Abstract
There is currently much interest in mate preferences for sexual ornaments. However, few studies have focused on individual variation in mate preference despite its importance for the rate and direction of sexual selection. Females of the sexually dimorphic stalk-eyed fly, Diasemopsis meigenii, exhibit an unambiguous rejection response towards unattractive males bearing small ornaments. We investigated individual mate preferences using repeated sequential sampling of female rejection or acceptance responses to a wide range of male ornament phenotypes. We found significant variation in the strength of individual preference. In addition, preference was positively associated with female eyespan, a condition-dependent trait putatively linked to visual acuity.
Keywords: individual mate preference, mate rejection, sexual selection, stalk-eyed fly
1. Introduction
Over the last decade there has been a great deal of interest in variation of mate preferences for sexual ornaments (Bakker & Pomiankowski 1995; Jennions & Petrie 1997; Badyaev & Qvarnström 2002). Such variation is important as it has consequences for the rate and direction of sexual selection (Turner & Burrows 1995). Species or population level variation has been used extensively to examine the evolutionary history of female mate preference and its coevolution with male ornaments (e.g. Wilkinson et al. 1998). In contrast, variation in preference between individuals is less well studied. Yet individual preferences can provide important insights into the selective forces that shape female mating decisions, since preference is predicted to be highly sensitive to both the costs of choice and the benefits derived from it (Pomiankowski 1987; Houle & Kondrashov 2002). Variation in preference among individuals can also be used to investigate the mechanisms underlying preference by measuring associations with other traits (e.g. Hingle et al. 2001a).
Most studies of female mate preference have focused on variation at the population or group level (reviewed in Jennions & Petrie 1997; Wagner 1998). However, extrapolation of such findings to the level of the individual can prove misleading, as individual preferences may differ widely in shape or form (Wagner et al. 1995; Wagner 1998). Selection may generate adaptive variation in individual preference if a female benefits from having preferences different from the population mean, for instance when the optimal strength of preference is dependent on the context of mate choice or the qualities of potential mates (Qvarnström 2001; Badyaev & Qvarnström 2002). In addition, selection can generate variance via the quality of the female, if, for example, female preferences are condition-dependent (Tomlinson & O'Donald 1996; Fawcett & Johnstone 2003). Despite its importance, only a few studies have successfully investigated individual preference variation (e.g. Wagner et al. 1995), while others have suffered from deficiencies in experimental design and choice of preference measure (reviewed in Wagner 1998).
Female preference should be distinguished, both conceptually and empirically, from female choice. Preference comprises the sensory and behavioural components that influence females to mate differentially with certain male phenotypes, whereas choice is the pattern of mating that is influenced not only by preference, but also other factors such as male availability and the costs of choice (Jennions & Petrie 1997). This limits the utility of typical experiments that assess ‘preference’ when females are given a choice between simultaneously presented males under conditions that exclude male–male competition. This design forces females to choose, which may misrepresent how females respond to the full range of male phenotypes (Wagner et al. 1995; Wagner 1998). In addition, excluding male–male competition often entails restricted access to males (e.g. held behind partitions or tethered), which may interfere with mating decisions (Candolin 1999; Nilsson & Nilsson 2000).
We therefore have adopted a ‘no-choice’ design, in which individual female preference functions are derived from their responses to sequentially presented males which vary in ornament size (Wagner 1998; Shackleton et al. 2005). In order to be exploited fully, no-choice tests need to assay females with a range of male phenotypes. Studies that simply employ two stimulus males (typically with extreme values of ornamentation) cannot accurately measure the strength of directional selection or detect stabilizing (e.g. Gerhardt 1991; Ritchie 1996; Hunt et al. 2005) or disruptive preference functions (e.g. Sappington & Taylor 1990; Greene et al. 2000). They also have limited power to resolve differences in the preference of individual females (Wagner 1998). It is also clear that the accuracy of preference functions will increase with the number of levels of a given ornament for which a female's response is measured. However, care needs to be taken in repeated sampling of female mating decisions to assess changes in female receptivity and/or preference through time (e.g. Collins 1995; Zeh et al. 1998).
