We documented indirect evidence of over 5800 tool manufactures on 1232 pandanus trees at 23 sites. We found that the strength of laterality in tool manufacture was correlated with design complexity in three ways: (i) the strongest effect size among the population-level edge biases for each design was for the more complex, stepped design, (ii) the strength of laterality at individual sites was on average greater for the stepped design than it was for the simpler wide and narrow, non-stepped designs, and (iii) there was a positive, but non-significant, trend for a correlation between the strength of laterality and the number of steps on a stepped tool. These three aspects together indicate that greater design complexity generally elicits stronger lateralization of crows' pandanus tool manufacture.
Keywords: laterality, New Caledonian crow, pandanus tool manufacture, task complexity
1. Introduction
Lateralized behaviour is widespread among animals and can reflect functional specialization of the cerebral hemispheres (Rogers & Andrew 2002). However, the factors that affect the strength of laterality at the population level are not well known. Healey et al.'s (1986) findings in humans led them to suggest there might be two kinds of lateralized manipulatory behaviour that are controlled by independent neural systems: (i) simple tasks like reaching and carrying an object that require limited fine motor skills and have a relatively weak, or non-existent, lateralized bias at the population level, and (ii) complex tasks like throwing and writing that require considerable fine motor skills and have a relatively strong lateralized bias across the population. In their review of manual lateralization in non-human primates, Fagot & Vauclair (1991) made a similar distinction. In relatively simple tasks (e.g. reaching), they considered that laterality is more likely to reflect hand preference than functional brain specialization and less likely to be at the population level. In relatively complex tasks, they suggested that handedness is more likely to reflect functional specialization of the brain and be at the population level. To summarize, population-level laterality is considered to generally reflect functional brain specialization. Consequently, the strength of population-level laterality in manipulatory tasks is predicted to positively correlate with task complexity.
Tool manufacture and tool use in non-humans seem to be ideal, closely related manual activities with which to investigate the relationship between strength of laterality and task complexity for several reasons: (i) the manipulatory actions in these two behaviours are different and can appear to separate into the relatively simple (tool use) and relatively complex (tool manufacture) kinds of behaviour distinguished above. For example, Rutledge & Hunt (2004) raised the possibility that the neural mechanism(s) underlying laterality in the tool manufacture of New Caledonian crows Corvus moneduloides might be different to those behind laterality in their tool use, (ii) they are usually lateralized (e.g. review by McGrew & Marchant 1997; Hunt et al. 2001; Rutledge & Hunt 2004; Lonsdorf & Hopkins 2005), and (iii) they may provide insight about the foundation for subsequent, human-like technological evolution.
Tool use in particular seems to elicit lateralized behaviour. In unimanual tasks, handedness in traditional human cultures Homo sapiens (Marchant et al. 1995), chimpanzees Pan troglodytes (McGrew & Marchant 1997; McGrew & Marchant 2001) and tufted capuchins Cebus apella (Westergaard et al. 1998) is expressed much more strongly in tool use than non-tool behaviours. Tool use in non-human primates is often strongly lateralized, and until recently the laterality was only known at the individual level in the wild (McGrew & Marchant 1997). Lonsdorf & Hopkins (2005) reported new field data and a re-analysis of previous findings that suggest population laterality of tool use in chimpanzees. New Caledonian crows (hereafter NC crows) are also strongly lateralized when they use tools angled in their bills, but this was only at the individual level in the 14 individuals studied (Rutledge & Hunt 2004; Weir et al. 2004). One contrast between non-human primates and NC crows is that ambilaterality in tool use appears to be rare, or absent, in the crows but occurs in primates (McGrew & Marchant 1997; Lonsdorf & Hopkins 2005). Tool use, then, is probably manipulatively demanding and generally requires lateralized use of the cerebral hemispheres.
