Abstract
Parasitic infection can influence a variety of behavioural mechanisms in animals, but little is known about the effects of infection on the cognitive processes underlying ecologically relevant behaviours. Here, we examined whether parasitic infection alters cognitive aspects of foraging in a social insect, the bumble-bee (Bombus impatiens). In controlled experiments, we assessed the ability of foraging bees to discriminate rewarding from non-rewarding flowers on the basis of colour and odour. We found that natural and experimental infection by a protozoan parasite (Crithidia bombi, which lives exclusively within the gut tract), impaired the ability of foragers to learn the colour of rewarding flowers. Parasitic infection can thus disrupt central nervous system pathways that mediate cognitive processes in bumble-bees and as a consequence, can reduce their ability to monitor floral resources and make economic foraging decisions. It is postulated that this infection-induced change to cognitive function in bumble-bees is the result of communication between immune and nervous systems. Parasitized animals, including invertebrates, can therefore show subtle behavioural changes that are nonetheless ecologically significant and reflect complex mechanisms.
Keywords: parasite, bumble-bee, foraging, cognition
1. Introduction
A variety of behavioural traits in animals can be altered by parasitic infection (Moore 2002; Klein 2003). For example, European minnows (Phoxinus phoxinus) infected by larvae of the cestode Ligula intestinalis show modifications to schooling behaviour (Barber & Huntingford 1996). The proximate mechanism underlying these behavioural changes in infected individuals often involves alterations to the function of the central nervous system (CNS). Parasites can alter host CNS function either directly by destroying neurons or secreting neuroactive substances or indirectly through neurochemical compounds produced by the host's immune system in response to infection (Adamo 1997, 2002; Thomas et al. 2005). Knowing whether parasitic infection alters CNS function through direct or indirect mechanisms is essential to fully understand the ecological and evolutionary ramifications of the corresponding behavioural changes for both host and parasite. However, differentiating the two mechanisms can be problematic, especially when the parasite resides in the host's CNS or is capable of secreting neuroactive compounds identical to those produced by its host. Indirect mechanisms are most likely to operate in host–parasite systems involving microparasites that are physically separated from the host CNS, elicit a host immune response (Thomas et al. 2005), and have a direct life cycle (i.e. infection-induced behavioural changes are not in an intermediate host; Kavaliers & Colwell 1995). Here, we use a protozoan microparasite of bumble-bees characterized by these properties to examine the effects of parasitic infection on CNS processes mediating behaviour.
Cognition, i.e. functions of the CNS concerned with information acquisition, retention and processing, plays an important role in behaviours that affect the survival and reproductive success of animals (Beltman et al. 2003; Mery & Kawecki 2003; Dukas 2004; Healy et al. 2005). Several psychological studies have indicated that parasitic infection has detrimental effects on cognition (Gibertini et al. 1995; Kavaliers et al. 1995; Cox & Holland 2001). For example, laboratory mice infected by a nematode parasite (Heligmosomoides polygyrus) show impairments to spatial learning (Kavaliers & Colwell 1995). Parasitic infection is also known to influence foraging, reproductive, social and anti-predator behaviours (Keymer & Read 1990; Kavaliers et al. 1999; Klein 2003), all of which contain cognitive elements (Dukas 1998). However, the effect of infection on the cognitive processes underlying ecologically relevant behaviours in animals has received minimal consideration. Moreover, there is little information on the influence of infection on cognitive function and behaviour in invertebrates.
In this study, we investigated whether parasitic infection alters cognitive aspects of foraging in a social insect, the bumble-bee Bombus impatiens. Bumble-bees (Bombus spp.) are an ideal model system for studying how parasites affect cognitive mechanisms underlying behaviour because ecologically meaningful behaviours are easily assayed in the laboratory. In the field, foraging bees rely heavily on sensorimotor, associative and spatial learning and memory to exploit floral resources (Laverty 1994; Cartar 2004; Gegear & Laverty 2005), so cognition is important to the success of their colonies. Bumble-bees also host a variety of parasites, and their immune responses to infection are well known (Moret & Schmid-Hempel 2001; Schmid-Hempel 2001). Crithidia bombi (Kinetoplastida: Trypanosomatidae) is common protozoan parasite that lives exclusively within the gut tract of bumble-bees (Schmid-Hempel 2001; Brown et al. 2003a,b). Generally, C. bombi is considered benign because infected individuals do not show increased mortality unless they are under severe food stress (Brown et al. 2000, 2003a,b). Nonetheless, C. bombi infections elicit a systemic immune response in bumble-bees (Brown et al. 2003a,b) and reduce the ability of foraging bees to exploit floral resources (Otterstatter et al. 2005). Although the proximate reason for this deficiency in foraging ability is currently unknown, one possibility is that C. bombi infections alter the cognitive processes necessary for bees to utilize floral information. Because C. bombi is a microparasite with a direct life cycle and is physically separated from the CNS of host bees, any infection-induced deficiencies in cognitive abilities would suggest indirect effects of parasitic infection on CNS function. We assessed the effect of C. bombi infection on cognitive function in B. impatiens foragers by comparing the ability of infected and uninfected individuals to discriminate profitable flowers on the basis of colour and odour cues.