Regardless of experimental design, surrogate measures of preference should accurately reflect female-mediated bias in male mating success. Surprisingly few studies have explicitly demonstrated a strong positive correlation between indirect measures of female preference (such as the degree of association or latency to mating with particular male phenotypes) and who a female prefers to mate with (Clayton 1990; Shackleton et al. 2005), and this association has not always been supported (Gabor 1999). An additional concern is that males can influence mating independently of females through, for example, variation in male mating ability (e.g. Rogers et al. 2005) or forced copulation (e.g. Cordero 1999). The presence of such male effects may inflate or diminish any estimates of the strength of female preference. Ideal species for studying preference are therefore those which perform easily distinguishable female-specific behaviours that indicate mating intent, such as solicitation of copulations or active rejection of unwanted suitors.
Stalk-eyed flies (Diptera: Diopsidae) are an important model system for the study of sexual selection (Wilkinson & Dodson 1997; Wilkinson 2001). They are characterized by the lateral displacement of eyes on elongate stalks in both sexes (Wilkinson & Dodson 1997). Sexual dimorphism in eyespan, where male eyespan is greater than that of females, has evolved on numerous occasions within the family (Baker & Wilkinson 2001), and such sex differences result from sexual selection both through male–male competition for access to females (Burkhardt & de la Motte 1983; Panhuis & Wilkinson 1999) and female mate choice (Burkhardt & de la Motte 1988; Wilkinson & Reillo 1994; Wilkinson et al. 1998; Hingle et al. 2001a,b). While considerable attention has been devoted to variation in male eyespan (David et al. 1998, 2000; Knell et al. 1999; Cotton et al. 2004a,b), no studies have explicitly investigated individual variation in mate preference in stalk-eyed flies.
In this study, we examine individual variation in female mate preference in the sexually dimorphic stalk-eyed fly, Diasemopsis meigenii (also referred to as Chaetodiopsis meigenii; Baker et al. 2001). Female D. meigenii exhibit an overt rejection response to dislodge males and resist unwanted matings. Rejection is unambiguous, allowing a female's preference for a particular male to be easily categorized. In addition, the rejection response is an entirely female behaviour, meaning that preference can be assayed in isolation from male effects. Using large-scale repeated sequential sampling of individual females' responses to a wide range of male phenotypes, we demonstrate the existence of variation in the strength of female preference for large male eyespan. We also control for female mating history, and test for any effects of prior exposure to males. We evaluate two female traits (eyespan and fecundity) putatively linked to preference in another species of stalk-eyed fly, Cyrtodiopsis dalmanni (Hingle et al. 2001a,b). We show that preference in D. meigenii is associated with female eyespan, a condition-dependent trait that affects visual acuity and hence the ability to discriminate between males.
2. Material and methods
(a) Production of experimental flies
The laboratory-adapted population of D. meigenii was collected from Nelspruit, South Africa in 2001, and maintained subsequently in cage culture (>200 individuals to minimize inbreeding) at 25 °C on a 12 : 12 h light : dark cycle, and fed pureed sweetcorn twice weekly. Fifteen minute artificial dawn and dusk periods were created by illumination from a single 60 W bulb, at the start and end of the light phase.
(b) Population preference for male eyespan
Individual virgin females (n=73) were isolated in containers (400 ml, containing a moist cotton base and food tray) at least 24 h prior to mating trials. Mating behaviour was observed for the first 1.5 h of each day (15 min dawn and 75 min full illumination). Each female was allowed one mating attempt with a single randomly chosen male (eyespan range=4.53–8.66 mm). Acceptance was defined as a male mounting the female and engaging genitalia. Female rejection behaviour was unambiguous comprising vigorous body shaking in an attempt to dislodge the male, often in conjunction with extension of the ovipositor prohibiting copulation. Occasionally, a mating occurred despite female rejection (five matings among 23 pairs showing female rejection behaviour). These individuals were excluded from the analyses. Thirteen males made no attempt to mount the female during the 1.5 h observation periods; these flies were classified separately from those that attempted a mating.
The eyespans (the distance between the outermost tips of the eyes; David et al. 1998) of each fly was measured using a video camera mounted on a monocular microscope and NIH image software (v.1.55, National Institutes of Health, Bethesda MD, USA).
(c) Individual preferences for male eyespan
Mature virgin males were anaesthetized over ice and their eyespan measured prior to experimentation. Males were allocated to one of seven eyespan size classes: 6.70–6.90, 7.00–7.20, 7.40–7.60, 7.61–7.80, 7.81–8.00, 8.10–8.30 and >8.40 mm (total range=6.72–8.72 mm). These size classes spanned the inflection point of the mating probability curve (i.e. when p(accept)=p(reject)=0.50) derived from the results of the population preference experiment (see below). Each size class contained 5–8 males, which were kept individually in 400 ml containers (as described above). Virgin females were also kept in 400 ml containers, lined with a dark blue paper base that allowed eggs laid during the experiment to be easily seen. Individuals of both sexes were isolated at least 24 h prior to the start of the experiment. Flies were fed three times a week on pureed sweetcorn.