To our knowledge, NC crows are the only non-human species for which an investigation of laterality in tool manufacture has been carried out in the wild. NC crows manufacture three specific tool designs from the barbed edges of Pandanus spp. leaves: wide, narrow and stepped designs (Hunt & Gray 2003; designs shown in figure 1) (see video of wide and stepped tool manufacture at http://language.psy.auckland.ac.nz/crows/video-clips.htm). The manufacture of stepped tools requires the highest number of manipulatory operations of the three designs (Hunt & Gray 2003). In contrast to their tool use, we previously found species-level laterality when NC crows manufacture stepped tools; most crows prefer to remove these tools from the left edges of leaves rather than the right edges (Hunt et al. 2001). We suggested that the relatively complex, sequential procedure required to manufacture these tools might explain the population-level effect. The rationale for this is evidence of a consistent (left) hemispheric bias in vertebrates for the processing of non-spatial, sequential programming (Bradshaw & Rogers 1993; Rogers 2002).
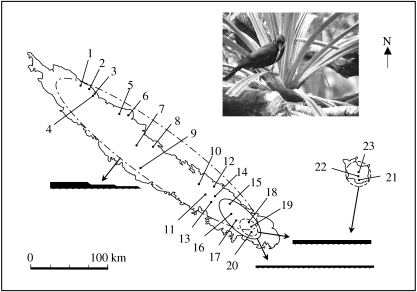
Figure 1.
The 23 sites on New Caledonia's Grande Terre and on Maré Island where we collected tool counterparts from pandanus leaves. Sites are grouped by each of the three tool designs, represented by the wide, narrow and stepped symbols. See table 1 for information on individual sites. The inset shows a NC crow at Pic Ningua in the process of removing a stepped tool from the left edge of a leaf, while standing behind the edge. Figure adapted from Hunt & Gray (2003).
NC crows provide us with an opportunity to investigate the extent of laterality in the manufacture of tools across closely related designs of differing complexity. The stepped design is more complex than the wide and narrow designs and may have evolved from incremental changes to simpler design(s) (Hunt & Gray 2003). A two-step tool, for example, requires four distinct cutting and ripping operations, but a wide tool only requires two such operations. By investigating laterality in NC crows' tool manufacture we can also control for any possible confounding effects associated with the manipulation of two independent limbs because they make tools with their bills. We previously published combined data on edge preference for stepped tool manufacture across 19 sites, but did not provide an analysis at individual sites (Hunt et al. 2001). In this paper, we examine edge preferences in the manufacture of wide, narrow and stepped tools at individual sites as well as across sites. Our main objectives were to determine if: (i) there was an overall bias for a particular leaf edge when NC crows manufacture wide and narrow tools, as we had found previously for stepped tools, (ii) strength of laterality was correlated with design complexity, and (iii) we could make inferences from the site data about the manufacture behaviour of individual NC crows.
2. Material and methods
(a) Sampling sites
The manufacture of a pandanus tool creates a matching outline, or ‘counterpart’, of its shape on the leaf edge, which provides a comprehensive record of tool manufacture over the past several years (Hunt 2000). We have verified by direct observation that a stepped tool counterpart is an accurate record of the shape of the completed tool (Hunt & Gray 2004). Most of the counterparts used in this paper were collected at 21 sites in 2000; the methods for their collection and the sites where they were found are in Hunt & Gray (2003). We collected additional counterparts in Parc Rivière Bleue in June 2002 (site 19 in figure 1) and on Maré (sites 22 and 23) from July to September 2003. The methods we used to collect the more recent counterparts were the same as those we usually used in 2000. That is, we walked along transects removing any counterparts that we could see on trees and reach from the ground. Parc Rivière Bleue is the only location that we know of in New Caledonia where the wide, narrow and stepped designs commonly co-occur, and counterparts of these three designs can sometimes even be found on the same tree (Hunt & Gray 2003). We took the opportunity in 2002 to collect more samples in Parc Rivière Bleue. In 2000, we collected counterparts along the southwest bank of Rivière Bleue, but in 2002 we collected them along the opposite river bank. On Maré, we collected counterparts at two new locations to increase the sample size of sites where wide tools are commonly made. One of the two new sites (site 22) was ca 5 km northwest of site 21 that was sampled in 2000. The second site (site 23) was just north of Rawa village, and ca 8 km north of site 22. The distances between sites on Maré and the mainland ensured that we sampled the tool manufacture of at least one different NC crow at each site.