2. Material and methods
(a) Bees and parasites
Individually marked bumble-bees (B. impatiens; Cresson) were trained to collect 30% sucrose (w/w) solution within a screened enclosure (2.2 m3). Bees entered the enclosure through a tube connected to their nest-box. Prior to experiments, we determined that source colonies were infected with C. bombi; however, the infection status of individual foragers was unknown. After learning trials (below), we sacrificed each tested bee and obtained an index of body size by measuring the radial cell of the right forewing at 25×. We also ground the entire gut tract in a microcentrifuge containing 100 μl of distilled water. A 10 μl drop of the gut sample was then transferred to a hemacytometer (Reichert Scientific, Buffalo, NY) to determine the concentration of C. bombi cells as a measure of infection intensity. Counts were carried out blind with respect to performance on the learning task.
(b) Flower types and arrays
Artificial ‘flowers’ were constructed by removing the caps from blue, yellow, orange and red 1.5 ml polypropylene microcentrifuge tubes (Fisher Scientific Canada) and fixing a circular (6 cm in diameter) collar of similarly coloured cardboard (Hilroy Canada) around the entrance of the tube. For trials involving olfactory discrimination, flowers were scented by placing a 3 μl mixture of a 1% solution, either geraniol or clove oil in pentane (Sigma-Aldrich, St Louis, MO), on the surface of the cardboard. To obtain sucrose reward from the flower, bees had to land on the top of the flower and crawl into the tube. We tested bees on an array of 40 flowers, distributed in eight rows of five (12 cm apart within rows and 6 cm between rows), fixed on a green background.
(c) Experiment 1
To determine whether C. bombi infection affects the ability of bees to discriminate rewarding from non-rewarding flowers on the basis of colour and odour cues together, we trained 22 bees from two colonies to collect sucrose from four flower types (yellow–geranium, yellow–clove, blue–geranium and blue–clove). Bees were trained by allowing them to visit an array of each flower type in succession for 20 foraging trips per type and then again the next day for three foraging trips per type. This procedure ensured that bees had recent experience with the properties and handling method of each type prior to testing. Immediately after training, bees were tested individually on an array containing 10 randomly distributed flowers of each of the four types, in which only one of the types was rewarded with 3 μl of 30% sucrose solution and the other three types contained the same volume of distilled water. The rewarding flower type was randomly selected for each bee and presented second during the training sequence to control for the potential effects of presentation order and cue preference on the choice behaviour of bees. We recorded the flower choices of each bee until it had reached a learning criterion of 80% visit frequency to the rewarding flower type over 30 consecutive visits (a performance level that was significantly greater than random choice; one-sample proportion test, Z=6.96, p<0.0001). The bee was considered to have made a choice when it landed on a flower and made contact with the entrance to the tube. Flowers were refilled immediately after being drained by the bee and replaced between bees. Upon reaching the learning criterion, the bee was captured in a sterilized plastic vial, chilled at 5 °C until immobile, and then checked for intensity of C. bombi infection.
We used repeated-measures logistic regression to determine (i) whether performance (proportion of visits to rewarding flowers) improved with experience (number of flower visits), (ii) whether performance was affected by C. bombi infection, including the explanatory variables colony, body size and rewarding flower type, and (iii) the relation between the proportion of colour or odour mistakes and intensity of infection. These analyses treat flower choice (rewarding or non-rewarding) and type of mistake (colour or odour) as binary dependent variables and account for covariation among repeated observations (flower visits) on individuals.