Females (n=47) were assayed for individual mate preference by scoring rejection or acceptance of 14 males (two males from each eyespan class). A single male was introduced to a female's container each day and her rejection or acceptance of a single mating attempt from the male was recorded. Once a female's response for a given male had been determined, the male was removed. Female virginity was maintained throughout the experiment by interrupting any mating attempts by dislodging males. The order in which particular male classes were presented over the study period was randomized (for each set of seven eyespan categories), and males were randomly chosen from within their eyespan size class (males typically performed one mating attempt per day, although occasionally they were used twice).
(d) Morphological and reproductive correlates of individual preferences
Female eyespan was measured at the end of the experiment (as described in §2b). Fecundity was estimated in two ways. First, the number of eggs laid on the blue paper base over the 14-day experimental period was recorded. Second, at the end of the experiment females were dissected in phosphate-buffered saline and the number of mature eggs contained within their ovaries counted. Total fecundity was defined as the sum of these two components.
(e) Statistical analysis
(i) Population preference for male eyespan
Some males (n=13) did not attempt to mate with females. We tested if male eyespan was associated with whether a male made a mating attempt using a t-test (assuming unequal variances). Among those males that did attempt to mate, we used logistic regression to determine the form of selection acting on male eyespan, as the dependent response variable was binomially distributed (Hardy & Field 1998). The female response was coded as 0 or 1 for rejection or acceptance of mating respectively. The significance of male eyespan as a predictor of mating success was tested using a likelihood ratio test of explained deviance (Hardy & Field 1998). The inflection point of the probability curve (when p(accept)=p(reject)=0.5) was used to describe the average eyespan size above which mating generally occurred and below which rejection was typically observed (among those males that attempted to mate).
(ii) Individual preferences and correlated traits
To identify variation between females in the strength of directional preference we evaluated the male eyespan×female identity interaction in a likelihood ratio test of explained deviance (the full model also comprised an intercept, and male eyespan and female identity main effects). A significant interaction indicated that the importance of male eyespan in determining the probability of rejection (i.e. preference) differs between females. Logistic regressions were also performed on individual females to identify those with significant versus non-significant directional preferences, using likelihood ratio tests of male eyespan effects. We also fitted cubic splines (Schluter 1988) to the data for each female to identify any females with significant non-directional (stabilizing or disruptive) preferences. Splines were generated for values of λ=−2 and −6, near the edges of the range of lambdas that minimized per-female cross-validation scores, and therefore represent conservative and liberal estimates of individual preference functions, respectively. Reliability of the fitted functions was evaluated via inspection of standard errors derived from 1000 bootstrapped replicates.
We explicitly tested for a change in female rejection behaviour and preference over the 14-day assay period using likelihood ratio tests of explained deviance for a model containing male eyespan, trial day, their interaction and an intercept. The trial day effect tests whether the average number of rejections changes over time and male eyespan×trial day interaction tests whether the importance of male eyespan in determining the probability of rejection (i.e. preference) changes over time.
Associations between female preference (significant versus non-significant) and morphological (eyespan) and reproductive (fecundity) characteristics were evaluated using t-tests, assuming equal or unequal variances where appropriate. We also calculated correlation coefficients between female eyespan and measures of fecundity. JMP v. 5.0.1a for the Macintosh (SAS Institute 1996) and the univariate spline fitter program v. 4.0 for Windows (http://www.zoology.ubc./ca/~schluter/splines.html) were used for all statistical analyses.