(b) Analysis
The analysis of the counterpart data is not straight forward because we do not know: (i) the number of NC crows responsible for the counterparts that we collected, that is, the pandanus tools manufactured, and (ii) the number of counterparts that each NC crow contributed. It is extremely difficult to observe NC crows manufacturing pandanus tools naturally in the wild because they live in tropical forest on difficult terrain and are wary of people. In over two years at our permanent study site on Maré we have rarely seen this behaviour. Establishing which NC crow made a tool can only come from direct observation of an individually identifiable bird. The two unknown aspects above can never be quantified in a survey of counterparts on leaves, but we can make realistic assumptions about them. NC crows are social birds that spend most of their time in family groups generally consisting of either mated pairs or parents with their young offspring (Hunt 1996). On Maré, we found that these core family units usually have different but overlapping foraging ranges where they live throughout the year (J. Holzhaider, G. R. Hunt & R. D. Gray, unpublished data). We also know that many NC crows at the site manufacture pandanus tools. It is therefore reasonable to assume that more than one NC crow was responsible for the counterparts that we collected at each site and that each individual made more than one tool. Given the necessarily qualitative aspect of these assumptions, our only option is to use a statistical test that either underestimates (when testing across sites) or overestimates a potential edge bias (when testing within or across sites).
A confounding effect when looking at edge preferences in pandanus tool manufacture is that the spiralling direction of leaves can significantly affect the frequency-of-use of left and right edges (Hunt 2000; Hunt et al. 2001). Hunt (2000) suggested that this spiral direction effect is caused by differential ease of access for NC crows to leaf edges. On anti-clockwise spiralling leaves, the trailing, or right, edge is more exposed, and the left edge is more exposed on clockwise spiralling leaves. Previous work showed that a bias for a particular edge at a site can exist over and above this effect (Hunt 2000; Hunt et al. 2001). We examined the effect of spiral direction on edge use at sites by using G-tests of independence on row and column tables. If there was a significant effect, we established whether it was a bias for the trailing or leading edge of a leaf.
We accounted for any spiral direction effect before testing for a bias in the use of left and right edges. We did this by organizing data (whether it was for individual sites or across sites) into a 2×2 row (clockwise or anti-clockwise leaf direction) and column (left or right edge) table. We then proportionately adjusted the cell and column totals to take into account the variation in the frequency of use of clockwise and anti-clockwise leaves. We finally used a G-test for goodness-of-fit (Sokal & Rohlf 1981) to see if the adjusted frequency of left and right edge use (observed distribution) differed from a 50/50 distribution (null distribution).
We tested for an overall edge bias across sites for each design using both a G-test for goodness-of-fit and a paired-samples t-test (after checking for normality of the data) to provide lower and upper bounds of probability. The G-test for goodness-of-fit gave a lower than expected p-value and therefore increased the chance of a type I error (false rejection of the null hypothesis of no edge bias). The overestimated p-value was because of the test assumption that each NC crow only contributed one manufacture, which inflated the sample size. In contrast, the paired-samples t-test viewed sites as the unit of analysis and consequently gave a higher than expected p-value, which increased the chance of a type II error (false acceptance of the null hypothesis). The p-value is underestimated because all counterparts at a site are assumed to be made by only one NC crow. We tested each design for an edge bias at individual sites only to obtain G-values to investigate the strength of edge biases, not to test the significance of edge biases because the values were based on inflated sample sizes. For the counterpart data, we assumed that on average the number of tools made by a NC crow which preferred right edges would be the same as that for a NC crow which preferred left edges.
We compared the strength of edge biases across sites between the three pandanus tool designs (i.e. at the population level). We calculated effect size indices from the t-values obtained from the paired-samples tests (upper bounds of probability) and the G-values from the goodness-of-fit tests (lower bounds). Using t-values, we calculated Cohen's standardized effect size d for each tool design (Cohen 1988). We used the formula d=t[2(1−r)/n]0.05, which took into account the correlation between groups (Dunlap et al. 1996). Using G-values, we calculated a ϕ coefficient of correlation for each design. can be written in terms of G (which is equivalent to χ2): =√(G/n). Both these indices were independent of sample size and correlation effects. We also compared the strength of the edge-use biases at sites between designs. We calculated ϕ-values from the G-values then used independent-sample t-tests to check for differences in the means of the ϕ-values between designs. Finally, we investigated if the strength of edge preference in stepped tool manufacture was related to the degree of design complexity of these tools, that is, the number of steps on a tool. The more steps on a tool the greater the number of sequential actions a NC crow employs to make it. We combined data across sites and only included counterparts when the number of steps was unambiguous. We then used goodness-of-fit tests (after accounting for variation in the use of clockwise and anti-clockwise leaves) to obtain G-values, which we again used to calculate ϕ-values.