(i) Results and discussion
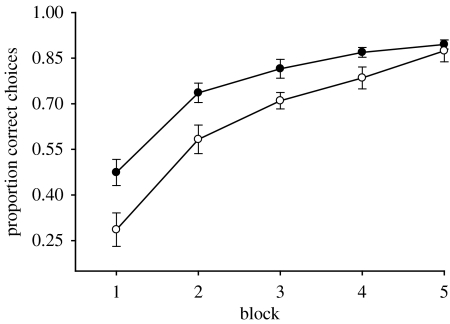
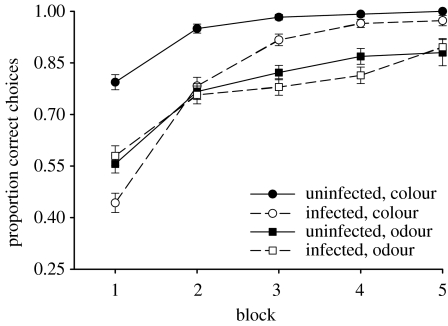
Of the bees tested, 12 bees were found to be naturally infected by C. bombi (C. bombi observed in gut) and 10 bees were uninfected (no C. bombi observed). Body size did not differ between infected and uninfected bees or between colonies (two-way ANOVA, infection status F1,21=0.95, p=0.34; source colony F1,21=0.128, p=0.27). Infected and uninfected bees both learned to discriminate rewarding from non-rewarding flowers on the basis of colour and odour cues. Performance, i.e. the proportion of visits to the rewarding flower type, increased from the first to last block of 30 flower visits (figure 1; infected bees G=8.11, p=0.0044; uninfected bees G=8.41, p=0.0037) and all bees reached the learning criterion of 80% visits to rewarding flowers. Infected and uninfected bees did not differ in the amount of time required to access flowers (mean±s.e. time from landing on the surface of the flower until reaching the bottom of the tube for the first-five flowers visited: infected =1.53±0.05 s, uninfected =1.59±0.04 s; ANOVA, F1,21=1.47, p=0.24). However, infected bees learned to discriminate among flowers more slowly, choosing a significantly smaller proportion of rewarding flowers over their first 150 flower visits than uninfected bees (G=5.55, p=0.018). Interestingly, compared to uninfected bees, infected bees made a significantly greater proportion of their visits to flowers of the wrong colour (figure 2; G=8.15, p=0.0043), but not flowers of the wrong odour (figure 2; G=0.01, p=0.96), suggesting that they were less able to learn the association between colour and reward. Indeed, there was a positive relationship between the proportion of colour errors made by infected bees over the 150 flower visits and their intensity of C. bombi infection (G=4.03, p=0.04). No such relationship was found for odour (G=0.04, p=0.84). The discrepancy between associating colour and odour cues with the rewarding flower type suggests that infected bees either had impairments only to colour discrimination learning or were unable to effectively combine visual information with olfactory information.
Figure 1.
The results of experiment 1 plotted as mean proportion of correct flower choices per block of 30 consecutive flower visits for infected (n=12; open circles) and uninfected (n=10 filled circles) bumble-bees. A correct choice is defined as a visit to a colour–odour combination containing sucrose reward. Error bars indicate standard error of the mean.
Figure 2.
The results of experiment 1 plotted as mean proportion of choices to flowers of the correct colour and odour per block of 30 consecutive flower visits for infected (n=12) and uninfected (n=10) bumble-bees. A correct choice is defined as a visit to a flower containing sucrose reward. Error bars indicate standard error of the mean.
(d) Experiment 2
The results of experiment 1 indicate that bumble-bees naturally infected by C. bombi have reduced cognitive performance. However, C. bombi infection did not necessarily cause the reduction as it is possible that cognitively deficient bees were more susceptible to infection. We, therefore, conducted another experiment, in which bees were artificially infected with C. bombi and their performance on a second flower-discrimination task was compared to bees that were not artificially infected. We used flowers that differed only in colour because the results of Experiment 1 suggested that colour learning was vulnerable to the effects of infection (figure 2).