3. Results
(a) Population preference for male eyespan
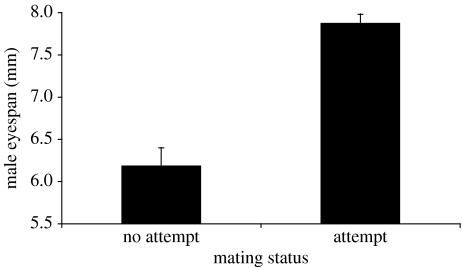
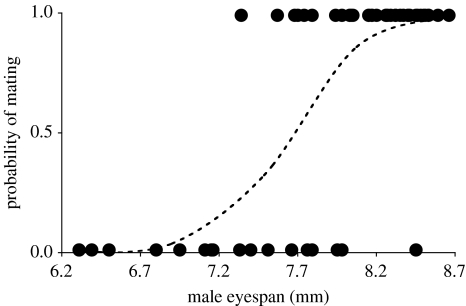
Males that failed to attempt a mating had significantly smaller eyespan than males that attempted to mate (irrespective of whether they succeeded or not; figure 1; eyespan mean (mm)± s.e.: no attempt=6.19±0.21, attempt=7.88±0.10, t13.71=5.14 (unequal variances), p<0.001). Among those males that attempted to mate, females exhibited strong preference for males with large eyespan (figure 2; =33.92, p<0.001). Males accepted as mates had significantly larger eyespan than those rejected (mean (mm) ±s.e.: accepted=8.19±0.05, rejected =7.25±0.15, t21.15=6.02 (unequal variances), p<0.001). The inflection point of the mating probability curve occurred at a male eyespan of 7.66 mm (figure 2); males with an eyespan <7.66 mm were more likely to be rejected by a female than accepted.
Figure 1.
The mean eyespan (±s.e.) of males that did not attempt a mating and males that did attempt a mating.
Figure 2.
The probability of being accepted as a mate in relation to male eyespan. Open circles represent the outcome of a single mating trial, and the dotted line represents the predicted probability of mating.
(b) Individual preferences for male eyespan
Individual females were tested against males with a similar range of eyespans, and there was no difference in the mean male eyespan across females (F46,611=0.018, p=1.00). Given this choice of comparable males, we found significant variation among females in the strength of their preferences (male eyespan×female identity interaction, =86.83, p<0.001). Of the 47 females assayed, 18 showed a significant directional preference for male eyespan, and in all but one case females preferred males with larger eyespan. This variation was caused by differences in the distribution of rejections (with respect to male eyespan), rather than the number of rejections, as females showing significant preference did not reject more often than females with non-significant preferences (mean probability of rejection per mating ±s.e.: significant preference=0.59±0.05, non-significant preference=0.64±0.04, t45=0.77, p=0.45).
We found no evidence for non-directional (stabilizing or disruptive) preference on male eyespan. Inspection of cubic spline curves (both conservative (λ=−2) and liberal (λ=−6)) and associated standard errors revealed that, in agreement with results from logistic regression, many preference functions of individual females were directional (λ=−2, n=15; λ=−6, n=26).
Females were assayed with a single male each day for 14 days. There was no significant change in the average probability (across all females) of rejection over this period (trial day =6.54, p=0.92), or any evidence of a change in preference for male eyespan resulting from the repeated sequential sampling design (male eyespan×trial day =15.57, p=0.27).
(c) Morphological and reproductive correlates of individual preferences
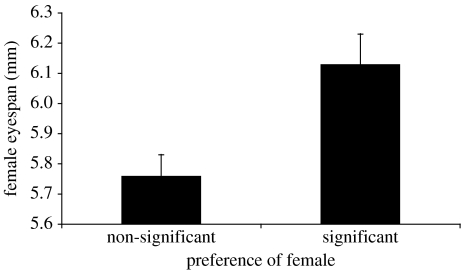
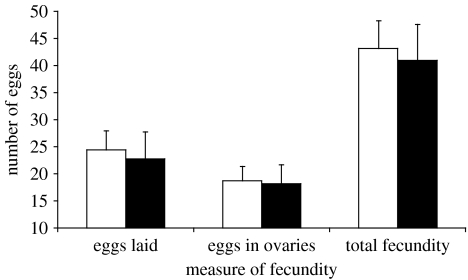
Females exhibiting significant preference for well-ornamented males had larger eyespans than females with non-significant preferences (figure 3; mean (mm) ±s.e.: significant preference=6.13±0.10, non-significant preference=5.76±0.07, t43.6=3.50 (unequal variances), p=0.001). In contrast we found no association between the presence of a preference and any of the estimates of fecundity (figure 4; mean eggs laid, t44=0.29, p=0.77; mean eggs in ovaries, t44=0.13, p=0.90; mean total fecundity, t44=0.26, p=0.79). Female eyespan and measures of fecundity were not significantly correlated with each other (female eyespan versus eggs laid, r=−0.21, p=0.16; eggs in ovaries, r=−0.11, p=0.47; total fecundity, r=−0.20, p=0.18).
Figure 3.
The mean eyespan (±s.e.) of females showing non-significant preferences and females exhibiting significant preferences.