The counterpart data are also problematic when it comes to inferring: (i) whether they represent the manufacture of a completed tool rather than only an attempt, and (ii) the number of completed tools. In the first case, uncompleted manufacture attempts commonly leave a strip of damaged material hanging from the leaf (fig. 3 in Hunt 1996 has a stepped tool example). The strip can then decay and fall from the leaf leaving a plausible counterpart, but this is much more likely to occur with attempted manufacture of narrow tools because of their narrowness (Hunt & Gray 2003). In the second case, a counterpart of a stepped or narrow tool probably represents the manufacture of a single tool, but recent observations on Maré show that a counterpart of a wide tool can represent the manufacture of more than one tool (G. R. Hunt 2004, personal observation). Therefore, wide-tool counterparts indicate the minimum number of tools manufactured at sites. We only include a design at sites when we had sufficient numbers of counterparts to analyse (greater than 30 counterparts). This gave us 19 sites where NC crows made stepped tools, six sites for narrow tools and five sites for wide tools (figure 1).
3. Results
(a) Presence of edge biases
NC crows' choice of a particular edge when manufacturing each design was affected by the direction in which leaves spiralled (wide tools: G1=71.8, p<0.0001, n=877; narrow tools: G1=42.5, p<0.0001, n=1136; stepped tools: G1=333.9, p<0.0001, n=3838). The G-tests for independence using the site data showed that there was either no spiral direction effect or a bias for the more exposed, trailing edge, but never a bias for the leading edge (table 1).
Table 1.
Summary of G-tests on data from 23 collection sites throughout New Caledonia. (The ‘edge’ columns give G-values for the goodness-of-fit tests and, in brackets, the edge bias in the site data at the 0.05 probability level: R, right edge; L, left edge; none, no edge bias. The ‘direction’ columns give results of the independence tests for any effect of leaf spiral direction: T, trailing edge bias; none, no spiral direction effect. Summary totals appear at the bottom of the table: A, anticlockwise-spiralling; C, clockwise-spiralling. ID numbers can be used to locate respective sites in figure 1. *p<0.05; *p<0.01; ***p<0.0001.)
| wide tools | narrow tools | stepped tools | |||||
|---|---|---|---|---|---|---|---|
| ID | site | edge | direction | edge | direction | edge | direction |
| 1 | Mt Ignambi | — | — | — | — | 7.3 (R) | T*** |
| 2 | Mt Colnett | — | — | — | — | 155.6 (L) | T*** |
| 3 | Mt Panié (I) | — | — | — | — | 308.6 (L) | T*** |
| 4 | Mt Panié (II) | — | — | — | — | 0.1 (—) | T*** |
| 5 | Mt Tonine | — | — | — | — | 157.7 (L) | none |
| 6 | Mt Köhîdagé | — | — | — | — | 22.3 (L) | T*** |
| 7 | Mt Aoupinié | — | — | — | — | 192.3 (R) | T** |
| 8 | Sommet Arago | — | — | — | — | 24.9 (L) | T*** |
| 9 | Mt Këiyöumâ | — | — | — | — | 46.9 (L) | T*** |
| 10 | Mt Nakada | — | — | — | — | 224.6 (L) | T*** |
| 11 | Pic Ningua | — | — | — | — | 39.1 (L) | T*** |
| 12 | Forêt de Saille | — | — | — | — | 65.3 (R) | T*** |
| 13 | Mt St Vincent | — | — | — | — | 153.7 (L) | T* |
| 14 | Pic Kambwi | — | — | — | — | 0.0 (—) | T*** |
| 15 | Mt Humboldt | — | — | 11.7 (R) | none | 25.2 (L) | T* |
| 16 | Mt Ouin | — | — | 29.5 (R) | T*** | 14.1 (L) | T*** |