We regularly removed newly emerged bees from their colony, marked each with a uniquely numbered tag for identification, and randomly assigned them to either ‘infected’ or ‘control’ treatments. Bees in the infected treatment were each fed≈100 000 C. bombi cells (haemocytometer count) in a drop of 50% sucrose solution, whereas bees in the control treatment were given only a drop of 50% sucrose solution. This dose produces infection intensities similar to those observed in naturally infected bees (M. C. Otterstatter, unpublished data) and does not overwhelm the immune system of bees (Allander & Schmid-Hempel 2000). In total, we marked 46 bees (26 infected, 20 control). Infected and control bees were then returned to their colony. We waited at least 9 days for infection to build up and then tested 22 bees (14 infected and eight control) on unscented blue, yellow, red and orange flowers as in the first experiment. Choice data for infected and control groups were analysed as in experiment 1, blind with respect to infection group, and including the explanatory variables body size, rewarding flower type and age.
(i) Results and discussion
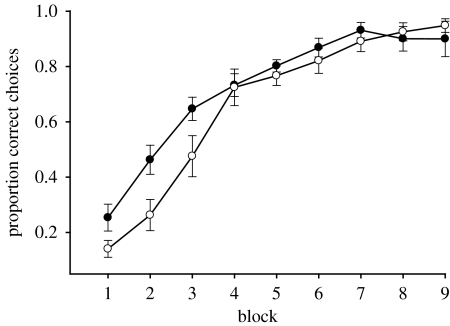
Infected and control bees differed significantly in their intensity of C. bombi infection (mean±s.e.; 27 149±3425 versus 3592±1351 cells μl−1; t-test, t17.9=−6.4, p<0.0001, Satterthwaite method for unequal variance), but not in age (15.0±0.8 versus 15.2±1.2 days; t22=0.16, p=0.88) or body size (2.71±0.04 versus 2.72±0.06 mm; t22=0.12, p=0.19). Infected and control bees both learned to choose flowers of the correct colour. Performance increased from the first to last block of 10 flower visits (figure 3; infected bees G=12.54, p=0.0004; uninfected bees G=6.94, p=0.0084) and all bees reached the 80% criterion within 90 visits. However, as in the first experiment, Infected bees made a significantly smaller proportion of visits to the rewarding flower type over their first 90 flower visits compared to control bees (G=4.64, p=0.031). This manipulative experiment confirms that the reduced cognitive performance of bees was indeed infection-induced, not a characteristic of bees prior to infection, and further reinforces our conclusion that infected bees are less able to learn flower discriminations on the basis of colour.
Figure 3.
The results of experiment 2 plotted as mean proportion of correct flower choices per block of 10 consecutive flower visits for bumble-bees in the infected (n=14; open circles) and control (n=8 filled circles) treatments. A correct choice is defined as a visit to a colour containing sucrose reward. Values were adjusted for the effects of bee size. Error bars indicate standard error of the mean.
3. General discussion
Few studies have considered the potential detrimental effects of parasitic infection on learning, memory and decision-making in animals, even though such cognitive processes underlie a wide variety of ecologically relevant behaviours (Dukas 1998; Shettleworth 1998). The results of our experiments show that the ability of bumble-bees to utilize floral information and make economic foraging decisions is impaired by infection with the intestinal parasite C. bombi. Bees infected naturally (experiment 1) and experimentally (experiment 2) by C. bombi learned to discriminate among flowers on the basis of colour more slowly than uninfected and control bees, respectively. In both experiments, infected bees eventually reached the performance levels of uninfected bees on the foraging task indicating that infection affected the ability of bees to acquire, but not retain, the reward properties of flowers. Our study, which is the first to show that parasitic infection alters choice behaviour in invertebrates by impairing cognitive function, adds to the growing evidence that infection can disrupt ecologically important aspects of cognition in animals (Gibertini et al. 1995; Kavaliers & Colwell 1995; Kavaliers et al. 1995; Cox & Holland 2001; Gegear et al. 2005).
Previous studies have shown that parasitic infection alters choice behaviour in animals (Milinski 1990; Pfennig & Tinsley 2002; Buchholz 2004; Mazzi 2004), but the mechanisms responsible for these effects have seldom been investigated. Typically, energetic constraints imposed by the parasite on the host have been invoked as an explanation for infection-induced changes to choice behaviour. In our experiments, infected bees made regular foraging trips to feeders prior to experiments, readily collected reward from each flower type during training, and sampled available flowers during testing. Furthermore, infected and uninfected bees did not differ in the amount of time required to access flowers. These observations suggest that infection-induced alterations to choice behaviour were not the result of an overall reduction in the physical condition of bees. Rather, our data suggest that infection impaired the ability of bees to utilize the floral information necessary to make economic choices. Behavioural studies of how parasites affect decision-making in host animals would benefit by considering the potential influence of infection on the ability of animals to acquire and manage information.