Figure 4.
Mean (±s.e.) values of measures of fecundity of females showing non-significant preferences (open bars) and females exhibiting significant preferences (black bars). Total fecundity=eggs laid+eggs in ovaries.
4. Discussion
We used female rejection of male mating attempts to quantify preference, as it was a reliable estimator of female-induced bias in male copulation success. The rejection response components (vigorous body shaking and extension of the ovipositor) are female-specific and occurred almost immediately upon mounting by the male, and so are unlikely to be influenced by male effects other than attractiveness. In addition, not all mating attempts elicited a struggle, so rejection is unlikely to have evolved through alternative mechanisms such as ‘sexual conflict’ or avoidance of ‘costs of mating’ (reviewed in Arnqvist & Rowe 2005). Resistance in D. meigenii is not a case of passive choice, where females set up a mating obstacle that only high quality males can overcome, but rather an active mechanism deployed to ward off unattractive males.
We established that female mate preference is a strong agent of sexual selection in D. meigenii. Males with large eyespan (>7.66 mm) were preferred as mates and achieved copulations without female resistance. In contrast, females actively rejected mating attempts by males with small ornaments. We also found that males with very small eyespan failed to even attempt a mating. Females may have actively avoided encounters with such males or, alternatively, small males may have been unable to attempt a mating, or be in too poor condition to be able to mate; further work is required to distinguish between these hypotheses. The conspicuous and exaggerated nature of male eyespan, coupled with the face-on method of mate inspection strongly implicates male eyespan as the focus of female mating decisions. Nonetheless, since male phenotypes were described only in terms of their eyespan, it is conceivable that any trait positively correlated with male eyespan could be the signal assessed by females.
We extended the population-level finding by investigating variation in individual mate preference. We assayed individual females 14 times and evaluated the strength of their preference over a broad range of male ornament sizes. Our use of repeated sequential sampling over the normal range of male phenotypes that females encounter gave us estimates of both the strength and form of individual preference functions. We controlled for female mating history by maintaining females as virgins throughout the experiment. This procedure did not cause changes in female mating behaviour over the assay period, as the average probability of rejecting a male and the average preference for large eyespan males did not change through time.
Individual females varied significantly in the strength of preference. Approximately 40% of females showed significant directional preference for large ornaments through consistent rejection of mating attempts by males with small eyespan; the remainder showed no significant bias of rejections toward small eyespan males. A single female preferred small eyespan males to large eyespan males; this may result from a low frequency of such females in the population or possibly a chance artefact from sampling a large number of individuals. Studies in other model systems have shown that female preference functions can sometimes be stabilizing (e.g. Gerhardt 1991; Ritchie 1996; Hunt et al. 2005) or disruptive (e.g. Sappington & Taylor 1990; Greene et al. 2000). However, we found no evidence for stabilizing or disruptive preferences in D. meigenii.
Previous work on another species of stalk-eyed fly, C. dalmanni, showed an association between preference and fecundity, whereby females reared on high quality corn copulated more frequently with large eyespan males and had higher fecundity than females reared on a poorer quality sucrose diet (Hingle et al. 2001b). We failed to find any relationship between preference and fecundity in the current study on D. meigenii. However, there is no a priori reason to assume that preference and fecundity are causally linked since both are important life-history traits, and likely to covary independently with overall female condition. In contrast to Hingle et al. (2001b), we maintained flies only on a high quality corn diet, so variation in female condition was likely to be low and hence yield a null correlation between preference and fecundity.
We did, however, find a strong association between the strength of female preference and female eyespan. Females exhibiting significant preference for males with large eyespan themselves had larger eyespan than females with non-significant preference. A similar relationship was found in C. dalmanni (Hingle et al. 2001a), although that study presented females with binary choices of males, and so only measured choice rather than preference functions. Female eyespan is likely to reflect a number of fitness components in stalk-eyed flies. For instance, female eyespan has been shown to be a condition-dependent trait in three Diopsid species (Knell et al. 1999; Cotton et al. 2004b), suggesting that females with larger eyespan and stronger preferences are in better condition as larvae than females with weaker preferences. In addition, female eyespan is highly allometric (Baker & Wilkinson 2001), indicating that females with strong preferences also enjoy the benefits associated with large body size; for example, fecundity is correlated with body size in wild-caught stalk-eyed flies (Cotton & Pomiankowski in preparation) and other insect taxa (Honek 1993).