| 17 | Mt Dzumac | — | — | 4.5 (R) | T** | 96.6 (L) | T*** |
| 18 | Mt Bleue | — | — | 1.6 (—) | T** | 23.1 (R) | T* |
| 19 | Rivière Bleue | 0.7 (—) | T*** | 2.5 (—) | none | 23.4 (R) | none |
| 20 | Mt Pouédihi | 0.5 (—) | T* | 10.6 (R) | none | — | — |
| 21 | Maré Island (I) | 0.1 (—) | T*** | — | — | — | — |
| 22 | Maré Island (II) | 8.4 (R) | T*** | — | — | — | — |
| 23 | Maré Island (III) | 2.0 (—) | none | — | — | — | — |
| summary totals | |||||||
| trees with A leaves | 117 | 95 | 387 | ||||
| trees with C leaves | 122 | 108 | 403 | ||||
| counterparts on LE | 405 | 458 | 2500 | ||||
| counterparts on RE | 472 | 678 | 1338 | ||||
| counterparts on A leaves | 409 | 527 | 1884 | ||||
| counterparts on C leaves | 468 | 609 | 1954 | ||||
When we combined data across sites, NC crows preferred left edges from which to remove stepped tools and right edges when manufacturing wide and narrow tools (wide tools: G1=8.0, p<0.01, n=877; narrow tools: G1=49.2, p<0.0001, n=1136; stepped tools: G1=345.3, p<0.0001, n=3838). The conservative paired-samples t-test showed no significant population-level edge bias for any of the three designs, but all the p-values closely approached significance (wide design: t=2.69, p=0.06, n=5; narrow design: t=2.21, p=0.08, n=6; stepped design: t=1.98, p=0.06, n=19). Given these upper and lower bounds of probability, the true p-value for a population-level edge effect for each design is likely to be less than 0.05.
(b) Strength of edge biases
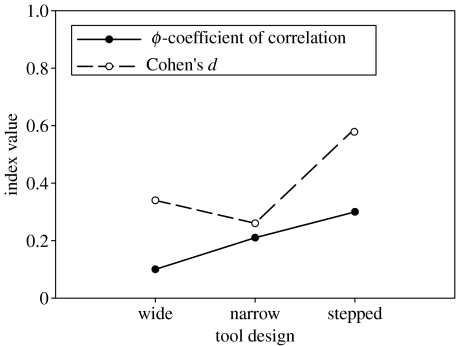
The above relatively high G-value of 345.3 for the stepped design suggested that there may be a correlation between the complexity of a pandanus tool design and strength of the population-level edge bias. Both the ϕ-values and the d-values showed that effect size was strongest for the more complex stepped design (figure 2).
Figure 2.
Indices of effect size for the population-level edge bias for each pandanus tool design. Cohen's d-values are based on the upper bounds of probability, and the ϕ-values are based on the lower bounds (see §2 for full explanation).
The G-values for the edge-bias tests at sites were noticeably smaller for the wide and narrow designs than they were for the stepped design (wide: 2.4±1.5 mean±s.e.m., n=5; narrow: 10.1±4.3, n=6; stepped: 83.2±20.5, n=19). To compare the strength of the edge biases at sites between the designs, we first converted the G-values to ϕ-values. The mean ϕ-values for the wide and narrow designs were not significantly different, so we combined these data to make a ‘non-stepped design’ (t=2.17, p=0.06, n=11). The mean ϕ-value for the stepped design was significantly larger than the mean ϕ-value for the non-stepped design (t=4.94, p<0.0001, n=30). That is, the edge biases at sites for stepped tool manufacture were on average much stronger than those for both wide and narrow tool manufacture.
We organized the counterpart data for the stepped design into categories with one, two, three or more than three steps to see if there was a relationship between the number of steps and the strength of the edge bias. There was a significant edge bias for each ‘step’ category (see G-values in table 2), after accounting for a significant spiral direction effect. The ϕ-values calculated from the G-values were positively related to the number of steps per tool, but the relationship only approached significance (r=0.92, p=0.077, n=4).
Table 2.