The observed modifications to foraging in infected bumble-bees are subtle but nonetheless have the potential to impose a significant ecological cost to bees in the field. In social bees, the reproductive success of the colony is directly related to the acquisition of floral resources by foragers. Given that plant species vary tremendously in the quality of floral rewards offered, bees that are better able to recognize and discriminate profitable species will acquire more resources and increase colony success. Our data suggest that infection by C. bombi could reduce colony success by impairing the ability of foragers to monitor and acquire floral resources. Moreover, although C. bombi is considered to be a benign parasite, food stress increases mortality in infected individuals (Brown et al. 2000, 2003a,b). Thus, the reductions in resource acquisition by infected foragers could also have detrimental effects on colony success by increasing mortality levels in infected nest bees. Considering that C. bombi parasitizes many bumble-bee species under natural conditions, a high prevalence of C. bombi could have a dramatic impact on host species diversity and abundance. Indeed, the prevalences of many parasites, including C. bombi have increased during recent years in some areas of eastern North America (Colla et al. in press), presenting a potential threat to bumble-bee populations.
Alterations to foraging behaviour in bumble-bees have important consequences for flowering plants. Most flowering plants rely on animals, such as bumble-bees, to ensure that pollen is transferred to an appropriate stigma for reproduction. Consequently, plant reproductive success is directly linked to the foraging decisions of pollinating animals. We found that infection by C. bombi increased the frequency with which bumble-bees moved between different flower types because they were less able to discriminate among floral cues. In an ecological context, such infection-induced behavioural changes in pollinating bees would increase reproductive costs to plants by decreasing the efficiency of pollen transfer and increasing the adverse effects of receiving heterospecific pollen (Waser 1983). Assuming that C. bombi infection alters flower-choice behaviour in species other than B. impatiens, a high frequency of infection could thus have a negative impact on plant species that rely on bumble-bees for pollination.
How can infection alter CNS function in bumble-bees despite the physical separation of C. bombi from relevant neural tissues? Because neuroactive compounds are rarely produced by microparasites such as C. bombi (Thomas et al. 2005), the modification to CNS function observed in infected bees was probably not due to the direct action of C. bombi but rather to the immune response of the bee itself. Indeed, C. bombi infections have been shown to elicit a systemic immune response in bumble-bees (Brown et al. 2003a,b). Such immune–nervous system interactions have been well established in vertebrates (Gibertini et al. 1995; Pugh et al. 1995; Maier & Watkins 1999). Our findings, and those of recent studies (Mallon et al. 2003; Riddell & Mallon 2005), suggest that such links also occur in invertebrates. Immune–nervous system connectivity can occur when products of an immune response to infection influence neural functioning (Klein 2003). For example, pro-inflammatory cytokines produced during an immune response have been shown to affect spatial learning in rodents (Gibertini et al. 1995). Similar compounds are produced during an immune response in invertebrates (Cooper 2003); however, the potential effects of these compounds on invertebrate CNS function have yet to be investigated. Alternatively, modifications to neural functioning can occur when the immune response competes for a resource required by the nervous system (Sheldon & Verhulst 1996; Maier & Watkins 1999; Adamo 2002; Klein 2003). Such trade-offs between the immune and nervous systems raise the intriguing possibility that many ecological decisions made by animals reflect a compromise between immune defence and cognitive function. The current study highlights the usefulness of bumble-bees as a system to examine potential immune–brain–behaviour interactions in invertebrates.
Acknowledgments
We thank D. F. Sherry, J. G. Burns, S. Colla, J. Manson and two anonymous reviewers for valuable comments on the manuscript. This work was supported by the Natural Sciences and Engineering Research Council of Canada.
Footnotes
R.J.G. would like to dedicate this contribution to the memory of Dr. T.M. Laverty.