Phenotypic correlations between preference and other female traits can also provide insight into the mechanisms underlying preference. There is good theoretical evidence that the role of female eyespan in mate discrimination is due, in part, to the mechanistic basis of diopsid vision. The number of ommatidia in the compound eyes increases with female eyespan (de la Motte & Burkhardt 1983) resulting in higher visual resolution for large eyespan females (Burkhardt & de la Motte 1983). Moreover, the higher number of ommatidia also gives rise to a larger field of binocular vision (de la Motte & Burkhardt 1983). This coupled with the greater distance between the laterally displaced eyes may result in increasingly sensitive stereoscopic vision in large eyespan females (Burkhardt & de la Motte 1983; de la Motte & Burkhardt 1983), providing a basis for more precise assessment of male ornaments. Indeed, our data are consistent with small eyespan females suffering from errors in perception through poorer visual acuity. Variation in preference between large and small eyespan females arose not through differences in the number of rejections, but rather differences in the distribution of rejections; large eyespan females rejected only small eyespan males, whereas small eyespan females rejected at random (with respect to male eyespan).
In conclusion, we have shown that female mate preference in the stalk-eyed fly D. meigenii may be responsible for the evolution of the exaggerated ornament found in this species. Preference, arising from the active rejection of mates with small ornaments, was highly variable between individuals. It was contingent on female eyespan, a condition-dependent trait putatively linked to visual acuity. There remains a pressing need to identify the relative contributions of genetic, environmental factors and their interaction to phenotypic variation in preference. In addition, experimental manipulation of condition and subsequent evaluation of links between preference and fecundity (sensu Hingle et al. 2001b) are essential to investigate the hypothesis that female preferences are condition-dependent. Future work on D. meigenii will address these unresolved issues.
Acknowledgments
This work was supported by a research grant from the BBSRC (to K.F. & A.P., and employing S.C.), a Commonwealth Scholarship from the Association of Commonwealth Universities (D.W.R.) and the Department of Biology, UCL (J.S.). The authors thank Martin Carr for discussion, Jerry Wilkinson for providing D. meigenii, and two anonymous referees for comments on the manuscript.
References
- Arnqvist G, Rowe L. Princeton University Press; Princeton, NJ: 2005. Sexual conflict. [Google Scholar]
- Badyaev A.V, Qvarnström A. Putting sexual traits into the context of an organism: a life-history perspective in studies of sexual selection. Auk. 2002;119:301–310. [Google Scholar]
- Baker R.H, Wilkinson G.S. Phylogenetic analysis of sexual dimorphism and eyespan allometry in stalk-eyed flies (Diopsidae) Evolution. 2001;55:1373–1385. doi: 10.1111/j.0014-3820.2001.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Baker R.H, Wilkinson G.S, DeSalle R. Phylogenetic utility of different types of molecular data used to infer evolutionary relationships among stalk-eyed flies (Diopsidae) Syst. Biol. 2001;50:87–105. 10.1080/106351501750107512 [PubMed] [Google Scholar]
- Bakker T.C.M, Pomiankowski A. The genetic basis of female mate preferences. J. Evol. Biol. 1995;8:129–171. 10.1046/j.1420-9101.1995.8020129.x [Google Scholar]
- Burkhardt D, de la Motte I. How stalk-eyed flies eye stalk-eyed flies: observations and measurements of the eyes of Cyrtodiopsis whitei (Diopsidae, Diptera) J. Comp. Physiol. A. 1983;151:407–421. 10.1007/BF00605457 [Google Scholar]
- Burkhardt D, de la Motte I. Big ‘antlers’ are favoured: female choice in stalk-eyed flies (Diptera, Insecta), field collected harems and laboratory experiments. J. Comp. Physiol. A. 1988;162:649–652. 10.1007/BF01342640 [Google Scholar]