Strength of laterality and the number of steps on stepped tools. (Data for counterparts with four or more steps were combined because of low sample sizes. All G-values were p<0.0001.)
| no. of steps | G for edge bias | ϕ-value | n |
|---|---|---|---|
| 1 | 23.5 | 0.17 | 782 |
| 2 | 151.6 | 0.34 | 1278 |
| 3 | 109.8 | 0.42 | 620 |
| 4, 5, 6 | 19.9 | 0.43 | 108 |
4. Discussion
Our findings provide the first evidence of a relationship between the degree of design complexity in tool manufacture and the strength of laterality. We draw this conclusion based on three positive correlations between strength of laterality and design complexity: (i) the strongest effect size among the population-level edge biases for each design was for the more complex, stepped design, (ii) the strength of laterality at individual sites was on average greater for the stepped design than it was for the simpler wide and narrow designs, and (iii) there was a non-significant trend for a correlation between strength of laterality and the number of steps on a stepped tool. These relationships are consistent with evidence in other studies that have found stronger lateralization of behaviour when task complexity is greater (Healey et al. 1986; Fagot & Vauclair 1991).
An interesting aspect of the population-level edge biases was that NC crows preferred right edges for both wide and narrow tool manufacture, and preferred left edges for stepped tool manufacture. An important question is do these different edge biases represent differently lateralized behaviour, or indeed laterality at all? Three free-living NC crows that we studied manufacturing wide and stepped tools provide clues about the answer. An NC crow at Pic Ningua, Grande Terre, had a strong bias for removing stepped tools from left edges and preferred to stand behind the leaf edge when doing so (see inset in figure 1) (Hunt & Gray 2004). Because the crow initially worked away from the trunk when it removed a tool, its head movements were mostly to the right to begin manufacture. The two remaining NC crows were from Maré and had no significant edge preference when they made wide tools, but they much preferred to stand facing the leaf edge when removing these tools (G. R. Hunt & R. D. Gray, unpublished data). These two crows had a (non-significant) tendency to use right edges more than left edges, which meant that they also tended to use head movements to the right rather than the left to begin manufacture. The behaviour of the three NC crows suggests that (i) individuals may generally be consistent in whether they stand facing or behind a leaf edge when removing a particular tool design, in which case edge biases indicate laterality in the direction of head movements for manufacture, and (ii) different edge biases may simply indicate different standing positions for manufacture rather than directionally different laterality. Observations of additional NC crows are needed to see if there is a consistent relationship between tool design, edge bias and standing position.
We could make four inferences about the manufacture behaviour of individual NC crows from the counterpart data. The strong edge biases at many sites for stepped tool manufacture suggest that NC crows can maintain a preference for either a right edge or a left edge when removing these tools. We recently provided the first direct evidence in support of a consistent left edge preference when we observed a NC crow manufacturing and using stepped tools at Pic Ningua (site 11 in figure 1) (Hunt & Gray 2004). The crow had a bias for left edges (74%) similar to that seen in counterparts that we collected at the site in 1997 (81%; Hunt 2000) and in 2000 (70%). We can also infer that most individual NC crows select the more exposed trailing edges because they are easier to access. This is because all significant spiral-direction effects were associated with a bias for these edges. The remaining two inferences that we can make are based on a significant effect for both spiral direction and edge use in many (n=18) of the 30 datasets in table 1 across the three designs. Fifteen of the 18 datasets were for stepped tools. In all the 18 cases, the bias for the preferred edge was strongest, or only existed, on leaves where that edge was easiest to access. For example, a bias for left edges was most obvious on clockwise leaves (where they are the trailing edges) and was reduced or absent on anti-clockwise leaves. The similarity in the way that the spiral direction of leaves affected edge biases suggests (i) a generally consistent bias for a particular edge across individual NC crows at a site, especially for stepped tools, and (ii) that most NC crows remove tools from both sides of a leaf. The three NC crows that we have studied in detail manufacturing pandanus tools all removed tools from both the left and right edges of leaves, albeit at different frequencies (Hunt & Gray 2004, unpublished data).