References
- Adamo S.A. How parasites alter the behaviour of their insect hosts. In: Beckage N, editor. Parasites and pathogens. Effects on host hormone and behaviour. Chapman & Hall; New York, NY: 1997. pp. 231–245. [Google Scholar]
- Adamo S.A. Modulating the modulators: parasites, neuromodulators and host behavioural change. Brain Behav. Evol. 2002;60:370–377. doi: 10.1159/000067790. 10.1159/000067790 [DOI] [PubMed] [Google Scholar]
- Allander K, Schmid-Hempel P. Immune defence reaction in bumble-bee workers after a previous challenge and parasitic coinfection. Funct. Ecol. 2000;14:711–717. 10.1046/j.1365-2435.2000.00476.x [Google Scholar]
- Barber I, Huntingford F.A. Parasite infection alters schooling behaviour: deviant positioning of helminth-infected minnows in conspecific groups. Proc. R. Soc. B. 1996;263:1095–1102. [Google Scholar]
- Beltman J.B, Haccou P, ten Cate C. The impact of learning foster species' song on the evolution of specialist avian brood parasitism. Behav. Ecol. 2003;14:917–923. 10.1093/beheco/arg082 [Google Scholar]
- Brown M.J.F, Loosli R, Schmid-Hempel P. Condition-dependent expression of virulence in a trypanosome infecting bumblebees. Oikos. 2000;91:421–427. 10.1034/j.1600-0706.2000.910302.x [Google Scholar]
- Brown M.J.F, Moret Y, Schmid-Hempel P. Activation of host constitutive immune defence by an intestinal trypanosome parasite of bumble bees. Parasitology. 2003a;126:253–260. doi: 10.1017/s0031182002002755. 10.1017/S0031182002002755 [DOI] [PubMed] [Google Scholar]
- Brown M.J.F, Schmid-Hempel R, Schmid-Hempel P. Strong condition-dependent virulence in a host–parasite system: reconciling genetic evidence with theory. J. Anim. Ecol. 2003b;72:994–1002. 10.1046/j.1365-2656.2003.00770.x [Google Scholar]
- Buchholz R. Effects of parasitic infection on mate sampling by female wild turkeys (Meleagris gallopavo): should infected females be more or less choosy? Behav. Ecol. 2004;15:687–694. 10.1093/beheco/arh066 [Google Scholar]
- Cartar R. Resource tracking by bumble bees: responses to plant-level differences in quality. Ecology. 2004;85:2764–2771. [Google Scholar]
- Colla, S. R., Otterstatter, M. C., Gegear, R. J. & Thomson J. D. In press. Plight of the bumble bee: pathogen spillover from commercial to wild population. Biol. Conserv
- Cooper E.L. Comparative immunology. Curr. Pharm. Design. 2003;9:119–131. doi: 10.2174/1381612033392297. 10.2174/1381612033392297 [DOI] [PubMed] [Google Scholar]
- Cox D.M, Holland C.V. Relationship between three intensity levels of Toxocara canis larvae in the brain and effects on exploration, anxiety, learning and memory in the murine host. J. Helminthol. 2001;75:33–41. doi: 10.1079/joh200028. [DOI] [PubMed] [Google Scholar]
- Dukas R. The University of Chicago Press; Chicago, IL: 1998. Cognitive ecology: the evolutionary ecology of information processing and decision making. [DOI] [PubMed] [Google Scholar]
- Dukas R. Evolutionary ecology of animal cognition. Annu. Rev. Ecol. Evol. Syst. 2004;35:347–374. 10.1146/annurev.ecolsys.35.112202.130152 [Google Scholar]
- Gegear R.J, Laverty T.M. Flower constancy in bumblebees: a test of the trait variability hypothesis. Anim. Behav. 2005;69:939–949. 10.1016/j.anbehav.2004.06.029 [Google Scholar]
- Gegear R.J, Otterstatter M.C, Thomson J.D. Does parasitic infection impair the ability of bumblebees to learn flower-handling techniques? Anim. Behav. 2005;70:209–215. 10.1016/j.anbehav.2004.09.025 [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein T.W. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-β. Brain Behav. Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. 10.1006/brbi.1995.1012 [DOI] [PubMed] [Google Scholar]
- Healy S.D, de Kort S.R, Clayton N.S. The hippocampus, spatial memory and food hording: a puzzle revisited. TREE. 2005;20:17–22. doi: 10.1016/j.tree.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Colwell D.D. Reduced spatial learning in mice infected with the nematode Heligmosomoides polygyrus. Parasitology. 1995;110:591–597. doi: 10.1017/s0031182000065318. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Colwell D.D, Galea L.A.M. Parasitic infection impairs spatial learning in mice. Anim. Behav. 1995;50:223–229. 10.1006/anbe.1995.0234 [Google Scholar]