- Candolin U. Male–male competition facilitates female choice in sticklebacks. Proc. R. Soc. B. 1999;266:785–789. 10.1098/rspb.1999.0706 [Google Scholar]
- Clayton N.S. Assortative mating in zebra finch subspecies, Taeniopygia guttata guttata and T.g. castanotis. Phil. Trans. R. Soc. B. 1990;330:351–370. [Google Scholar]
- Collins S.A. The effect of recent experience on female choice in zebra finches. Anim. Behav. 1995;49:479–486. 10.1006/anbe.1995.0062 [Google Scholar]
- Cordero A. Forced copulations and female contact guarding at a high male density in a calopterygid damselfly. J. Insect Behav. 1999;12:27–37. 10.1023/A:1020972913683 [Google Scholar]
- Cotton S, Fowler K, Pomiankowski A. Condition-dependence of sexual ornament size and variation in the stalk-eyed fly Cyrtodiopsis dalmanni (Diptera: Diopsidae) Evolution. 2004a;58:1038–1046. doi: 10.1111/j.0014-3820.2004.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Cotton S, Fowler K, Pomiankowski A. Heightened condition dependence is not a general feature of male eyespan in stalk-eyed flies (Diptera: Diopsidae) J. Evol. Biol. 2004;17:1310–1316. doi: 10.1111/j.1420-9101.2004.00754.x. 10.1111/j.1420-9101.2004.00754.x [DOI] [PubMed] [Google Scholar]
- David P, Hingle A, Greig D, Rutherford A, Pomiankowski A, Fowler K. Male sexual ornament size but not asymmetry reflects condition in stalk-eyed flies. Proc. R. Soc. B. 1998;265:2211–2216. 10.1098/rspb.1998.0561 [Google Scholar]
- David P, Bjorksten T, Fowler K, Pomiankowski A. Condition-dependent signalling of genetic variation in stalk-eyed flies. Nature. 2000;406:186–188. doi: 10.1038/35018079. 10.1038/35018079 [DOI] [PubMed] [Google Scholar]
- de la Motte I, Burkhardt D. Portrait of an Asian stalk-eyed fly. Naturwissenschaften. 1983;70:451–461. 10.1007/BF01079611 [Google Scholar]
- Fawcett T.W, Johnstone R.A. Mate choice in the face of costly competition. Behav. Ecol. 2003;14:771–779. 10.1093/beheco/arg075 [Google Scholar]
- Gabor C. Association patterns of sailfin mollies (Poecilia latipinna): alternative hypotheses. Behav. Ecol. Sociobiol. 1999;46:333–340. 10.1007/s002650050627 [Google Scholar]
- Gerhardt H.C. Female mate choice in treefrogs: static and dynamic acoustic criteria. Anim. Behav. 1991;42:615–635. [Google Scholar]
- Greene E, Lyon B.E, Muehter V.R, Ratcliffe L, Oliver S.J, Boag P.T. Disruptive sexual selection for plumage coloration in a passerine bird. Nature. 2000;407:1000–1003. doi: 10.1038/35039500. 10.1038/35039500 [DOI] [PubMed] [Google Scholar]
- Hardy I.C.W, Field S.A. Logistic analysis of animal contests. Anim. Behav. 1998;56:787–792. doi: 10.1006/anbe.1998.0833. 10.1006/anbe.1998.0833 [DOI] [PubMed] [Google Scholar]
- Hingle A, Fowler K, Pomiankowski A. Size-dependent mate preference in the stalk-eyed fly Cyrtodiopsis dalmanni. Anim. Behav. 2001a;61:589–595. doi: 10.1098/rspb.2001.1647. 10.1006/anbe.2000.1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingle A, Fowler K, Pomiankowski A. The effect of transient food stress on female mate preference in the stalk-eyed fly Cyrtodiopsis dalmanni. Proc. R. Soc. B. 2001b;268:1239–1240. doi: 10.1098/rspb.2001.1647. 10.1098/rspb.2001.1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honek A. Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos. 1993;66:483–492. [Google Scholar]
- Houle D, Kondrashov A.S. Coevolution of costly mate choice and condition-dependent display of good genes. Proc. R. Soc. B. 2002;269:97–104. doi: 10.1098/rspb.2001.1823. 10.1098/rspb.2001.1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J, Brooks R, Jennions M.D. Female mate choice as a condition-dependent life history trait. Am. Nat. 2005;166:79–93. doi: 10.1086/430672. 10.1086/430672 [DOI] [PubMed] [Google Scholar]
- Jennions M.D, Petrie M. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. 1997;72:283–327. doi: 10.1017/s0006323196005014. 10.1017/S0006323196005014 [DOI] [PubMed] [Google Scholar]