Lonsdorf & Hopkins's (2005) report of population-level handedness in the use of tools by free-living chimpanzees appears to confirm that tool use had little to do with the early evolution of handedness in humans. However, the factor(s) that drove human handedness to the extreme levels seen in modern humans remain controversial. Explanations include selection for efficient manual behaviour unknown in non-humans like precision throwing (Calvin 1983). An alternative view is that the extreme right-handedness is a by-product of the evolution of language (Deacon 1997; Corballis 2003) or, more generally, the efficient use of an enlarged neocortex (Steele 2001). The possibility that selection for tool manufacture skills, rather than those for tool use, might have helped shape human handedness is rarely considered. In fact, little is known at all about how right-handedness evolved in association with manual skills. Toth (1985) reported population-level right-handedness in the stone knapping of Homo 1.4–1.9 million years ago, but determining handedness from flaking evidence is very problematic (Pobiner 1999). Early humans, though, must have relied heavily on bimanual skills for object-related manipulation like manufacturing stone and wood tools. In non-tool-related tasks, bimanual activities in captive chimpanzees better elicit a population-level division of skill between the hands than do unimanual ones (Hopkins & Pearson 2000; Hopkins et al. 2003, 2004). Tool manufacture, then, is likely to be relatively neurologically demanding and lateralized at the population level. In NC crows, tool manufacture is consistently lateralized across the population but this does not appear to be the case for tool use. Our current findings show that the strength of the laterality in NC crows' tool manufacture is dependent on the degree of complexity of the manufacture. We propose that tool manufacture may help shape population-level lateralization of fine motor skills in species that develop sophisticated tools.
Acknowledgements
Staff in the Political Section of the provincial administration on Maré provided valuable help with access to forest and W. Wadrobert kindly allowed us to work on his family's land in Wabao District. C. Lambert (Province Sud), C. Papineau (Province Nord) and D. Houmbouy (Province des Iles Loyauté) gave us permission to collect counterparts in New Caledonia, and E. DuTailly provided us with accommodation and assistance in Nouméa. We thank R. B. Rutledge, B. Bulman-Fleming and two referees for their insightful comments on drafts of this work. This research was funded by an Auckland University Emerging Research Activities Grant (G.R.H.) and the New Zealand Marsden Fund (R.D.G. & G.R.H.).
References
- Bradshaw J.L, Rogers L.J. Academic Press; San Diego, CA: 1993. The evolution of lateral asymmetries: language, tool use, and intellect. [Google Scholar]
- Calvin W.H. A stone's throw and its launch window: timing precision and its implications for language and hominid brains. J. Theor. Biol. 1983;104:121–135. doi: 10.1016/0022-5193(83)90405-8. 10.1016/0022-5193(83)90405-8 [DOI] [PubMed] [Google Scholar]
- Cohen J. 2nd edn. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- Corballis M.C. From mouth to hand: gesture, speech, and the evolution of right-handedness. Behav. Brain Sci. 2003;26:199–260. doi: 10.1017/s0140525x03000062. 10.1017/S0140525X03000062 [DOI] [PubMed] [Google Scholar]
- Deacon T. Allen Lane The Penguin Press; London, UK: 1997. The symbolic species: the co-evolution of language and the human brain. [Google Scholar]
- Dunlap W.P, Cortina J.M, Vaslow J.B, Burke M.J. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol. Meth. 1996;1:170–177. 10.1037/1082-989X.1.2.170 [Google Scholar]
- Fagot J, Vauclair J. Manual laterality in nonhuman primates: a distinction between handedness and manual specialization. Psychol. Bull. 1991;109:76–89. doi: 10.1037/0033-2909.109.1.76. 10.1037/0033-2909.109.1.76 [DOI] [PubMed] [Google Scholar]
- Healey J.M, Liederman J, Geschwind N. Handedness is not a unidimensional trait. Cortex. 1986;22:33–53. doi: 10.1016/s0010-9452(86)80031-4. [DOI] [PubMed] [Google Scholar]