- Kavaliers M, Colwell D.D, Choleris E. Parasites and behaviour: an ethopharmacological analysis and biomedical implications. Neurosci. Biobehav. Rev. 1999;23:1037–1045. doi: 10.1016/s0149-7634(99)00035-4. 10.1016/S0149-7634(99)00035-4 [DOI] [PubMed] [Google Scholar]
- Keymer A.E, Read A.F. Behavioural ecology: the impact of parasitism. In: Barnard C.J, Behnke J.M, editors. Parasitism and host behaviour. Taylor & Francis; London: 1990. pp. 37–61. [Google Scholar]
- Klein S.L. Parasite manipulation of the proximate mechanisms that mediate social behaviour in vertebrates. Physiol. Behav. 2003;79:441–449. doi: 10.1016/s0031-9384(03)00163-x. 10.1016/S0031-9384(03)00163-X [DOI] [PubMed] [Google Scholar]
- Laverty T.M. Bumble bee learning and flower morphology. Anim. Behav. 1994;47:531–545. 10.1006/anbe.1994.1077 [Google Scholar]
- Mallon E.B, Brockmann A, Schmid-Hempel P. Immune response inhibits associative learning in insects. Proc. R. Soc. B. 2003;270:2471–2473. doi: 10.1098/rspb.2003.2456. 10.1098/rspb.2003.2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S.F, Watkins L.R. Bidirectional communication between the brain and the immune system: implications for behaviour. Anim. Behav. 1999;57:741–751. 10.1006/anbe.1998.1068 [Google Scholar]
- Mazzi D. Parasites make pipefish careless. J. Evol. Biol. 2004;17:519–527. doi: 10.1111/j.1420-9101.2004.00704.x. 10.1111/j.1420-9101.2004.00704.x [DOI] [PubMed] [Google Scholar]
- Mery F, Kawecki T.J. A fitness cost of learning ability in Drosophila melanogaster. Proc. R. Soc. B. 2003;270:2465–2469. doi: 10.1098/rspb.2003.2548. 10.1098/rspb.2003.2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milinski M. Parasitism and host behaviour. Taylor & Francis; London: 1990. Parasites and host decision-making; pp. 95–116. [Google Scholar]
- Moore J. Oxford series in ecology and evolution. Oxford University Press; New York, NY: 2002. Parasites and the behavior of animals. [Google Scholar]
- Moret Y, Schmid-Hempel P. Immune defence in bumble-bee offspring. Nature. 2001;414:509. doi: 10.1038/35107138. 10.1038/35107138 [DOI] [PubMed] [Google Scholar]
- Otterstatter M.C, Gegear R.J, Colla S, Thomson J.D. Effects of parasitic mites and protozoa on the flower constancy and foraging rate of bumble bees. Behav. Ecol. Sociobiol. 2005;58:383–389. 10.1007/s00265-005-0945-3 [Google Scholar]
- Pfennig K.S, Tinsley R.C. Different mate preferences by parasitized and unparasitised females potentially reduces sexual selection. J. Evol. Biol. 2002;15:399–406. 10.1046/j.1420-9101.2002.00406.x [Google Scholar]
- Pugh C.R, Fleshner M, Watkins L.R, Maier S.F, Rudy J.W. The immune system and memory consolidation: a role for the cytokine IL-1-β. Neurosci. Biobehav. Rev. 1995;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Riddell, C. E. & Mallon, E. B. In press. Insect psychoneuroimmunology: Immune response reduces learning in protein starved bumblebees (Bombus terrestris). Brain Behav. Immun [DOI] [PubMed]
- Schmid-Hempel P. On the evolutionary ecology of host–parasite interactions: addressing the question with regard to bumblebees and their parasites. Naturwissenschaften. 2001;88:147–158. doi: 10.1007/s001140100222. 10.1007/s001140100222 [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. TREE. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Shettleworth S.J. Oxford University Press; New York, NY: 1998. Cognition, evolution and behavior. [Google Scholar]
- Thomas F, Adamo S, Moore J. Parasitic manipulation: where are we and where should we go? Behav. Processes. 2005;68:185–199. doi: 10.1016/j.beproc.2004.06.010. 10.1016/j.beproc.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Waser N.M. The adaptive nature of floral traits: ideas and evidence. In: Real L.A, editor. Pollination biology. Academic Press; Orlando: 1983. pp. 241–285. [Google Scholar]