- Knell R.J, Fruhauf A, Norris K.A. Conditional expression of a sexually selected trait in the stalk-eyed fly Diasemopsis aethiopica. Ecol. Entomol. 1999;24:323–328. 10.1046/j.1365-2311.1999.00200.x [Google Scholar]
- Nilsson S.O, Nilsson G.E. Free choice by female sticklebacks: lack of preference for male dominance traits. Can. J. Zool. 2000;78:1251–1258. 10.1139/cjz-78-7-1251 [Google Scholar]
- Panhuis T.M, Wilkinson G.S. Exaggerated eyespan influences male contest outcome in stalk-eyed flies. Behav. Ecol. Sociobiol. 1999;46:221–227. 10.1007/s002650050613 [Google Scholar]
- Pomiankowski A. The costs of choice in sexual selection. J. Theor. Biol. 1987;128:195–218. doi: 10.1016/s0022-5193(87)80169-8. [DOI] [PubMed] [Google Scholar]
- Qvarnström A. Context-dependent genetic benefits from mate choice. Trends Ecol. Evol. 2001;16:5–7. doi: 10.1016/s0169-5347(00)02030-9. [DOI] [PubMed] [Google Scholar]
- Ritchie M.G. The shape of female mating preferences. Proc. Natl Acad. Sci. USA. 1996;93:14 628–14 631. doi: 10.1073/pnas.93.25.14628. 10.1073/pnas.93.25.14628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D.W, Baker R.H, Chapman T, Denniff M, Pomiankowski A, Fowler K. Direct and correlated responses to artificial selection on male mating frequency in the stalk-eyed fly Cyrtodiopsis dalmanni. J. Evol. Biol. 2005;18:642–650. doi: 10.1111/j.1420-9101.2004.00860.x. 10.1111/j.1420-9101.2004.00860.x [DOI] [PubMed] [Google Scholar]
- Sappington T.W, Taylor O.R. Disruptive sexual selection in Colias eurytheme butterflies. Proc. Natl Acad. Sci. USA. 1990;87:6132–6135. doi: 10.1073/pnas.87.16.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D. Estimating the form of natural selection on a quantitative trait. Evolution. 1988;42:849–861. doi: 10.1111/j.1558-5646.1988.tb02507.x. [DOI] [PubMed] [Google Scholar]
- Shackleton M.A, Jennions M.D, Hunt J. Fighting success and attractiveness as predictors of mate mating success in the black field cricket, Teleogryllus commodus: the effectiveness of no-chioce tests. Behav. Ecol. Sociobiol. 2005;58:1–8. 10.1007/s00265-004-0907-1 [Google Scholar]
- Tomlinson I.P.M, O'Donald P. The influence of female viability differences on the evolution of mate choice. Heredity. 1996;77:303–312. 10.1038/sj.hdy.6880180 [Google Scholar]
- Turner G.F, Burrows M.T. A model of sympatric speciation by sexual selection. Proc. R. Soc. B. 1995;260:287–292. [Google Scholar]
- Wagner W.E. Measuring female mating preferences. Anim. Behav. 1998;55:1029–1042. doi: 10.1006/anbe.1997.0635. 10.1006/anbe.1997.0635 [DOI] [PubMed] [Google Scholar]
- Wagner W.E, Murray A, Cade W.H. Phenotypic variation in the mating preferences of female field crickets, Gryllus integer. Anim. Behav. 1995;49:1269–1281. 10.1006/anbe.1995.0159 [Google Scholar]
- Wilkinson G.S. Genetic consequences of sexual selection in stalk-eyed flies. In: Dugatkin L.A, editor. Model systems in behavioural ecology. Integrating conceptual, theoretical, and empirical approaches. Princeton University Press; Princeton, NJ: 2001. pp. 72–91. [Google Scholar]
- Wilkinson G.S, Dodson G.N. Function and evolution of antlers and eye stalks in flies. In: Choe J, Crespi B, editors. The evolution of mating systems in insects and arachnids. Cambridge University Press; Cambridge, UK: 1997. pp. 310–328. [Google Scholar]
- Wilkinson G.S, Reillo P.R. Female preference response to artificial selection on an exaggerated male trait in a stalk-eyed fly. Proc. R. Soc. B. 1994;255:1–6. [Google Scholar]
- Wilkinson G.S, Kahler H, Baker R.H. Evolution of female mating preferences in stalk-eyed flies. Behav. Ecol. 1998;9:525–533. [Google Scholar]
- Zeh J.A, Newcomer S.D, Zeh D.W. Polyandrous females discriminate against previous mates. Proc. Natl Acad. Sci. USA. 1998;95:13 732–13 736. doi: 10.1073/pnas.95.23.13732. 10.1073/pnas.95.23.13732 [DOI] [PMC free article] [PubMed] [Google Scholar]