- Hopkins W.D, Pearson K. Chimpanzee (Pan troglodytes) handedness: variability across multiple measures of hand use. J. Comp. Psychol. 2000;114:126–135. doi: 10.1037/0735-7036.114.2.126. 10.1037/0735-7036.114.2.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W.D, Hook M, Braccini S, Schapiro S.J. Population-level right handedness for a coordinated bimanual task in chimpanzees: replication and extension in a second colony of apes. Int. J. Primatol. 2003;24:677–689. doi: 10.1023/A:1023752816951. 10.1023/A:1023752816951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W.D, Wesley M.J, Izard M.K, Hook M, Schapiro S.J. Chimpanzees (Pan troglodytes) are predominantly right-handed: replication in three populations of apes. Behav. Neurosci. 2004;118:659–663. doi: 10.1037/0735-7044.118.3.659. 10.1037/0735-7044.118.3.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt G.R. Manufacture and use of hook-tools by New Caledonian crows. Nature. 1996;379:249–251. 10.1038/379249a0 [Google Scholar]
- Hunt G.R. Human-like, population-level specialization in the manufacture of pandanus tools by New Caledonian crows Corvus moneduloides. Proc. R. Soc. B. 2000;267:403–413. doi: 10.1098/rspb.2000.1015. 10.1098/rspb.2000.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt G.R, Gray R.D. Diversification and cumulative evolution in New Caledonian crow tool manufacture. Proc. R. Soc. B. 2003;270:867–874. doi: 10.1098/rspb.2002.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt G.R, Gray R.D. Direct observations of pandanus-tool manufacture and use by a New Caledonian crow (Corvus moneduloides) Anim. Cogn. 2004;7:114–120. doi: 10.1007/s10071-003-0200-0. 10.1007/s10071-003-0200-0 [DOI] [PubMed] [Google Scholar]
- Hunt G.R, Corballis M.C, Gray R.D. Laterality in tool manufacture by crows. Nature. 2001;414:707. doi: 10.1038/414707a. 10.1038/414707a [DOI] [PubMed] [Google Scholar]
- Marchant L.F, McGrew W.C, Eibl-Eibesfeldt I. Is human handedness universal? Ethological analyses from three traditional cultures. Ethology. 1995;101:239–258. [Google Scholar]
- McGrew W.C, Marchant L.F. Using the tools at hand: manual laterality and elementary technology in Cebus spp. and Pan spp. Int. J. Primatol. 1997;18:787–810. 10.1023/A:1026347913888 [Google Scholar]
- McGrew W.C, Marchant L.F. Ethological study of manual laterality in the chimpanzees of the Mahale mountains, Tanzania. Behaviour. 2001;138:329–358. 10.1163/15685390152032497 [Google Scholar]
- Lonsdorf E.V, Hopkins W.D. Wild chimpanzees show population-level handedness for tool use. Proc. Natl Acad. Sci. USA. 2005;102:12 634–12 638. doi: 10.1073/pnas.0505806102. 10.1073/pnas.0505806102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobiner B.L. The use of stone tools to determine handedness in hominids. Curr. Anthropol. 1999;40:90–92. [Google Scholar]
- Rogers L.J. Lateralization in vertebrates: its early evolution, general pattern and development. Adv. Stud. Behav. 2002;31:107–161. 10.1016/S0065-3454(02)80007-9 [Google Scholar]
- Rogers L.J, Andrew R.J, editors. Comparative vertebrate lateralization. Cambridge University Press; Cambridge, UK: 2002. [Google Scholar]
- Rutledge R, Hunt G.R. Lateralized tool use in wild New Caledonian crows. Anim. Behav. 2004;67:327–332. 10.1016/j.anbehav.2003.07.002 [Google Scholar]
- Sokal R.R, Rohlf F.J. 2nd edn. W.H. Freeman & Co; New York, NY: 1981. Biometry. [Google Scholar]
- Steele J. When did directional asymmetry enter the record? Proc. Br. Acad. 2001;106:153–168. [Google Scholar]
- Toth N. Archaeological evidence for preferential right-handedness in the lower and middle Pleistocene, and its possible implications. J. Hum. Evol. 1985;14:607–614. [Google Scholar]
- Westergaard G.C, Kuhn H.E, Suomi S.J. Laterality of hand function in tufted capuchin monkeys (Cebus apella): comparison between tool use actions and spontaneous non-tool actions. Ethology. 1998;104:119–125. [Google Scholar]
- Weir A.A.S, Kenward B, Chappell J, Kacelnik A. Lateralization of tool use in New Caledonian crows (Corvus moneduloides) Proc. R. Soc. B. 2004;271:S344–S346. doi: 10.1098/rsbl.2004.0183. 10.1098/rspb.2004.2803 [DOI] [PMC free article] [PubMed] [Google Scholar]